
Thermal ablation for hepatocellular carcinoma: what’s new in 2019
Introduction
Liver cancer is currently the sixth most common cancer and fourth leading cause of cancer-related death worldwide. Hepatocellular carcinoma (HCC) is the most common primary liver tumor and its incidence has been increasing worldwide in recent decades (1-3). Due to the complex nature of this disease, many factors are considered when determining the best treatment option for HCC, including tumor extent and location, the underlying liver function, presence or absence of extra-hepatic disease, patient performance status, and co-morbidities. For this reason, all potential curative therapies for HCC (e.g., ablation, resection, transplantation) should be utilized as first-line treatments, whenever feasible (4).
Thermal ablation for HCC is accepted as a curative treatment option in many HCC treatment guidelines because of the excellent outcomes performed in a more minimally invasive manner (4-8). In fact, during the past decade, many cohort and comparative studies have shown that ablation for early HCC provides promising survival gains comparable to those of surgical resection (9-17). Given this, EASL-EROTC guidelines recently recommended ablation as a first line option for very early stage (single, smaller than 2 cm in diameter) HCC rather than surgical resection (4).
From a technical standpoint, complete and accurate ablation is essential to achieve the best outcomes after ablation for HCC. Therefore, many technological advances are continually introduced to improve upon the effectiveness of thermal ablation. In an attempt to tailor therapy to a specific patient and tumor (e.g., size, location, histology, etc.), diverse energy sources are now used. Microwave ablation (MWA) has recently gained popularity around the world because of its intrinsic advantages of faster ablation with high temperature and less susceptibility to heat sink effect when compared to radiofrequency ablation (RFA) (18-26). In addition, novel guidance systems using fusion or contrast-enhanced ultrasound techniques have become more common, especially in countries where sonographic guidance is the predominant imaging guidance modality (27-29). Furthermore, the benefits of “no-touch” techniques using multiple RF electrodes to achieve sufficient ablative margins without the need for direct tumor puncture have recently been reported (30-35). Lastly, antiviral treatment has contributed to overall improved outcomes after thermal ablation for HCC by decreasing tumor recurrence (36).
The purpose of this review, therefore, is to highlight the current state of ablation in HCC treatment guidelines and update on recently introduced therapeutic outcomes and new advances in ablation-related techniques.
Role of ablation in current HCC management guidelines
All of the major hepatology societies have proposed HCC management guidelines to assess the scientific evidence and to provide the best guidance for clinicians treating these patients. The Asia-Pacific, American, and European societies have all recently published updated their guidelines (4-8) and these are presented below.
The Asia-Pacific Association of the Study of Liver (APASL) Guidelines currently recommend local ablation for the following conditions: (I) Child-Pugh class A or B patients with HCC (<3 in number and smaller than 3 cm in diameter); (II) for 2 cm or smaller in Child-Pugh class A or B cirrhosis, RFA is a first-line treatment (5); and (III) where cases are concerning the safety after RFA, ethanol injection can be considered.
The current American Association of the Study of Liver Disease (AASLD) HCC guidelines states the following: (I) thermal ablation is the best modality for HCCs smaller than 3 cm in diameter, although MWA now has the potential to show better tumoral control than RFA; (II) resection, local ablation, or transarterial chemoembolization (TACE) can be considered to minimize the risk of tumor progression as a ‘bridge’ to transplant; and (III) RFA or MWA may be considered as first-line treatment in very early-stage disease (BCLC 0) (6).
The European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC) Clinical Practice Guidelines suggest the following: (I) RFA is recommended as a first-line treatment for very early-stage disease (tumors <2 cm diameter); (II) RFA has been adopted as an alternative first-line option for patients with early-stage HCC, considering survival benefit similar to that of surgery in RCTs and meta-analyses; (III) MWA is comparable to RFA, but has the potential advantage for being able to treat 3–5-cm tumors but the reduced impact of the cooling effect of large vessels remains unknown; (IV) for exophytic tumors or tumors abutting the gallbladder, liver hilum, or intestine, laparoscopic surgery may be better than thermal ablation and (V) thermal ablation is superior to ethanol injection except for small lesions (4).
