Efficacy of cyclophosphamide, doxorubicin, and cisplatin for adenoid cystic carcinoma, and their relationship with the pre-chemotherapy tumor growth rate
Introduction
Adenoid cystic carcinoma (ACC) is a rare cancer that occurs mainly in the salivary gland. ACC accounts for approximately 10% of salivary neoplasms and 1% of head and neck cancer cases (1). The most common sites of ACC are the minor salivary gland and submaxillary sinus (2). ACC is an indolent tumor that grows slowly, but commonly metastasizes to the lungs and bones (3,4).
The standard treatment for ACC is surgical resection and/or radiotherapy according to the tumor site, stage, and histologic grade (5,6). However, approximately 60% of patients who undergo resection eventually experience relapse with or without distant metastasis, and they have a poor prognosis (7). Chemotherapy is administered for advanced, relapsed, or metastatic ACC; however, ACC does not respond well to palliative chemotherapy (8). Although several phase II studies on palliative cytotoxic chemotherapy for ACC have been reported, the response rate is mostly <20% (8,9). Therefore, the role of palliative chemotherapy for ACC is still controversial because of its chemo-resistance and the lack of large-sized studies evaluating this issue.
Patients with metastatic ACC usually show an indolent asymptomatic clinical course without active treatment. However, metastatic lesions eventually grow rapidly and the patient becomes symptomatic, although many metastatic ACC lesions usually present as stable disease without active chemotherapy till a certain time point. Therefore, it is not easy for clinicians to decide when to initiate chemotherapy. Based on the results of several phase II studies, some experts prefer to administer chemotherapy when the tumor starts growing rapidly or when the patient starts showing symptoms. However, there are no data regarding the pre-chemotherapy growth velocity and the optimal timing for initiating chemotherapy. Furthermore, when physicians encounter a stable disease rate in phase II trials, it is difficult to distinguish between natural stable disease without chemotherapy and active disease control owing to chemotherapy (10).
Therefore, in this study, we evaluated (I) the efficacy of cyclophosphamide, doxorubicin, and cisplatin (CAP) combination chemotherapy, and (II) analyzed the relationship between the pre-chemotherapy tumor growth rate (P-TGR) and treatment outcomes of CAP.
Methods
Patient population
We retrospectively reviewed the medical records of patients diagnosed with ACC and treated with CAP chemotherapy at Seoul National University Hospital (SNUH) between November 2015 and December 2018. The diagnosis was confirmed pathologically.
We included patients aged 18 years or older, who had a measurable lesion according to the Response Evaluation Criteria for Solid Tumors (RECIST 1.1) (11), an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0-2, and adequate hematologic, hepatic, and renal functions.
Treatment schedule and calculation of the tumor growth rate
Patients were treated with palliative CAP, with 750 mg/m2 cyclophosphamide, 60 mg/m2 cisplatin, and 40 mg/m2 doxorubicin, all administered intravenously on day 1 every 3 weeks. Chemotherapy was continued for a maximum of 6 cycles owing to the cumulative cardiac toxicity of doxorubicin. Response evaluation was performed according to the RECIST 1.1. P-TGR was defined as the difference in the sum of the largest diameter of the target lesion per unit of time between the pre-baseline and baseline CT images: (S0 – Spre)/(T0 – Tpre), where S0 and Spre indicate the sum of the largest diameter of target lesions at pre-baseline and baseline, while T0 and Tpre indicate the pre-baseline and baseline time (12). Tumor shrinkage% was defined as the largest difference in the sum of the target lesion between baseline and after CAP chemotherapy.
Progression-free survival (PFS) was defined as the time between the first day of CAP to the date of disease progression or death. Overall survival (OS) was defined as the time between the first day of CAP to the date of death. Median PFS, OS, and follow-up period were calculated using the Kaplan-Meier method. The relationship between P-TGR and tumor shrinkage% after CAP was analyzed by using the Pearson method. Statistical analysis was performed using R version 3.5.3 (R Development Core Team, https://www.r-project.org/).
Ethical considerations
The study protocol was reviewed and approved by the Institutional Review Board of SNUH (approval number: H-1812-059-993). This study was conducted according to the guidelines for biomedical research and Declaration of Helsinki. The need for informed consent from patients was waived owing to the retrospective nature of the study.
Results
Patient characteristics
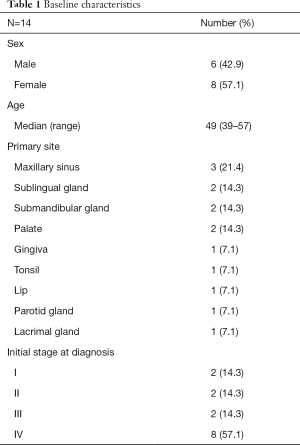
A total of 14 patients (6 men, 8 women) were enrolled in this study. The baseline characteristics of the patients are summarized in Table 1. The median patient age was 49 years (range, 39-57 years). The most common primary sites of ACC were the sublingual gland and the maxillary sinus in 3 patients. Eight patients were had stage IV ACC at the time of diagnosis. All patients included in this study received prior local treatment before CAP chemotherapy, except for 1 patient: 10 patients underwent surgical treatment and 13 patients received prior radiotherapy. Five patients received other systemic treatment including chemotherapy and tyrosine kinase inhibitors. The median time from initial diagnosis of ACC to CAP chemotherapy was 51.0 months (range, 1.8–268.6 months). The median follow-up duration was 29.2 months.
