Risk factors for developing hepatocellular carcinoma in Egypt
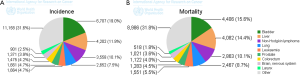
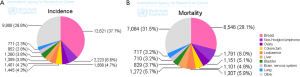
Incidence
In Egypt, hepatocellular carcinoma (HCC) is the second most common cancer in men and the 6th most common cancers in women (Figures 1,2) (1). Hospital-based studies from Egypt have reported an overall increase in the relative frequency of all liver-related cancers in Egypt, from approximately 4% in 1993 to 7.3% in 2003 (2). This rising incidence (3) may be due to high prevalence of hepatitis C virus (HCV) and its complications (4) and the fact that people born 20 years ago or earlier in Egypt has not been vaccinated against hepatitis B virus (HBV) (5). Investigations in Egypt have shown the increasing importance of HCV infection in the etiology of liver cancer, estimated to account for 40-50% of cases, and the declining influence of HBV and HBV/HCV infection (25% and 15%, respectively) (2,6). The rising incidence of HCC in Egypt could be also explained through improvements in screening programs and diagnostic tools (7), as well as the increased survival rate among patients with cirrhosis allowing time for some of them to develop HCC. The higher HCC incidence among urban residents could represent better access to medical facilities, resulting in an underestimate of HCC in rural populations.
Environmental risk factors
Cirrhosis
It has been recognized that the most important clinical risk factor for the development of HCC is cirrhosis. Approximately 80% of HCCs develop in cirrhotic livers (8). The high rate of co-existing cirrhosis in HCC patients and the emergence of HCC in prospectively followed cirrhosis patients have led to the assumption that pre-existing cirrhosis is an important prerequisite for hepatocarcinogenesis, although some HCCs do arise in the absence of cirrhosis (9).
Viral hepatitis and HCC (Table 1)
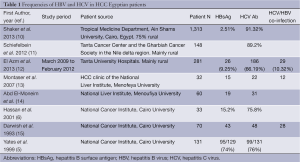
Full table
Although HBV is considered worldwide as a major risk factor for liver cirrhosis and HCC, the prevalence of HBV infection in Egypt has been declining over the last two decades (16). It was found that occult HBV infection and the HBV genotype B or D may influence the outcome of HBV infection leading to the development of HCC and may be strongly associated with HCV in liver carcinogenesis. A decrease in the immune status may result in HBV reactivation in anti-HBs positive patients undergoing chemotherapy (17).
Egypt has possibly the highest HCV prevalence worldwide (18), estimated among the general population to be around 14% (19). Studies of the HCV genome confirmed a uniquely high proportion of genotype 4 (over 90%) in Egypt (20,21). Yet, much of the HCV prevalence data are limited by variability in and selectivity of the populations studied, inconsistent HCV testing methods, and a lack of data regarding mode of transmission. A strong correlation between HCV infection and intravenous treatment for schistosomiasis was frequently reported (22). Schistosomiasis, trematode blood flukes, is endemic in tropical areas of Africa, South America, Asia and the Caribbean. Only S. japonicum which is not present in Egypt has been classified as possibly carcinogenic in humans (23). Since chronic HCV does not typically lead to carcinogenesis for 10-30 years following infection, the rates of liver cancer can be expected to continue increasing until the cohort of intravenous antischistosomal treatment related infected individuals has worked its way through (2,24). This suggests that the true burden of liver cancer in Egypt has yet to be realized.
Chronic HCV infection mostly leads to hepatic cirrhosis before developing HCC (25). HCV is a RNA virus and hence cannot integrate into the host genome. The carcinogenesis of HCV-associated HCC is proposed to be a multistep process involving upregulation of inflammatory cytokines and induction of oxidative stress from chronic hepatitis, fibrosis, liver regeneration, and, ultimately, the development of cirrhosis (26). Moreover, HCV may play a direct role in hepatic carcinogenesis through involvement of viral gene products in inducing liver cell proliferation (27).
