
The value of sonographically guided fine-needle aspiration in the diagnosis of small lymph
Introduction
The lesions of superficial lymphadenopathies are complicated and can be divided into two categories: neoplastic and non-neoplastic (1). Thapa et al. (1) found that 25.4% of lymph node lesions were neoplastic and that 74.6% were non-neoplastic. In addition, 70% of lymph node lesions were located in the neck. Plukker et al. believed that lymph nodes with short-axis diameters less than 1 cm, clear boundaries, uniform internal echogenicity and normal lymphatic portals are largely benign (2). Another study, however, indicated that lymph nodes with short-axis diameters less than 1 cm that contain micrometastases and without abnormal imaging findings are difficult to distinguish from nonmetastatic lymph nodes (3). Ultrasound-guided lymph node core-needle biopsy (CNB) and fine-needle aspiration (FNA) are widely applied in the diagnosis of superficial lymphadenopathies. CNB can retrieve more tissue but is not suitable for some elderly patients in poor health and with poor coagulation function. CNB cannot effectively retrieve small lymph node samples. Ultrasound-guided FNA cytology examination is minimally invasive, simple and fast (4), rarely causes complications and is associated with very good patient compliance. However, due to the limited number of cells retrieved and the high false-negative rate associated with FNA of lymph nodes with smaller short-axis diameters, Ewing et al. suggested that the size of lymph nodes affects FNA biopsies and that the corresponding results therefore have limited significance (5). This study aimed to investigate the diagnostic sensitivity, specificity and accuracy of ultrasound-guided FNA for lymph nodes with different short-axis diameters, analyze the diagnostic value of ultrasound-guided FNA, and determine whether a short-axis diameter ≤1 cm influences FNA results.
Methods
Subjects
Ultrasound-guided FNA examinations of 1,725 patients showing abnormal lymph nodes during routine sonographic examination were performed in our hospital from December 2015 to June 2017. Abnormal ultrasound findings include an irregular lymph node morphology, an unclear boundary, a ratio of long-axis diameter to short-axis diameter >2, eccentric or absent lymphatic portals, thickening of cortical bands, and abnormal blood flow. Among the cases, 483 were excluded from the study due to missed follow-ups and lack of a final diagnosis, and 1,242 cases were enrolled, including 662 males and 580 females aged 13–92 years, with an average age of 56.22±12.7 years. Among these, 1,061 cases of cervical and supraclavicular lymph nodes, 2 cases of subclavian bone, 161 cases of axillary lymph nodes, and 18 cases of inguinal lymph nodes were included. The distribution of the 1,242 cases is shown in Table 1. The primary tumors in patients with enlarged lymph nodes are shown in Table 2. All patients signed an informed consent form before undergoing ultrasound-guided biopsy, and coagulation function was examined.
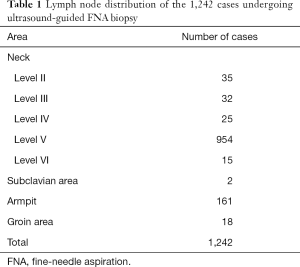
Full table
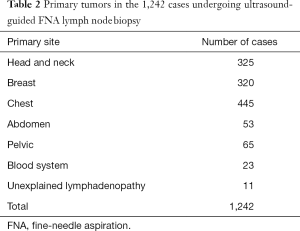
Full table
Apparatus, biopsy procedure and final diagnostic results
Apparatus
The real-time color ultrasound scanner used for ultrasound-guided biopsy was the ALOKA 10 diagnostic ultrasonic system (Aloka Co., Ltd., Japan), and the probe used was a 5- to 12-MHz linear array Transducer. For CNB, 18-G biopsy needles with lengths of 6 or 10 cm by C. R. Bard, Ltd., USA were used. For FNA biopsy, Sonopsy-C1 vacuum aspiration biopsy needles (21 G) produced by Hakko Co., Ltd., Japan were used.