According to a recent comparative review of the three major HCC treatment guidelines (37), in AASLD guideline, locoregional treatment can be considered for cirrhotic patients with HCC (e.g., T2 or T3, no vascular involvement), if not candidates for transplantation or resection while in the APASL guideline, ablation is recommended as an alternative to resection for Child-Pugh class A or B patients with ≤3 tumors that are each ≤3 cm. Furthermore, ablation is recommended as a first-line treatment for very early stage (single, ≤2 cm). In EASL guideline, ablation is considered as the “standard of care” for unresectable BCLC 0 and A tumors, an alternative to resection in single tumors that are 2 to 3 cm, and even as a potential first-line therapy for resectable BCLC-0 HCCs with favorable locations. Therefore, ablation as an alternative to resection has been validated by comparable outcomes and with minimal morbidity (Figure 1).

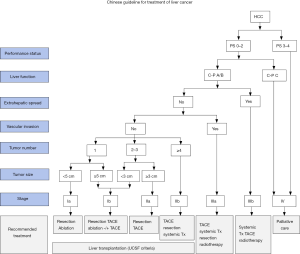
In addition to the guidelines discussed above, Chinese HCC management guidelines proposed by the National Health and Family Planning Commission of the People’s Republic of China state the following: First, local ablation is recommended for a single tumor <5 cm or up to three tumors <3 cm without vascular invasion or extrahepatic metastasis as the outcomes are similar in patients with Child-Pugh class A or B. The tumor-free survival rate of RFA, however, is slightly lower than that of surgical resection for patients with tumors that are ≤3 cm. Second, local ablation is often considered in combination with TACE for patients with unresectable tumors (3–7 cm). Third, RFA is the most commonly used minimally invasive therapy in China for liver cancer due to its many advantages including ease of use, favorable efficacy, and cost-effectiveness (Figure 2) (7).
Finally, the Korean Liver Cancer Association (KLCA)-National Cancer Center (NCC) Korea Practice Guidelines for the Management of HCC recommend RFA with the following options: (I) as an alternative to resection in patients with a single nodular HCC (≤3 cm), considering comparable outcomes and better safety; (II) RFA is superior to ethanol injection except for smaller (<2 cm) tumor or unfavorable location for RFA; (III) TACE combined with RFA is recommended for 3–5 cm unresectable HCCs and (IV) MWA and cryoablation are expected to provide comparable outcomes and safety compared to RFA (8).
Based on the extensive, accumulated clinical experience and the scientific evidence regarding HCC ablation since 1990, all currently available HCC management guidelines from major academic societies accept ablation therapy as a curative treatment option for early stage HCC, especially for patients whose hepatic reserve is insufficient and for those who have a co-morbidity that prohibits resection. Furthermore, ablation therapy could be an adjunctive option as a combined treatment for intermediate stage HCC. However, there is no specific recommendation regarding ablation energy or guiding modality due to limited evidence so far (4-8,37).
Updated therapeutic outcomes of thermal ablation for HCC
During the past two decades, several randomized controlled studies and meta-analysis studies have proven the clinical benefit and safety of thermal ablation for HCC when compared to surgical resection (9-18). In fact, three randomized controlled trials of patients with up to 3 HCC tumors (smaller than 5 cm in diameter) compared the outcomes between RFA and resection. One trial demonstrated a survival benefit for resection over RFA, but the other two trials showed no significant difference in recurrence-free and overall survival rates (16-18). Most meta-analyses also demonstrated that ablation has similar overall survival but inferior local tumor control rates compared to resection. However, two recent meta-analyses showed that resection had better recurrence-free and overall survival compared to RFA (38,39). According to the interim report of the SURF study from Japan, there was no significant difference in 3-year recurrence-free survival and overall survival between resection and RFA for early-stage HCC (40).