Full table
Treatment outcomes
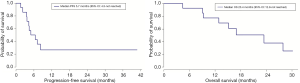
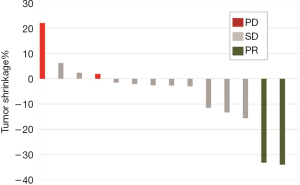
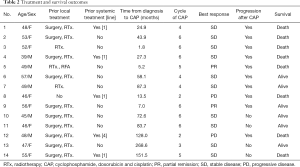
The patients received a median of 5 cycles of CAP (range, 2–6 cycles). Figure 1 shows the waterfall graph for the tumor shrinkage% after CAP chemotherapy. Two patients achieved partial response (PR) and 10 patients showed stable disease (71.4%). Overall response rate was 14.3% (95% CI, −4.0% to 32.6%), and the disease control rate was 85.7% (95% CI, 67.4% to 104.0%). Eight patients died of ACC disease progression. Table 2 summarizes the detailed outcome of each patient. Median PFS was 5.7 months (95% CI: 4.3 to not reached). Median OS was 23.4 months (95% CI: 12.9 to not reached; Figure 2).
Full table
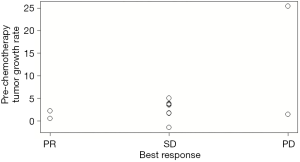
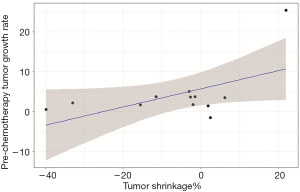
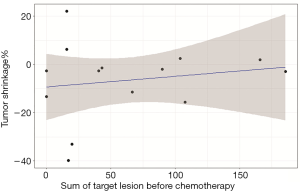
Patients who achieved PR had a low P-TGR (Figure 3). P-TGR and tumor shrinkage% according to the RECIST 1.1 were positively correlated with a correlation coefficient of 0.56 (P=0.06; Figure 4). However, the sum of the target lesion before CAP chemotherapy was not correlated with the tumor shrinkage% (Figure 5).
Discussion
In this study, palliative CAP chemotherapy had a modest anti-cancer effect, with a response rate of 14.3% and median PFS of 5.7 months. A low P-TGR was associated with the response to CAP chemotherapy.
Chemotherapy for ACC was performed mainly for disease stabilization at the time of rapid progression of systemic metastasis. Anticancer drugs such as cisplatin, and 5-fluorouracil have been used alone or in combination to treat ACC (13-15). Several small-sized phase II studies have reported the effect of mitoxantrone, vinorelbine, paclitaxel, and gemcitabine (16-19). However, the overall response rate of these anticancer drugs for ACC is <20% (8,9,20). Although patients with salivary cancer treated with CAP showed a response rate of approximately 25–30%, all cases of salivary cancer not only ACC, were included, and only a few studies with ACC cases have been performed (15,21,22). In the present study, only 2 of 14 patients achieved PR. However, 10 patients (71.4%) had stable disease without any severe adverse effects. Therefore, CAP combination chemotherapy may be considered for ACC treatment.
ACC is an indolent cancer, with patients showing symptoms after long periods. In some cases, patients with multiple metastasis showed long-term survival of >10 years (23-25). In patients with metastatic ACC without symptoms, there is no definite consensus for when to start systemic chemotherapy. Physicians often follow the wait-and-watch approach to determine the appropriate time for chemotherapy and initiate chemotherapy when the tumor starts growing rapidly. Thus, it is difficult to decide when to start chemotherapy for ACC patients. In our study, patients who had a lower P-TGR showed a good response to CAP chemotherapy. The patients who achieved PR were treatment naïve patients and two patients with PD were took systemic chemotherapy before CAP chemotherapy. Three of five patients who treated with other systemic chemotherapy were experienced SD. Although previous treatment is not consistent, our study suggests that early initiation of chemotherapy for patients with a low P-TGR may be more helpful for disease control.
Our study has several limitations. This was a single-center retrospective study, with a small number of patients. Moreover, the prior administered treatments varied between patients.
Nevertheless, considering the rare incidence of ACC, it is difficult to conduct a randomized phase III trial for evaluating the effect of chemotherapy. Our study confirmed the effect of CAP chemotherapy for ACC and determined the relationship between the P-TGR and treatment response. Owing to the limited research about when chemotherapy for ACC should be started, this study has the strength of being able to determine the timing for the initiation of chemotherapy. However, further studies are needed to determine the role of CAP chemotherapy and prognostic factors under homogeneous clinical conditions.