Aflatoxins and HCC
There is suggestive evidence for an additional etiologic role of aflatoxin in hepatocarcinogenesis in Egypt. Aflatoxins are toxic and carcinogenic metabolites of moulds, mainly Aspergillus flavus and parasiticum that contaminate a variety of agricultural commodities, particularly peanuts, maize and cottonseed, in countries with hot and humid climates. Aflatoxin B1 (AFB1) is the major metabolite produced by these moulds. Aflatoxins are classified as human carcinogens based on sufficient evidence of carcinogenicity (28).
Dilber et al. detected a significant higher percent of aflatoxins in the serum of Egyptian patients with HCC compared to their controls; with a two-fold increased risk (29). Also, Rahman El-Zayadi et al. examined 200 HCC cases and 120 healthy controls and detected AFB1 in 17% of the HCC cases compared to 9.4% of the healthy controls (risk ratio =2) (30). The level of AFB1 was significantly higher in patients having multiple lesions and also in patients presenting with tumor size more than 5 cm. This may be related to the effect of AFB1 as predisposing factor affecting all the liver homogenously (31). El-Kafrawy et al. documented the presence of p53 codon 249 mutations associated with aflatoxin exposure in a sample of HCC tumor tissues analyzed by gene chip analysis in Egypt (32). Generally, in human cancer, in more than 50% of tumors, p53 is mutated and these mutations occur at the third position of codon 249 with the GC-TA transversion (33,34). Both aflatoxin exposure and HCV were strongly correlated with liver disease progression to stage G3S3, that was indicative of HCC (35).
Role of pesticides in the etiology of HCC
Occupational exposure pesticides may have a contributory role in the etiology or progression of HCC. A major segment of the Egyptian population (i.e., around 26%) is employed in agriculture (36) and uses pesticides routinely to control insects, weeds, rodents, and fungal infections of crops and livestock. Studies suggested that exposures to organophophorus and carbamate pesticides, as a result of increasing discharge of untreated industrial wastes and agricultural irrigation waste water, are additive risk factors to current HCV and HBV infection among rural males (37,38). Future investigation should address the possible hepatocarcinogenicity of pesticides using biomarkers of exposure and other techniques to better estimate dose-response relationships (35).
Alcohol, coffee, smoking, OCPs
Alcohol consumption increases the risk of HCC primarily through the development of cirrhosis. It has been suggested that heavy alcohol consumption of >80 g/d ethanol for at least five years increases the risk of HCC by nearly 5-fold (39). Epidemiological studies suggested a strong synergistic effect of alcohol on both HBV and HCV infections in developing HCC (40). Egyptian surveys have found a gradual increase in the consumption of alcohol, leading to the prediction that this will be the most common form of substance misuse in the coming years (41).
Coffee consumption may have a potentially favorable effect on the prevention of liver diseases, including liver cirrhosis and HCC (42,43). Some components in coffee, including diterpenes, cafestol, and kahweol, may act as blocking agents via modulation of multiple enzymes involved in carcinogenic detoxification as demonstrated in animal models and cell culture systems (44-46). Moreover, coffee constituents modify the xenotoxic metabolism thorough induction of glutathione-S-transferase and inhibition of N-acetyltransferase (47,48).
The effect of tobacco in the development of HCC is biologically plausible, due to the carcinogenic potential of several of the ingredients in tobacco that are metabolized in the liver (49). A Korean study has found a 50% increase in the risk of primary liver cancer for current male smokers compared to never smokers (50). However, a population based case-control study from the United States did not observe a significantly increased risk of primary liver cancer among current male smokers (51). A prospective study of 12,008 men observed that smoking significantly increased the risk of HCC only in anti-HCV-positive patients but not in those who were anti-HCV-negative when compared to anti-HCV-negative nonsmoking individuals (52). In Egypt, a preliminary case-control study showed significantly higher percentage of HCC patients used to smoke for more than 20 years, more than 20 cigarettes/day and heavier than those in the controls (53). Bakir et al. reported that smoking was found in 64% of Egyptian patients with HCC compared to 38% in patients with liver cirrhosis and 39% in controls (54). Another study revealed that tobacco smoking was a common risk factor of HCC among both cirrhotic and noncirrhotic patients (55). According to WHO statistics 2009, an estimated 40% of Egyptian males above the age of 15 years are smokers.