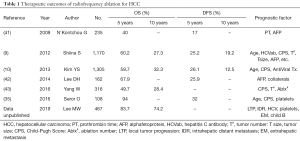
The two largest cohort studies of RFA reported similar overall survival rates at 5 and 10 years, while there were large differences in local tumor control rates depending on the treatment strategy for insufficient ablative margin (9,10) (Table 1). However, more recently, studies showed significant improvement in overall survival (approaching 80% at 5 years) due to technological advances and post-ablation antiviral therapy (36,44). A recent cohort investigation of 301 potentially transplantable patients with single HCC ≤3 cm treated by RFA as first line demonstrated that 5-year survival rates after RFA were 79.0% in the HCC ≤2 cm group [vs. 70.9% in the HCC >2 cm group (P=0.01)]. Prognostic factors of post-RFA recurrence outside Milan criteria were larger tumor (>2 cm) and level of serum alpha-fetoprotein (44,45).
Full table
While tumor size and location are well-proven factors regarding the procedure feasibility and tumor control rate, tumor aggressiveness, the addition of a non-hypervascular hepatobiliary phase, hypointense nodule on gadoxetic acid-enhanced MRI was recently found to be an important imaging biomarker related to survival after ablation. For this reason, Lee et al proposed prediction models for microvascular invasion using clinical and imaging variables. For example, in patients with high tumor markers (e.g., AFP, PIVKA II, etc.) and MRI findings suggesting microvascular invasion (e.g., peritumoral enhancement, hepatobiliary peritumoral hypodensity, etc.), the early recurrence rate was higher in the RFA group than resection group (46). Another study reported that non-hypervascular hepatobiliary phase (HBP) hypointense nodules on liver MRI can be a predictor for recurrence-free survival after RFA and surgery. The 5-year recurrence free survival rates were significantly lower in RFA (51%) than resection (65%) group in patients without those nodules, while the same study showed similar results in the RFA (28%) and resection (34%) groups in patients with those nodules. For this reason, surgery may be the more appropriate treatment modality for even very early stage HCC, if there was not associated with that kind of nodules on pre-treatment MRI (47).
In terms of the “no touch” technique, Mohkam et al. recently reported a comparative study between no-touch multibipolar RFA (NTM-RFA, n=77) and liver resection (LR, n=62) for single HCC between 2 and 5 cm. In fact, they found no significant difference in local recurrence rates at one and three years between the two groups (RFA 5.5%, 10.0% vs. resection 1.9%, 1.9%). For disease-free recurrence and overall survival at 3 years, there was also no significant difference between the both groups (RFA, 40.8% vs. resection, 56.4%; RFA, 86.7% vs. resection, 91.4%) (48).
In 2019, one interesting study reported contemporary treatment trends and outcomes from the United States National Cancer Database by comparing RFA with surgical resection for HCC. A total of 18,296 patients were evaluated (resection, n=10,085 vs. RFA, n=8,211). RFA was found to be superior to resection in terms of hospital stay duration, unplanned readmission, and 1- and 3-month mortality. For HCC with smaller than 1.5 cm in diameter, RFA and surgical resection yield similar survival rates (49). However, as Child-Pugh score which represents important predict factor for cirrhosis patients survival was not calculated in this program, more dedicate study is needed in future.
Many clinical studies on MWA for HCC have been published during the last decade (50). However, most of these studies were retrospective in nature and from single institutions. There were only two RCTs that compare MWA with RFA have been reported (21,51). The reported therapeutic outcomes of MWA for HCC were promising as follows: technical success rates ranged from 88% to 95%; progression-free survival rates were ≤92 and 5-year survival rates ranged from 43% to 60% (51). Several retrospective comparative studies also showed that MWA provided better local tumor control than RFA, but failed to prove a survival benefit (19,22-24). In fact, one meta-analysis that included 16 studies involving 2,062 patients found that either MWA or RFA can be used for effective local therapy for HCC because there was no difference in the outcomes including local tumor progression, overall and disease-free survival as well as adverse events (20).
In 2017, a phase III RCT in 405 patients with HCCs (<5 cm) were reported to compare the outcomes and safety of MWA with RFA. There was no significant difference in the technique effectiveness and 5 years local tumor progression (MWA, 99.6%, 11.4% vs. RFA, 98.8%, 19.7%) For 5-year disease-free and overall survival rates, there was no significant difference between the both groups (MWA; 36.7%, 67.3% vs. RFA; 24.1%, 72.7%). The major complication rates were 3.4% for MWA and 2.5% for RFA. They concluded that both MWA and RFA are suitable options for early-stage HCC and MWA is more promising due to its higher thermal efficiency (51).