Biopsy method
The patient was placed in the supine or lateral position to fully expose the location of the lymph node to be punctured. General ultrasound scanning was performed, the optimal puncture position was selected, the depth of needle insertion was measured, and the puncture site on the body surface was located. The puncture site was wiped with a general disinfectant and injected with 2% lidocaine for local anesthesia.
FNA
A 5-mL syringe with a 21-G needle was manually inserted until the needle tip reached the edge of the lymph node while avoiding important tissues and organs, such as large blood vessels, nerves and the trachea, under color Doppler ultrasound guidance. After confirming the target, the needle was inserted into the lymph node cortex, and then the needle was set at a 2-mL scale to maintain negative pressure in the syringe. After rotating and aspirating the syringe 3–5 times, the direction of the needle was changed such that aspiration was performed in a fan shape at different trajectories in the lymph node. The needle was withdrawn once tissue was present in the syringe. The puncture site was covered with sterile gauze and pressure was applied with appropriate force for 10 min. Patients who showed no adverse reactions after 20 min of observation were released.
CNB
The skin of the puncture site was first pierced by a thick, 12-G needle tip, and then, the surgeon inserted the needle into the puncture point while avoiding large blood vessels, nerves and the trachea under ultrasound guidance. The biopsy gun was quickly pressed and the needle was withdrawn to complete the biopsy. After confirming that a sufficient sample was obtained, the collected specimen was quickly fixed with formaldehyde solution and sent for histological examination. The puncture site was covered with sterile gauze, and pressure was applied with appropriate force for 10 min. Patients who showed no adverse reactions after 30 min of observation were released.
Interpretation of ultrasound-guided lymph node FNA results and the final diagnostic criteria
For ultrasound-guided FNA cytological diagnosis, the presence of cancer cells and atypical cells was regarded as a positive result; otherwise, the result was considered negative. Among the 1,725 patients who underwent ultrasound-guided FNA biopsy, 988 also underwent CNB, and the CNB results were regarded as the final diagnosis. The 737 patients who did not undergo CNB were followed-up. The follow-up duration was between 6 and 22 months. The criteria for the diagnosis of lymph nodes were no significant changes in lymph node size and a benign internal structure (n=200). If lymph nodes were enlarged or the internal structure was changed during the follow-up, patients were subjected to additional CNB or FNA, and the results were used as the final diagnosis. Among the cases that underwent a second FNA biopsy, cancer cells in the lymph nodes accompanied by lymph node enlargement were identified in 2 cases, and the results of the second FNA biopsy were used as the final diagnosis. Negative results were found in the lymph nodes by a second ultrasound-guided FNA biopsy in 137 cases. Due to the presence of enlarged lymph nodes, these patients were followed-up again and eventually excluded from the study. Additionally, another 346 cases were excluded due to loss to follow-up or the presence of enlarged lymph nodes but no pathological data. Ultimately, 1,242 patients were included in this study.
Lymph node grouping
The 1,242 cases of biopsied lymph nodes were divided into two groups according to the length of the short-axis diameters (short-axis diameters ≤1 cm and >1 cm) to investigate the diagnostic value of FNA biopsy.
The 883 cases of lymph nodes with short-axis diameters ≤1 cm were divided into three groups according to the following diameters to investigate the diagnostic value of FNA biopsy: 0.7 cm ≤ short-axis diameter ≤1 cm, 0.5 cm ≤ short-axis diameter <0.7 cm, and short-axis diameter <0.5 cm.
Statistical analysis
SPSS24 statistical software was used for the statistical analysis. The Chi-square test was performed to assess the diagnostic value of ultrasound-guided FNA in the different groups. P<0.05 was used to define statistically significant differences.
Results
Lymph node size
The short-axis diameters of the lymph nodes in the study ranged from 0.3 to 5.7 cm, with an average diameter of 0.93±0.52 cm. The average short-axis diameter of each group is shown in Table 3.