Oral contraceptives (OCs) appear to be associated with the development of benign liver tumors such as hepatic hemangioma, hepatocellular adenoma or focal nodular hyperplasia (56). Malignant transformation can occur within the context of hepatic adenomas after 11 years mean duration of OCs use (57). The frequency of HCC among hepatic adenomas appears to vary from 5% to 18% (58,59). In Egypt, 10.8% of married women aged 15-49 years were relying on OCs (60).
Host-related risk factors
Obesity
The prevalence of obesity has increased to epidemic proportions over the last three decades. According to WHO statistics 2008, an estimated 46.3% of females in this age group are said to be obese, in comparison with approximately 22.5% of Egyptian males. Excess body mass is classified as overweight if the body mass index (BMI) is >25 and <30 kg/m2, or obese if the BMI is ≥30 kg/m2. Both are associated with a higher risk of developing all cancers, including liver cancer (61). Those patients who were overweight had a 17% increase in risk of developing HCC, whereas obese patients had an 89% increase in risk (62). Thus, surveillance is important for diagnosis of asymptomatic HCC among this population.
Diabetes mellitus (DM)
A positive correlation between the history of diabetes mellitus and HCC was observed (63). Some possible mechanisms explained this association. Most non-insulin dependent diabetics show hyperinsulinemia. Thus, insulin or its precursors may interact with liver cells to stimulate mitogenesis or carcinogenesis (64,65). Another possible pathway is that a p53 mutation (an apoptotic factor) was noted frequently in HCC patients with diabetes rather than non-diabetics, this might provide an evidence for a molecular mechanism involving this common association (66).
According to WHO statistics 2008, an estimated 7.4% of Egyptian females and 7% of Egyptian males above the age of 25 years are said to have elevated blood glucose. An Egyptian study revealed high prevalence of DM in liver cirrhosis and HCC but no statistically significant difference in prevalence of DM between HCC and liver cirrhosis patients (54).
Nonalcoholic fatty liver disease (NAFLD)
NAFLD is being diagnosed with increasing frequency as a manifestation of the metabolic syndrome, obesity and diabetes mellitus type 2. The key process in NAFLD that predisposes patients to HCC is the development of NASH. The diagnosis of NASH relies on a biopsy with a histopathology showing features of steatosis, hepatocellular injury (ballooning, Mallory bodies), and fibrosis (67). The presence of NASH places patients at risk for progressive fibrosis and subsequent cirrhosis. The pathophysiology of hepatic carcinogenesis in patients with NAFLD-NASH has not been completely elucidated (68). But initial research suggests that excess fatty acid supply and hepatocellular steatosis elicit increased fatty acid oxidation with subsequent enhanced reactive oxidative stress (69). This process further promotes the release of proinflammatory cytokines, prooncogenic signals and epigenetic changes. Importantly, these cascades of events may take place in the absence of cirrhosis. In fact, case reports have been published where HCC arose in patients with NASH in the absence of cirrhosis (70).
Most population-based cohort and case-control studies support the link between NAFLD and HCC by showing that patients who are obese and have diabetes mellitus type 2 are twice as likely to develop HCC compared to non-obese and nondiabetic patients (71-74). An Egyptian epidemiological study over last 15 years including 1,759 HCC patients found that 5.3% of patients had suffered from NASH (75).
NAFLD-NASH is an emerging risk factor for HCC with the potential to contribute and eventually overtake HCV as the main risk factor for HCC given the galloping rates of obesity and diabetes in the world (76). Efforts should continue to better understand the link of NAFLD-NASH with HCC.