TACE is currently the standard of care treatment for intermediate-stage HCC in most management guidelines and when combined with ablation, has been proven better than TACE alone in many comparative studies and meta-analyses. One such study by Nouso et al. recently compared overall survival between RFA and TACE group in intermediate-stage HCC. After propensity score matching, the 3 years overall survival rates were higher in RFA group (70%) than in the TACE group (52%) (52).
HBV is identified as one of the most important risk factors in HCC development. Recently, anti-HBV treatment has been reported can prevent HCC recurrence after local therapies. A retrospective cohort study from Taiwan reported that HCC recurrence rates were significantly lower in patients received anti-HBV therapy compared with rates in untreated group after RFA (41.8% vs. 51.4%) (53). Sohn et al. demonstrated that oral antiviral treatment affected the overall survival after RFA for HBV-related HCC. There was significant difference in overall survival at 5 years between the control and treatment group (77.2% vs. 93.5%) (36). Chen et al. also reported the timely treatment with sustained virologic response using PegINF/RBV can decrease tumor recurrence after RFA for HVC related HCC (54).
As HCC is a complex malignancy with poor hepatic reserve and tendency for recurrence even after curative treatment, a multidisciplinary approach (MDT) is the utmost important to provide the best outcome for the patients with HCC. One recent retrospective study with 6,619 patients with HCC claims that the 5-year survival rate was significantly higher in MDT group compared to that of control group (71.2% vs. 49.4%) (55). Thus, MDT approach may be the promising option to improve the patient’s survival which warrants prospective validation.
Technical advancement
Early detection of smaller tumors has increased due to “state-of-the-art” imaging techniques. Magnetic resonance imaging (MRI) with liver-specific contrast agents now allows imagers to evaluate the hepatobiliary phase besides the arterial and portal venous phase similar to conventional extracellular contrast agents. The diagnostic performance of Gd-EOB-DTPA-enhanced MRI with diffusion-weighted imaging for small HCCs, were reported with a sensitivity of 91–93% (56). Gd-EOB-DTPA-enhanced MRI by adding hypointensity on the hepatobiliary phase can increase sensitivity for diagnosing subcentimeter HCCs (56-59).
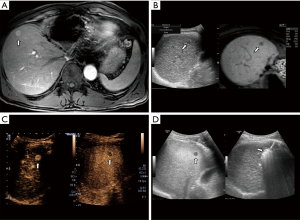
Contrast-enhanced US (CEUS) is also gaining acceptance as a useful imaging tool to diagnose HCC and further characterize indeterminate nodules with equivocal enhancement on CT or MRI. Furthermore, the long temporal window on the post-vascular phase of Sonazoid®-enhanced ultrasound provides improved performance for US guided ablation for HCC. Sonazoid®-enhanced ultrasound can localize the index tumor in over 80% of inconspicuous tumors with fusion imaging (60) (Figure 3). Contrast-enhanced US (CEUS) can be used as an imaging modality for post-ablation assessment after ablation (61).
Fusing imaging systems using a magnetic field generator and position sensor in ultrasound probes are now standard tools for ultrasound-guided HCC ablation. For this reason, an operator can more confidently recognize an inconspicuous tumor on real-time US image by comparing the fused CT/MRI-acquired images along the same sectional plane (61-64). The detectability for small <2 cm tumors can be increased with reducing the probability of mistargeting by using fusion imaging system (65). Percutaneous fusion US imaging with MRI was technically feasible in two-thirds of patients with subcentimeter (<1 cm) recurrent HCC, showing 98.4% of technique efficacy (66). Fusion conventional US combined with Gd-EOB-DTPA-enhanced MRI (hepatobiliary phase) can increase the sensitivity over conventional or CEUS techniques, although small and subcapsular tumors are still difficult to accurately localize due to liver deformation during breathing (67-69).