Full table
FNA diagnosis and final diagnosis
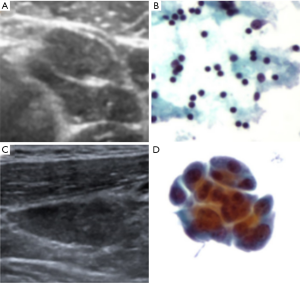
The results for the FNA cytology diagnosis of 1,242 lymph nodes are shown in Table 4. Based on the cytological diagnosis, 700 cases were positive, and 542 cases were negative. In addition, 988 cases underwent CNB, among which 699 cases were positive and 289 cases were negative, based on histological diagnosis. Among the 254 cases without CNB, 2 cases were positive (Figure 1) and 252 cases were negative (Figure 2) according to follow-up and second biopsy or final diagnosis results. The final diagnosis results corresponded to 700 positive cases and 542 negative cases. Among the 1,242 cases, FNA examinations revealed 692 true-positive cases (Figure 3), 533 true-negative cases (Figure 4), 8 false-negative cases (Figure 5) and 9 false-positive cases (Figure 6) according to the final diagnosis criteria.
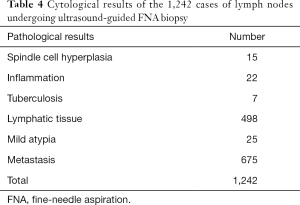
Full table

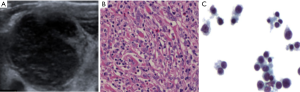
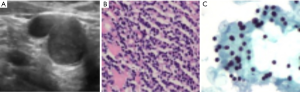
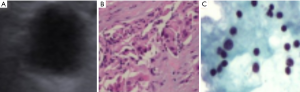
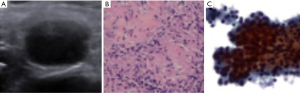
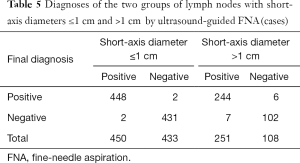
Diagnostic results of ultrasound-guided FNA for lymph nodes with short-axis diameters ≤1 and >1 cm
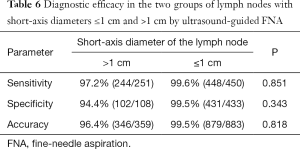
The diagnostic results of the lymph nodes with short-axis diameters ≤1 and >1 cm by ultrasound-guided FNA are shown in Table 5. The diagnostic efficacy is shown in Table 6. No significant differences in sensitivity, specificity and accuracy were observed between the two groups (P>0.05).
Full table
Full table
Diagnoses of different groups of lymph nodes with a short-axis diameter <1 cm by ultrasound-guided FNA
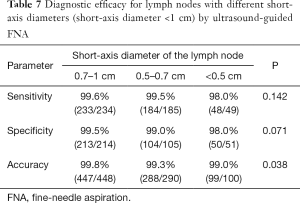
The diagnostic efficacy in the three groups of lymph nodes (0.7 cm ≤ short-axis diameter ≤1 cm, 0.5 cm ≤ short-axis diameter <0.7 cm and short-axis diameter <0.5 cm) by ultrasound-guided FNA is shown in Table 7. A significant difference in accuracy was found among the three groups (P<0.05), but no significant differences in diagnostic sensitivity and specificity were observed (P>0.05).