Iron overload
Hereditary hemochromatosis, a rare autosomal recessive genetic disorder characterized by excess iron absorption, is caused by mutations in the HFE gene and/or other mutations in the iron metabolism machinery (77). The estimated prevalence of Hereditary hemochromatosis in Egypt is around 0.5% (78). The altered iron metabolism seen in hereditary hemochromatosis leads to excess iron storage in the liver and the subsequent development of liver cell damage. Several studies have shown that the diagnosis of hereditary hemochromatosis confers a consistent and markedly elevated risk for the development of HCC (79-81). An Egyptian study revealed that the frequencies of HD and DD genotype of H63D mutation were significantly increased among HCC patients compared to control group and to cirrhosis group (82).
In fact, patients with excess total body iron secondary to other etiologies such as β thalassemia or iron overload in people of African descent have been shown to have a higher risk of HCC in the absence of genetic hemochromatosis (83,84). Regardless of etiology, surveillance for HCC should be undertaken in case of iron overload (85).
Autoimmune hepatitis (AIH)
AIH is a disease of unknown etiology affecting females mainly (86). It is an inflammation of the liver that occurs when immune cells mistake the liver’s normal cells for harmful invaders and attack them. The risk of HCC among AIH patients with cirrhosis is 1.9% per year. This is comparable to HCC risk among patients with cirrhosis secondary to HBV, HCV or alcohol-related liver disease (87). In Egypt, an epidemiological study over last 15 years including 1,759 HCC patients found that 0.5% of patients had suffered from AIH (75). HCC incidence of about 1% has been reported from different geographic areas among chronic AIH dependent liver cirrhosis (88-90).
Others
Epidemiology studies revealed that severe alpha1 antitrypsin deficiency (A1ATD) is a significant risk factor for cirrhosis and HCC unrelated to HBV or HCV infections. However, predisposition to HCC in moderate A1ATD is rare, and probably occurs in combination with HBV and/or HCV infections or other unknown risk factors (91). It is proposed that accumulation of polymers of A1ATD variants in endoplasmic reticulum of hepatocytes leads to damage of hepatocytes by gain-of-function mechanism (92). The increased frequency of mutant A1AT deficiency alleles together with the existance of HFE mutant alleles among HCV liver cirrhosis Egyptian patients may warrant us to do further studies assessing their relevance for risk stratification for disease progression (93).
Hereditary Tyrosinemia is an autosomal recessive inborn error of tyrosine metabolism caused by a deficiency of fumarylacetoacete hydrolase (FAH). Hepatomegaly with focal hepatic lesions is the commonest presentation. It is increasingly recognized among Egyptian children; this may be explained by the high rate of consanguinity among Egyptians (94). Tyrosinemia might be complicated by the development of HCC (95). Thus, dietary or pharmacological management of hereditary tyrosinemia might offer a strategy for prevention of HCC in these cases (96).
Conclusions
As in many developing countries, Egypt is undergoing an epidemiologic transition. With increasing urbanization, smoking rates, environmental exposures, aging and life style changes, in addition to the heavy burden of HCV, it is likely that HCC will continue to rise in the next few years. However, with wider use of Hepatitis B vaccination, the importance of HBV will decrease in the future. As HCV related HCCs are on the increase in many geographical areas, a safe and effective vaccine that prevents and treats HCV infection is urgently required. Other possible risk factors of HCC such as DM and obesity deserve more concern for their rapid increasing worldwide. Such review should help define the complex aetiology of HCC, enabling policy makers to create targeted and more efficient prevention and screening programs. Our review produced important preliminary insights that can be used to develop more refined, prospective analyses of HCC magnitude and risk in Egypt.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- GLOBOCAN 2008 database (version 1.2). Available online: http://globocan.iarc.fr
- El-Zayadi AR, Badran HM, Barakat EM, et al. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol 2005;11:5193-8. [PubMed]
- Lehman EM, Soliman AS, Ismail K, et al. Patterns of hepatocellular carcinoma incidence in Egypt from a population-based Cancer registry. Hepatol Res 2008;38:465-73. [PubMed]
- Egyptian Ministry of Health. Egyptian Ministry of Health Annual Report: 2007. Available online: http://www.mohp.gov.eg/main (accessed 2 May 2008).