Another technological development is electromagnetic (EM) tracking, a system for US guided tumor ablation that has been introduced, but for various reasons, has not increased dramatically in popularity. At present, there are several different types of EM tracking including coaxial system with EM-guiding trocar, detachable EM position sensor at handle of electrode, and disposable EM sensor at active tip of electrode (70,71).
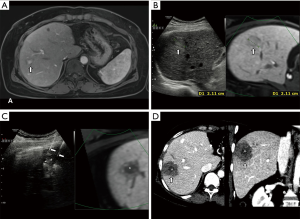
While radiofrequency energy was the most popular energy source for thermal ablation of HCC in the past decade, its limitations include a smaller ablation volume and the potential risk of heat sink effect from adjacent vessels that can ultimately lead to tumor recurrence. The “no touch” technique, with multiple RF electrodes, is drawing attention by demonstrating favorable outcomes compared to traditional tumor insertion techniques using a single electrode (Figure 4). Furthermore, dual-channel RF generators with high power have also been introduced to the market. Many investigations has been introduced to increase the ablation performance using radiofrequency energy including multiple clustered, multi-tined expandable, cooled-wet perfused, multiple switching systems (31,33,72). Once such study by Woo et al. reported the favorable local control rate of 89% for small- and medium-sized HCC by using a monopolar, multiple-electrode switching system (31). In addition, a phase III study using lyso-thermosensitive liposomal doxorubicin with RFA demonstrated the potential to improve survival with RFA dwell time of ≥45 minutes for a solitary lesion. The following OPTIMA study has closed and is awaiting follow-up results (73).
A third-generation microwave ablation system incorporating antenna cooling and high-power generation is spreading widely around the world. This system provides a larger ablation zone with higher temperatures in a given time period compared to RFA (74). Several studies report better local tumor control rates over RFA, but the evidence proving clear survival benefit over RFA is currently insufficient. While MWA does have some clear advantages over RFA, including shorter ablation time, less pain and less heat sink effect (18-26,50,51,75,76), further investigation is warranted to compare RFA with MWA in terms of survival benefit.
Other ablative technologies are used for HCC treatment. Although, cryoablation may be safer for peri-ductal tumors and allows for easy monitoring of the ablation zone during the procedure with an iceball. However, it usually requires multiple applicators for a certain ablation volume, and the clinical outcome evidence over thermal ablation remains limited (77-79). In addition, irreversible electroporation (IRE) is a promising new ablative technique. It mechanism of action uses high-voltage, low-energy direct current to create nanopores within the cell membrane by passage of electrons through adjacent cells. Although clinical data regarding use of IRE to treat HCC are also very limited, a recent study reported an 18-month recurrence-free survival for patients with unresectable HCCs treated by IRE (80,81). HIFU treatment is also a well-known, non-invasive technique that has mainly been used for advanced HCC in China. However, it is not as popular because of the longer procedure time and limited therapeutic window caused by the thoracic cage and respiratory motion (82). One recently introduced ablation technique is histotripsy, which can fractionate tissue through a mechanical, non-invasive ultrasonic ablation process that precisely controls acoustic cavitation while utilizing real-time US guidance (83).
Research on the relationship between ablation and the immune system continues to emerge as the era of immunotherapy evolves as a new paradigm in the field of oncology. Many recent studies have evaluated immuno-ablation (i.e., abscopal effect) in which the use of ablation technology releases immune-related antigens that evoke a transitional immune response. Several studies have reported the relationship of ablation and ficolin-3, macrophage migration inhibitory factor (MIF), and circulating T-cell subsets (84-86).
Conclusions
Image-guided tumor ablation using thermal energy for early-stage HCC has been accepted as a curative treatment option in all HCC treatment guidelines. Microwave ablation is gaining popularity due to its more effective ablation performance with high temperature heating and lack of heat sink effect. However, more evidence of real survival gain is required for MWA to replace RFA. Therapeutic outcomes are improved due to current technical advances in fusion US, CEUS, and antiviral treatment. Cryoablation and IRE are promising tools for safe ablation, but more clinical evidence is needed. Lastly, immuno-ablation has the potential to maximize therapeutic outcomes after ablation. However, further study into this very interesting and exciting area is warranted.