In conclusion, the results of this study suggested that CAP chemotherapy might be a suitable treatment option for ACC. A low P-TGR was associated with a good response to CAP chemotherapy. Early initiation of chemotherapy could be helpful to control metastatic ACC, although the appropriate timing of chemotherapy should be considered carefully.
Acknowledgments
We thank the patients included in the current study.
Funding: This study was supported by a grant from the Korea Health Technology R&D Project “Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer” through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (MHW), Republic of Korea (grant number HI17C2085).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2020.03.07). BK serves as an unpaid editorial board member of Chinese Clinical Oncology from Mar 2019 to Feb 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was reviewed and approved by the Institutional Review Board of SNUH (approval number: H-1812-059-993).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin: a clinicopathologic study of 242 cases. Am J Surg 1974;128:512-20. [Crossref] [PubMed]
- Spiro RH. Salivary neoplasms: overview of a 35‐year experience with 2,807 patients. Head Neck Surg 1986;8:177-84. [Crossref] [PubMed]
- Bradley PJ. Distant Metastases from Salivary Glands Cancer. ORL J Otorhinolaryngol Relat Spec 2001;63:233-42. [Crossref] [PubMed]
- Fordice J, Kershaw C, El-Naggar A, et al. Adenoid Cystic Carcinoma of the Head and Neck. Arch Otolaryngol Head Neck Surg 1999;125:149. [Crossref] [PubMed]
- Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg 1992;164:623-8. [Crossref] [PubMed]
- CancerResearch. Head and neck cancers statistics. Cancer Res UK 2019.
- Howard DJ, Lund VJ. Reflections on the management of adenoid cystic carcinoma of the nasal cavity and paranasal sinuses. Otolaryngol Head Neck Surg 1985;93:338-41. [Crossref] [PubMed]
- Papaspyrou G, Hoch S, Rinaldo A, et al. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck 2011;33:905-11. [Crossref] [PubMed]
- Laurie SA, Ho AL, Fury MG, et al. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol 2011;12:815-24. [Crossref] [PubMed]
- Keam B, Kim SB, Shin SH, et al. Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer 2015;121:2612-7. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Schramm VL, Srodes C, Myers EN. Cisplatin Therapy for Adenoid Cystic Carcinoma. Arch Otolaryngol 1981;107:739-41. [Crossref] [PubMed]
- Hill ME, Constenla DO, A’Hern RP, et al. Cisplatin and 5-fluorouracil for symptom control in advanced salivary adenoid cystic carcinoma. Oral Oncol 1997;33:275-8. [Crossref] [PubMed]
- de Haan LD, De Mulder PHM, Vermorken JB, et al. Cisplatin‐based chemotherapy in advanced adenoid cystic carcinoma of the head and neck. Head Neck 1992;14:273-7. [Crossref] [PubMed]
- Mattox DE, Von Hoff DD, Balcerzak SP. Southwest Oncology Group study of mitoxantrone for treatment of patients with advanced adenoid cystic carcinoma of the head and neck. Invest New Drugs 1990;8:105-7. [Crossref] [PubMed]
- Airoldi M, Pedani F, Succo G, et al. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer 2001;91:541-7. [Crossref] [PubMed]
- Gilbert J, Li Y, Pinto HA, et al. Phase II trial of taxol in salivary gland malignancies (E1394): A trial of the Eastern Cooperative Oncology Group. Head Neck 2006;28:197-204. [Crossref] [PubMed]
- van Herpen CML, Locati LD, Buter J, et al. Phase II study on gemcitabine in recurrent and/or metastatic adenoid cystic carcinoma of the head and neck (EORTC 24982). Eur J Cancer 2008;44:2542-5. [Crossref] [PubMed]
- Licitra L, Cavina R, Grandi C, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma: A phase H trial of 22 patients. Ann Oncol 1996;7:640-2. [Crossref] [PubMed]
- Licitra L, Cavina R, Grandi C. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. Ann Oncol 1996;7:640. [Crossref] [PubMed]
- Creagan ET, Woods JE, Rubin J, et al. Cisplatin‐based chemotherapy for neoplasms arising from salivary glands and contiguous structures in the head and neck. Cancer 1988;62:2313-9. [Crossref] [PubMed]
- Umeda M, Nishimatsu N, Masago H, et al. Tumor-doubling time and onset of pulmonary metastasis from adenoid cystic carcinoma of the salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:473-8. [Crossref] [PubMed]
- Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg 1997;174:495-8. [Crossref] [PubMed]
- Vikram B, Strong EW, Shah JP, et al. Radiation therapy in adenoid-cystic carcinoma. Int J Radiat Oncol Biol Phys 1984;10:221-3. [Crossref] [PubMed]