- Yates SC, Hafez M, Beld M, et al. Hepatocellular carcinoma in Egyptians with and without a history of hepatitis B virus infection: association with hepatitis C virus (HCV) infection but not with (HCV) RNA level. Am J Trop Med Hyg 1999;60:714-20. [PubMed]
- Hassan MM, Zaghloul AS, El-Serag HB, et al. The role of hepatitis C in hepatocellular carcinoma - A case control study among Egyptian patients. J Clin Gastroenterol 2001;33:123-6. [PubMed]
- el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2001;5:87-107. [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [PubMed]
- Gao J, Xie L, Yang WS, et al. Risk factors of hepatocellular carcinoma--current status and perspectives. Asian Pac J Cancer Prev 2012;13:743-52. [PubMed]
- Shaker MK, Abdella HM, Khalifa MO, et al. Epidemiological characteristics of hepatocellular carcinoma in Egypt: a retrospective analysis of 1313 cases. Liver Int 2013;33:1601-6. [PubMed]
- Schiefelbein E, Zekri AR, Newton DW, et al. Hepatitis C virus and other risk factors in hepatocellular carcinoma. Acta Virol 2012;56:235-40. [PubMed]
- El Azm AR, Yousef M, Salah R, et al. Serum anti-P53 antibodies and alpha-fetoprotein in patients with non-B non-C hepatocellular carcinoma. Springerplus 2013;2:69. [PubMed]
- Montaser LM, Abbas OM, Saltah AM, et al. Circulating AFP mRNA as a Possible Indicator of Hematogenous Spread of HCC Cells: A Possible Association with HBV Infection. J Egypt Natl Canc Inst 2007;19:48-60. [PubMed]
- Abd El-Moneim E, Younis FA, Allam N, et al. Gene deletion of glutathione S-transferase M1 and T1 and risk factors of hepatocellular carcinoma in Egyptian patients. Egypt J Immunol 2008;15:125-34. [PubMed]
- Darwish MA, Issa SA, Aziz AM, et al. Hepatitis C and B viruses, and their association with hepatocellular carcinoma in Egypt. J Egypt Public Health Assoc 1993;68:1-9. [PubMed]
- Khattab MA, Eslam M, Sharwae MA, et al. Seroprevalence of hepatitis C and B among blood donors in Egypt: Minya Governorate, 2000-2008. Am J Infect Control 2010;38:640-1. [PubMed]
- Hassan ZK, Hafez MM, Mansor TM, et al. Occult HBV infection among Egyptian hepatocellular carcinoma patients. Virol J 2011;8:90. [PubMed]
- Mohamoud YA, Mumtaz GR, Riome S, et al. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis 2013;13:288. [PubMed]
- El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Egypts: Ministry of Health. Cairo: El-Zanaty and Associates, and Macro International; 2009.
- Ray SC, Arthur RR, Carella A, et al. Genetic epidemiology of hepatitis C virus throughout egypt. J Infect Dis 2000;182:698-707. [PubMed]
- Tanaka Y, Agha S, Saudy N, et al. Exponential spread of hepatitis C virus genotype 4a in Egypt. J Mol Evol 2004;58:191-5. [PubMed]
- Halim AB, Garry RF, Dash S, et al. Effect of schistosomiasis and hepatitis on liver disease. Am J Trop Med Hyg 1999;60:915-20. [PubMed]
- Chou YH, Chiou HJ, Tiu CM, et al. Duplex doppler ultrasound of hepatic schistosomiasis japonica: a study of 47 patients. Am J Trop Med Hyg 2003;68:18-23. [PubMed]
- Halim AB, Garry RF, Dash S, et al. Effect of schistosomiasis and hepatitis on liver disease. Am J Trop Med Hyg 1999;60:915-20. [PubMed]
- Donato F, Tagger A, Chiesa R, et al. Hepatitis B and C virus infection, alcohol drinking, and hepatocellular carcinoma: a case-control study in Italy. Brescia HCC Study. Hepatology 1997;26:579-84. [PubMed]
- Liang TJ, Heller T. Pathogenesis of hepatitis C-associated hepatocellular carcinoma. Gastroenterology 2004;127:S62-71. [PubMed]
- El-Nady GM, Ling R, Harrison TJ. Gene expression in HCV-associated hepatocellular carcinoma--upregulation of a gene encoding a protein related to the ubiquitin-conjugating enzyme. Liver Int 2003;23:329-37. [PubMed]
- IARC. Monographs, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs, 1987;1-42:supplement 7. Lyon: IARC Press.