Acknowledgments
Funding: This work was supported by Natural Science Foundation of Jiangsu Province (BK20161064) and China Postdoctoral Science Foundation (2015M581837).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Alazawi W, Cunningham M, Dearden J, et al. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther 2010;32:344-55. [Crossref] [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD Guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer 2018;7:235-60. [Crossref] [PubMed]
- Korean Liver Cancer Association (KLCA); National Cancer Center (NCC). 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol 2019;20:1042-113. [Crossref] [PubMed]
- Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569-77. [Crossref] [PubMed]
- Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 2013;58:89-97. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection vs. percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300-7. [Crossref] [PubMed]
- Qi X, Zhao Y, Li H, et al. Management of hepatocellular carcinoma: an overview of major findings from meta-analyses. Oncotarget 2016;7:34703-51. [PubMed]
- Majumdar A, Roccarina D, Thorburn D, et al. Management of people with early- or very early-stage hepatocellular carcinoma. Cochrane Database Syst Rev 2017;3:CD011650. [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802. [Crossref] [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [Crossref] [PubMed]
- Wang Y, Luo Q, Li Y, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PloS One 2014;9:e84484. [Crossref] [PubMed]
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 2011;79:124-30. [Crossref] [PubMed]
- Potretzke TA, Ziemlewicz TJ, Hinshaw JL, et al. Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol 2016;27:631-8. [Crossref] [PubMed]
- Huo YR, Eslick GD. Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis. J Vasc Interv Radiol 2015;26:1139-1146.e2. [Crossref] [PubMed]
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331-7. [Crossref] [PubMed]
- Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PloS One 2013;8:e76119. [Crossref] [PubMed]
- Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82:1379-84. [Crossref] [PubMed]
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 2012;22:1983-90. [Crossref] [PubMed]
- Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol 2008;19:1087-92. [Crossref] [PubMed]
- Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 2012;35:868-74. [Crossref] [PubMed]
- Kang TW, Lee MW, Song KD, et al. Ultrasound-guided radiofrequency ablation using a new electrode with an electromagnetic position sensor for hepatic tumors difficult to place an electrode: a preliminary clinical study. Cardiovasc Intervent Radiol 2017;40:1891-8. [Crossref] [PubMed]
- Ahn SJ, Lee JM, Lee DH, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol 2017;66:347-54. [Crossref] [PubMed]
- Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography 2014;33:227-39. [Crossref] [PubMed]
- Chang W, Lee JM, Yoon JH, et al. No-touch radiofrequency ablation using multiple electrodes: an in vivo comparison study of switching monopolar versus switching bipolar modes in porcine livers. PLoS One 2017;12:e0176350. [Crossref] [PubMed]
- Woo S, Lee JM, Yoon JH, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology 2013;268:589-600. [Crossref] [PubMed]
- Choi JW, Lee JM, Lee DH, et al. Switching monopolar radiofrequency ablation using a separable cluster electrode in patients with hepatocellular carcinoma: a prospective study. PLoS One 2016;11:e0161980. [Crossref] [PubMed]
- Yoon JH, Lee JM, Hwang EJ, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol 2014;15:235-44. [Crossref] [PubMed]
- Hocquelet A, Aubé C, Rode A, et al. Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol 2017;66:67-74. [Crossref] [PubMed]
- Seror O, N’Kontchou G, Nault JC, et al. Hepatocellular carcinoma within Milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology 2016;280:611-21. [Crossref] [PubMed]
- Sohn W, Kang TW, Choi SK, et al. Effect of oral antiviral treatment on long-term outcomes of radiofrequency ablation therapy for hepatitis B virus-related hepatocellular carcinoma. Oncotarget 2016;7:47794-807. [Crossref] [PubMed]
- Foerster F, Galle PR. Comparison of the current international guidelines on the management of HCC. JHEP Reports 2019;1:114-9. [Crossref]
- Lin Y, Wen Q, Guo L, et al. A network meta-analysis on the efficacy and prognosis of different interventional therapies for early-stage hepatocellular carcinoma. Int J Hyperthermia 2018;35:450-62. [Crossref] [PubMed]
- Xu XL, Liu XD, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology 2018;287:461-72. [Crossref] [PubMed]
- Izumi N, Hasegawa K, Nishioka Y, et al. A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). J Clin Oncol 2019;37:abstr 4002.