Full table
Discussion
Accurate cancer staging is key to determining an individualized treatment plan and to predict prognosis, while lymph node evaluation is key to cancer staging. Clearly determining whether lymph node metastasis is present has guiding significance for surgical resection and selection of radiotherapy and chemotherapy regimens. The short-axis diameters of metastatic lymph nodes are often considered to be greater than 1 cm (3), but for some sites, especially the head and neck, only 8% to 37.5% of metastatic lymph nodes have a short-axis diameter greater than 1 cm. Therefore, increasing the detection rate of metastatic lymph nodes with short-axis diameters ≤1 cm is important for selecting treatment plans and predicting a patient’s prognosis. However, diagnosing the nature of suspected lymph nodes by relying solely on clinical manifestations, imaging studies and laboratory tests is often difficult. Pathological examination is the gold standard for the diagnosis of such diseases. Currently, surgical resection, histological analysis based on ultrasound-guided core-needle puncture or cytological analysis based on FNA are used to obtain lymph node tissue (5). The advantage of surgical biopsy is that the materials are complete and easily meet the needs of pathological examination. However, the trauma caused by this type of surgical biopsy is relatively extensive, the procedure is very costly, and patients’ compliance is poor. Ultrasound-guided CNB can generally obtain a sufficient specimen and preserve the surrounding tissue structure, allowing immunohistochemistry analysis based on histomorphological examination, resulting in high diagnostic efficiency (6-9). However, for lymph nodes with small short-axis diameters, the application of CNB is very limited and is associated with complications such as hemorrhage and nerve injuries (10,11). Cytological examination via ultrasound-guided FNA has the advantages of producing minimal trauma, simple and rapid execution (12), very good patient compliance and a low incidence of serious complications. Additionally, ultrasound-guided FNA allows biopsy of suspicious lymph nodes in complicated structures such as posterior pharyngeal space under endoscopic ultrasound guidance. Lymphadenopathy can be identified by observing cell morphological abnormalities such as the nuclear membrane, nucleolus and nuclear-cytoplasm ratio (4,9,13,14).
Most existing studies of lymph nodes with short diameters ≤1 cm have indicated that FNA can be performed in cases with absent lymphatic portals, local cortical thickening and changes in the blood supply pattern in the primary lymph node drainage area (15,16). However, Plukker et al. believe that the results of FNA are limited due to the limited number of cells obtained by FNA (2). Furthermore, Ewing et al. believe that FNA is not suitable for lymph nodes with short-axis diameters ≤1.2 cm due to relatively high false-negative rates (5).
In the current study, we found that the sensitivities, specificities and accuracies of ultrasound-guided FNA were 99.6%, 99.5% and 99.5% for lymph nodes with short-axis diameters ≤1 cm and 97.2%, 94.4% and 96.4% for lymph nodes with short-axis diameters >1 cm, respectively. No significant differences were observed between the two groups (P>0.05). The sensitivities of ultrasound-guided FNA for the three groups of lymph nodes with short-axis diameters ≤1 cm were 99.6%, 99.5% and 98.0%, respectively. The specificities were 99.5%, 99.0% and 98.0%, respectively. The accuracies were 99.8%, 99.3% and 99.0%, respectively, with significant differences detected among the three groups (P<0.05). No significant differences in sensitivity and specificity were observed among the three groups (P>0.05). Our findings suggest that for lymph nodes with short-axis diameters ≤1 cm, the accuracy decreases as the short-axis diameter decreases, but FNA still has high diagnostic accuracy.
A limitation of this study is that all lymph nodes that underwent FNA were identified by routine ultrasound examination, and the results of routine ultrasound examination were affected by the expertise of the sonographers. Additionally, not all of the lymph nodes were diagnosed by CNB histological examination, and some cases of enlarged lymph nodes identified at the follow-ups were excluded due to a lack of second biopsy results, which may have affected our findings.