- Dilber MS, Phelan A, Aints A, et al. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther 1999;6:12-21. [PubMed]
- Rahman El-Zayadi A, Abaza H, Shawky S, et al. Prevalence and epidemiological features of hepatocellular carcinoma in Egypt-a single center experience. Hepatol Res 2001;19:170-9. [PubMed]
- El-Farrash MA, Abdel-Wahab M, Rizk MS. Serum Aflatoxin level as a predictor of Hepatocarcinogenesis in HCV-infected Egyptians. Egyptian J Med Microbiol 2008;17:83-90.
- El-Kafrawy SA, Abdel-Hamid M, El-Daly M, et al. p53 mutations in hepatocellular carcinoma patients in Egypt. Int J Hyg Environ Health 2005;208:263-70. [PubMed]
- Smela ME, Currier SS, Bailey EA, et al. The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis 2001;22:535-45. [PubMed]
- Lasky T, Magder L. Hepatocellular carcinoma p53 G > T transversions at codon 249: the fingerprint of aflatoxin exposure? Environ Health Perspect 1997;105:392-7. [PubMed]
- Anwar WA, Khaled HM, Amra HA, et al. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: possibilities for prevention. Mutat Res 2008;659:176-84. [PubMed]
- Assaad R. Labour Supply, Employment and Unemployment in the Egyptian Economy 1988- 2006, ERF working paper 0701, 2007.
- Ezzat S, Abdel-Hamid M, Eissa SA, et al. Associations of pesticides, HCV, HBV, and hepatocellular carcinoma in Egypt. Int J Hyg Environ Health 2005;208:329-39. [PubMed]
- Badawi AF, Michael MS. Risk factors for hepatocellular carcinoma in Egypt: the role of hepatitis-B viral infection and schistosomiasis. Anticancer Res 1999;19:4565-9. [PubMed]
- Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323-31. [PubMed]
- Brechot C, Nalpas B, Feitelson MA. Interactions between alcohol and hepatitis viruses in the liver. Clin Lab Med 1996;16:273-87. [PubMed]
- Okasha A. eds. Substance use in a major public health hazard. In: Proceedings of the First Egyptian International Conference on Addiction and Drug Abuse. Cairo: Ministry of Health, 1996.
- Kono S, Shinchi K, Imanishi K, et al. Coffee and serum gamma-glutamyltransferase: a study of self-defense officials in Japan. Am J Epidemiol 1994;139:723-7. [PubMed]
- Shimazu T, Tsubono Y, Kuriyama S, et al. Coffee consumption and the risk of primary liver cancer: pooled analysis of two prospective studies in Japan. Int J Cancer 2005;116:150-4. [PubMed]
- Yu MW, Chen CJ. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res 1993;53:790-4. [PubMed]
- Cavin C, Holzhäuser D, Constable A, et al. The coffee-specific diterpenes cafestol and kahweol protect against aflatoxin B1-induced genotoxicity through a dual mechanism. Carcinogenesis 1998;19:1369-75. [PubMed]
- Majer BJ, Hofer E, Cavin C, et al. Coffee diterpenes prevent the genotoxic effects of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and N-nitrosodimethylamine in a human derived liver cell line (HepG2). Food Chem Toxicol 2005;43:433-41. [PubMed]
- Bravi F, Bosetti C, Tavani AA, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology 2007;46:430-5. [PubMed]
- Huber WW, Parzefall W. Modification of N-acetyltransferases and glutathione S-transferases by coffee components: possible relevance for cancer risk. Methods Enzymol 2005;401:307-41. [PubMed]
- Marrero JA, Fontana RJ, Fu S, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol 2005;42:218-24. [PubMed]
- Yun YH, Jung KW, Bae JM, et al. Cigarette smoking and cancer incidence risk in adult men: National Health Insurance Corporation Study. Cancer Detect Prev 2005;29:15-24. [PubMed]
- Zhu K, Moriarty C, Caplan LS, et al. Cigarette smoking and primary liver cancer: a population-based case-control study in US men. Cancer Causes Control 2007;18:315-21. [PubMed]
- Sun CA, Wu DM, Lin CC, et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan.