- N'Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475-83. [Crossref] [PubMed]
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900-9. [Crossref] [PubMed]
- Yang W, Yan K, Goldberg SN, et al. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol 2016;22:2993-3005. [Crossref] [PubMed]
- Lee DH, Lee JM, Kang TW, et al. Clinical Outcomes of Radiofrequency Ablation for Early Hypovascular HCC: A Multicenter Retrospective Study. Radiology 2018;286:338-49. [Crossref] [PubMed]
- Doyle A, Gorgen A, Muaddi H, et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol 2019;70:866-73. [Crossref] [PubMed]
- Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg 2019. [Epub ahead of print].
- Lee DH, Lee JM, Yu MH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MR can help determine the treatment method for HCC. Eur Radiol 2019;29:3122-31. [Crossref] [PubMed]
- Mohkam K, Dumont PN, Manichon AF, et al. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm. J Hepatol 2018;68:1172-80. [Crossref] [PubMed]
- Uhlig J, Sellers CM, Stein SM, et al. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. Eur Radiol 2019;29:2679-89. [Crossref] [PubMed]
- Vogl TJ, Nour-Eldin A, Hammerstingl RM, et al. Microwave ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms - review article. Rofo 2017;189:1055-66. [Crossref] [PubMed]
- Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut 2017;66:1172-3. [Crossref] [PubMed]
- Nouso K, Kariyama K, Nakamura S, et al. Application of radiofrequency ablation for the treatment of intermediate-stage hepatocellular carcinoma. J Gastroenterol Hepatol 2017;32:695-700. [Crossref] [PubMed]
- Lee TY, Lin JT, Zeng YS, et al. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology 2016;63:1517-27. [Crossref] [PubMed]
- Chen YC, Teng W, Hsieh YC, et al. Timely eradication of HCV viremia by PegIFN/RBV is crucial in prevention of post RFA recurrence in CHC-HCC patients. J Formos Med Assoc 2019;118:1239-46. [Crossref] [PubMed]
- Sinn DH, Choi GS, Park HC, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One 2019;14:e0210730. [Crossref] [PubMed]
- Choi JW, Lee JM, Kim SJ, et al. Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR Images and their value as an imaging biomarker. Radiology 2013;267:776-86. [Crossref] [PubMed]
- Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology 2012;264:761-70. [Crossref] [PubMed]
- Yu MH, Kim JH, Yoon JH, et al. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 2014;271:748-60. [Crossref] [PubMed]
- Hwang J, Kim SH, Lee MW, et al. Small (≤2 cm) hepatocellular carcinoma in patients with chronic liver disease: comparison of gadoxetic acid-enhanced 3.0 T MRI and multiphasic 64-multirow detector CT. Br J Radiol 2012;85:e314-e322. [Crossref] [PubMed]
- Min JH, Lim HK, Lim S, et al. Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol 2014;20:61-70. [Crossref] [PubMed]
- Nishigaki Y, Hayashi H, Tomita E, et al. Usefulness of contrast-enhanced ultrasonography using sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res 2015;45:432-40. [Crossref] [PubMed]
- Minami Y, Chung H, Kudo M, et al. Radiofrequency ablation of hepatocellular carcinoma: value of virtual CT sonography with magnetic navigation. AJR Am J Roentgenol 2008;190:W335-41. [Crossref] [PubMed]
- Song KD, Lee MW, Rhim H, et al. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol 2013;201:1141-7. [Crossref] [PubMed]
- Lee MW, Kim YJ, Park HS, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol 2010;194:W396-400. [Crossref] [PubMed]
- Lee MW, Rhim H, Cha DI, et al. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1-3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol 2013;24:958-65. [Crossref] [PubMed]