Conclusions
Ultrasound-guided FNA has high diagnostic value for metastatic lymph nodes. In particular, ultrasound-guided FNA has high accuracy for diagnosing small lymph nodes and can achieve an accurate diagnosis for lymph nodes that cannot be accessed by CNB.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2019.08.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a retrospective study, we collected all cases in hospital computer system (or called PACS), so we do not have ethics committee information. And this study have no conflict with anybody and do not broke any human ethics. All patients signed an informed consent form before undergoing ultrasound-guided biopsy, and coagulation function was examined.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thapa S, Ghosh A, Ghartimagar D, et al. Histopathological Analysis of Non-Neoplastic Superficial Lymphadenopathies. Kathmandu Univ Med J 2017;15:51-5. (KUMJ). [PubMed]
- Plukker JT, van Westreenen HL. Staging in oesophageal cancer. Best Pract Res Clin Gastroenterol 2006;20:877-91. [Crossref] [PubMed]
- Liu J, Wang Z, Shao H, et al. Improving CT detection sensitivity for nodal metastases in oesophageal cancer with combination of smaller size and lymph node axial ratio. Eur Radiol 2018;28:188-95. [Crossref] [PubMed]
- McElroy C, Velilla R, Chaudhary H, et al. Fine-needle aspiration diagnosis of squamous cell carcinoma in a lymph node involved with small lymphocytic lymphoma: case report and review of the literature. Diagn Cytopathol 2009;37:48-50. [Crossref] [PubMed]
- Ewing DE, Layfield LJ, Joshi CL, et al. Determinants of False-Negative Fine-Needle Aspirates of Axillary Lymph Nodes in Women with Breast Cancer: Lymph Node Size, Cortical Thickness and Hilar Fat Retention. Acta Cytol 2015;59:311-4. [Crossref] [PubMed]
- Vandervelde C, Kamani T, Varghese A, et al. A study to evaluate the efficacy of image-guided core biopsy in the diagnosis and management of lymphoma--results in 103 biopsies. Eur J Radiol 2008;66:107-11. [Crossref] [PubMed]
- Roussel F, Dalion J, Benozio M. The risk of tumoral seeding in needle biopsies. Acta Cytol 1989;33:936-9. [PubMed]
- Han F, Xu M, Xie T, et al. Efficacy of ultrasound-guided core needle biopsy in cervical lymphadenopathy: A retrospective study of 6,695 cases. Eur Radiol 2018;28:1809-17. [Crossref] [PubMed]
- Awwad A, Tiwari S, Sovani V, et al. Reliable EGFR mutation testing in ultrasound-guided supraclavicular lymph node fine-needle aspirates: a cohort study with diagnostic performance analysis. BMJ Open Respir Res 2015;2:e000075. [Crossref] [PubMed]
- Lachar WA, Shahab I, Saad AJ. Accuracy and cost-effectiveness of core needle biopsy in the evaluation of suspected lymphoma: a study of 101 cases. Arch Pathol Lab Med 2007;131:1033-9. [PubMed]
- Goel G, Janaki PD, Smitha NV, et al. Role of Axillary Ultrasound, Fine Needle Aspiration Cytology and Sentinel Lymph Node Biopsy in clinically N0 Breast Cancer. Indian J Surg Oncol 2016;7:407-12. [Crossref] [PubMed]
- Das DK, Bhambhani S, Pant JN, et al. Superficial and deep-seated tuberculous lesions: fine-needle aspiration cytology diagnosis of 574 cases. Diagn Cytopathol 1992;8:211-5. [Crossref] [PubMed]
- Altuwairgi O, Baharoon S, Alkabab Y, et al. Ultrasound-guided core biopsy in the diagnostic work-up of tuberculous lymphadenitis in Saudi Arabia, refining the diagnostic approach. Case series and review of literature. J Infect Public Health 2014;7:371-6. [Crossref] [PubMed]
- de Larrinoa AF, del Cura J, Zabala R, et al. Value of ultrasound-guided core biopsy in the diagnosis of malignant lymphoma. J Clin Ultrasound 2007;35:295-301. [Crossref] [PubMed]
- Dabirmoghaddam P, Sharifkashany S, Mashali L. Ultrasound-guided fine needle aspiration cytology in the assessment of cervical metastasis in patients undergoing elective neck dissection. Iran J Radiol 2014;11:e7928. [Crossref] [PubMed]
- Borgemeester MC, van den Brekel MW, van Tinteren H, et al. Ultrasound-guided aspiration cytology for the assessment of the clinically N0 neck: factors influencing its accuracy. Head Neck 2008;30:1505-13. [Crossref] [PubMed]