- Am J Epidemiol 2003;157:674-82.Abdou Moustafa EF, Galal GM, Aly A, et al. Smoking and the risk of hepatocellular carcinoma among Egyptian patients. A preliminary case-control study. Arab J Gastroenterol 2009;10:AB53-60.
- Bakir AS, Ali-Eldin ZA. Is diabetes mellitus a risk factor for hepatocellular carcinoma in Egyptian patients? J Am Sci 2012;8:353-8.
- Morsy KH, Hasanain AF, Kobeisy MA. Risk factors of hepatocellular carcinoma: are they the same among cirrhotic and noncirrhotic patients in upper Egypt? J Arab Soc Med Res 2011;6:103-10.
- Tajada M, Nerín J, Ruiz MM, et al. Liver adenoma and focal nodular hyperplasia associated with oral contraceptives. Eur J Contracept Reprod Health Care 2001;6:227-30. [PubMed]
- Ito M, Sasaki M, Wen CY, et al. Liver cell adenoma with malignant transformation: a case report. World J Gastroenterol 2003;9:2379-81. [PubMed]
- Zucman-Rossi J, Jeannot E, Van Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology 2006;43:515-24. [PubMed]
- Micchelli ST, Vivekanandan P, Boitnott JK, et al. Malignant transformation of hepatic adenomas. Mod Pathol 2008;21:491-7. [PubMed]
- Awadalla HI. Contraception use among Egyptian women: results from Egypt demographic and health survey in 2005. J Reprod Infertil 2012;13:167-73. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 2007;97:1005-8. [PubMed]
- Lagiou P, Kuper H, Stuver SO, et al. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst 2000;92:1096-9. [PubMed]
- Adami HO, Chow WH, Nyren O, et al. Excess risk of primary liver Cancer in patients with diabetes mellitus. J Natl Cancer Inst 1996;88:1472-7. [PubMed]
- Moore MA, Park CB, Tsuda H. Implications of the hyperinsulinaemia-diabetes-cancer Link for preventive efforts. Eur J Cancer Prev 1998;7:89-107. [PubMed]
- Hsu HC, Peng SY, Lai PL, et al. Allelotype and loss of heterozygosity of p53 in primary and recurrent hepatocellular carcinomas. A study of 150 patients. Cancer 1994;73:42-7. [PubMed]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003;37:1202-19. [PubMed]
- Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134-40. [PubMed]
- Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut 2010;59:1303-7. [PubMed]
- Hai S, Kubo S, Shuto T, et al. Hepatocellular carcinoma arising from nonalcoholic steatohepatitis: report of two cases. Surg Today 2006;36:390-4. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003;348:1625-38. [PubMed]
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4:369-80. [PubMed]
- Mori S, Yamasaki T, Sakaida I, et al. Hepatocellular carcinoma with nonalcoholic steatohepatitis. J Gastroenterol 2004;39:391-6. [PubMed]
- Hashimoto E, Yatsuji S, Tobari M, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol 2009;44 Suppl 19:89-95. [PubMed]
- Saleh SM, Elhosay YA, Ezzat WM, et al. Hepatocellular carcinoma and possible related risk factors. Research & Reviews in BioSciences 2012;6(4-5).
- Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepatic Medicine Evidence and Research 2012;4:19-37.
- Powell LW, Subramaniam VN, Yapp TR. Haemochromatosis in the new millennium. J Hepatol 2000;32:48-62. [PubMed]
- US Census Bureau, International Data Base, 2004. Available online: http://www.census.gov/population/international/data/idb/informationGateway.php
- Elmberg M, Hultcrantz R, Ekbom A, et al. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology 2003;125:1733-41. [PubMed]
- Yang Q, Mcdonnell SM, Khoury MJ, et al. Hemochromatosis-associated mortality in the United States from 1979 to 1992: an analysis of multiple-cause mortality data. Ann Intern Med 1998;129:946-53. [PubMed]
- Fracanzani AL, Conte D, Fraquelli M, et al. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology 2001;33:647-51. [PubMed]
- Gharib AF, Karam RA, Pasha HF, et al. Polymorphisms of hemochromatosis, and alpha-1 antitrypsin genes in Egyptian HCV patients with and without hepatocellular carcinoma. Gene 2011;489:98-102. [PubMed]
- Mandishona E, Macphail AP, Gordeuk VR, et al. Dietary iron overload as a risk factor for hepatocellular carcinoma in Black Africans. Hepatology 1998;27:1563-6. [PubMed]
- Borgna-Pignatti C, Vergine G, Lombardo T, et al. Hepatocellular carcinoma in the thalassaemia syndromes. Br J Haematol 2004;124:114-7. [PubMed]
- Moyo VM, Makunike R, Gangaidzo IT, et al. African iron overload and hepatocellular carcinoma (HA-7-0-080). Eur J Haematol 1998;60:28-34. [PubMed]
- Meza-Junco J, Montaño-Loza AJ, Martínez-Benitez B, et al. Hepatocellular carcinoma in patients with autoimmune liver diseases: two case reports and literature review. Ann Hepatol 2007;6:122-6. [PubMed]
- Dawn BM, Todd S, Kim SI, et al. eds. Biochemistry and molecular biology. Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins, 2007.
- Wong RJ, Gish R, Frederick T, et al. Development of hepatocellular carcinoma in autoimmune hepatitis patients: a case series. Dig Dis Sci 2011;56:578-85. [PubMed]
- Teufel A, Weinmann A, Centner C, et al. Hepatocellular carcinoma in patients with autoimmune hepatitis. World J Gastroenterol 2009;15:578-82. [PubMed]
- Geramizadeh B, Nikeghbalian S, Shamsaifar A, et al. Hepatocellular carcinoma in two patients with autoimmune hepatitis, a single center experience and review of the literature. Hepat Mon 2013;13:e7957. [PubMed]
- Topic A, Ljujic M, Radojkovic D. Alpha-1-antitrypsin in pathogenesis of hepatocellular carcinoma. Hepat Mon 2012;12:e7042. [PubMed]
- Carlson JA, Rogers BB, Sifers RN, et al. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J Clin Invest 1989;83:1183-90. [PubMed]
- Settin A, El-Bendary M, Abo-Al-Kassem R, et al. Molecular analysis of A1AT (S and Z) and HFE (C282Y and H63D) gene mutations in Egyptian cases with HCV liver cirrhosis. J Gastrointestin Liver Dis 2006;15:131-5. [PubMed]
- El-Karaksy H, Fahmy M, El-Raziky M, et al. Hereditary tyrosinemia type 1 from a single center in Egypt: clinical study of 22 cases. World J Pediatr 2011;7:224-31. [PubMed]
- Montalto G, Cervello M, Giannitrapani L, et al. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci 2002;963:13-20. [PubMed]
- Ashorn M, Pitkänen S, Salo MK, et al. Current strategies for the treatment of hereditary tyrosinemia type I. Paediatr Drugs 2006;8:47-54. [PubMed]