- Song KD, Lee MW, Rhim H, et al. Percutaneous US/MRI fusion-guided radiofrequency ablation for recurrent subcentimeter hepatocellular carcinoma: technical feasibility and therapeutic outcomes. Radiology 2018;288:878-86. [Crossref] [PubMed]
- Kunishi Y, Numata K, Morimoto M, et al. Efficacy of fusion imaging combining sonography and hepatobiliary phase MRI with Gd-EOB-DTPA to detect small hepatocellular carcinoma. AJR Am J Roentgenol 2012;198:106-14. [Crossref] [PubMed]
- Lim S, Lee MW, Rhim H, et al. Mistargeting after fusion imaging-guided percutaneous radiofrequency ablation of hepatocellular carcinomas. J Vasc Interv Radiol 2014;25:307-14. [Crossref] [PubMed]
- Kang TW, Lee MW, Choi SH, et al. A novel electrode with electromagnetic tip tracking in ultrasonography-guided radiofrequency ablation: A phantom, ex vivo, and in vivo experimental study. Invest Radiol 2015;50:81-7. [Crossref] [PubMed]
- Hakime A, Barah A, Deschamps F, et al. Prospective comparison of freehand and electromagnetic needle tracking for US-guided percutaneous liver biopsy. J Vasc Interv Radiol 2013;24:1682-9. [Crossref] [PubMed]
- Seror O, N'Kontchou G, Tin-Tin-Htar M, et al. Radiofrequency ablation with internally cooled versus perfused electrodes for the treatment of small hepatocellular carcinoma in patients with cirrhosis. J Vasc Interv Radiol 2008;19:718-24. [Crossref] [PubMed]
- Lee JM, Han JK, Kim HC, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol 2007;42:676-83. [Crossref] [PubMed]
- Tak WY, Lin SM, Wang Y, et al. Phase III HEAT study adding Lyso-Thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res 2018;24:73-83. [Crossref] [PubMed]
- Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation—what should you use and why? Radiographics 2014;34:1344-62. [Crossref] [PubMed]
- Kamal A, Elmoety AAA, Rostom YAM, et al. Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial. J Gastrointest Oncol 2019;10:562-71. [Crossref] [PubMed]
- Shiina S, Sato K, Tateishi R, et al. Percutaneous ablation for hepatocellular carcinoma: comparison of various ablation techniques and surgery. Can J Gastroenterol Hepatol 2018;2018:4756147. [Crossref] [PubMed]
- Rong G, Bai W, Dong Z, et al. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One 2015;10:e0123065. [Crossref] [PubMed]
- Xu J, Noda C, Erickson A, et al. Radiofrequency ablation vs. cryoablation for localized hepatocellular carcinoma: a propensity-matched population study. Anticancer Res 2018;38:6381-6. [Crossref] [PubMed]
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015;61:1579-90. [Crossref] [PubMed]
- Mafeld S, Wong JJ, Kibriya N, et al. Percutaneous irreversible electroporation (IRE) of hepatic malignancy: a bi-institutional analysis of safety and outcomes. Cardiovasc Intervent Radiol 2019;42:577-83. [Crossref] [PubMed]
- Tian G, Zhao Q, Chen F, et al. Ablation of hepatic malignant tumors with irreversible electroporation: A systematic review and meta-analysis of outcomes. Oncotarget 2017;8:5853-60. [PubMed]
- Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235:659-67. [Crossref] [PubMed]
- Knott EA, Swietlik JF, Longo KC, et al. Robotically-assisted sonic therapy for renal ablation in a live porcine model: initial preclinical results. J Vasc Interv Radiol 2019;30:1293-302. [Crossref] [PubMed]
- Shen S, Peng H, Wang Y, et al. Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis. BMC Cancer 2018;18:117. [Crossref] [PubMed]
- Sugimoto K, Kakimi K, Takeuchi H, et al. Irreversible electroporation versus radiofrequency ablation: comparison of systemic immune responses in patients with hepatocellular carcinoma. J Vasc Interv Radiol 2019;30:845-853.e6. [Crossref] [PubMed]
- Zhou Y, Xu X, Ding J, et al. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. J Cancer Res Ther 2018;14:40-5. [Crossref] [PubMed]


