
Cutaneous T-cell lymphoma (CTCL), rare subtypes: five case presentations and review of the literature
Introduction
The vast majority of cutaneous T-cell lymphomas (CTCL) are encompassed by mycosis fungoides (MF) and CD30+ lymphoproliferative disorder (LPD), however rare distinct CTCLs have been defined. The current edition of the World Health Organization (WHO) includes 12 CTCL subtypes with discrete diagnosable clinical, histologic and phenotypic features (1). The clinicopathologically most well defined and most common CTCL is MF, which categorically includes a number of disease variants as well as Sézary Syndrome. The primary cutaneous CD30+ LPDs are the second most common CTCLs and include both primary cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis. The remaining CTCLs can be conceptualized as the rare variants, each of which represent only 1–2% of all cutaneous lymphoma cases (2). The rare CTCLs include entities with an indolent clinical course: subcutaneous panniculitis-like T-cell lymphoma (SPTCL), primary cutaneous CD4+ small/medium T-cell LPD (SMPTC-LPD) and primary cutaneous acral CD8+ T-cell lymphoma as well as aggressive subtypes: primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma (CD8+ PCAETL) and primary cutaneous gamma /delta T-cell lymphoma (PCGDTCL) (1,3).
In the current review article, we discuss these rare CTCL subtypes and their management focusing on case presentations from the multidisciplinary cutaneous lymphoma clinic at Memorial Sloan Kettering Cancer Center.
Case number 1
A 62-year-old female with history of malignant melanoma in-situ presented with a 6-month history of a single asymptomatic nodule (Figure 1) on her left neck. A biopsy showed a nodular lymphoid infiltrate in the dermis. Immunohistochemical stains showed that the predominant lymphocytic population was CD3+, CD4+, and CD7+ with a subset of cells expressing PD-1 and scattered intermingled CD8+ T-cells. A smaller population of CD20+ B-cells was also noted. Positron emission tomography-computed tomography (PET-CT) scanning showed no evidence of extracutaneous disease and complete blood count (CBC) was normal. The findings were most in keeping with a primary cutaneous CD4+ SMPTC-LPD. The lesion was excised with complete remission with no recurrence on follow-up 2.5 years later.
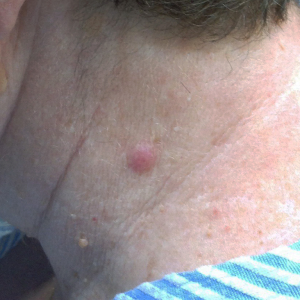
Primary cutaneous CD4+ SMPTC-LPD
Primary cutaneous CD4+ SMPTC-LPD is a rare indolent T cell lymphoproliferative disorder that is limited to the skin. It represents approximately 2% of all cases of cutaneous lymphomas and is associated with an excellent prognosis (3-6). The 2016 revision of the WHO classification of lymphoid neoplasms changed the terminology from primary cutaneous CD4+ small/medium sized pleomorphic T-cell lymphoma to “lymphoproliferative disorder” in order to reflect the limited clinical risk and uncertain significance of a malignant diagnosis in this localized disease (1).
SMPTC-LPD presents clinically as a slow-growing solitary erythematous papule, plaque, nodule or tumor. Multiple lesions, multifocal disease (4,7) or ulceration are much less common presentations (6) and should be accepted within this diagnostic category with caution. SMPTC-LPD is most often located on the face, neck or the upper trunk. It usually appears in adults; however, it can be seen also in children (5,6,8).
Histologically, dense infiltrates of small to medium-sized pleomorphic lymphocytes are identified in either a nodular or diffuse pattern that involve the dermis, and which can infiltrate the subcutis with or without epidermotropism (5,9). In most cases, numerous reactive cells accompany the neoplastic lymphocytes and include B cells, histiocytes, plasma cells, and eosinophils (4,10). The lesional cells are CD3+, CD4+, βF1+ T cells that are typically negative for CD30 and cytotoxic molecules (6,11). Partial or complete loss of pan T-cell markers CD7 and rarely CD5 can be seen (5,6,12). The neoplastic cells are characteristically identified to have a T-follicular helper (Tfh) phenotype by expression of PD-1, less often by BCL-6 and CXCL-13 (4,13). Molecular studies demonstrate clonal rearrangements of T-cell receptor (TCR) genes in the majority of cases (4,6).
SMPTC-LPD usually do not extend beyond the skin and subcutis (5,9,12), however because of histopathologic overlap with peripheral T-cell lymphoma, unspecified (10) or lymphoma subtypes that may express PD-1 and follicular T-cell markers, such as angioimmunoblastic T-cell lymphoma (AITL) (9), we often complete work-up and staging procedures at initial diagnosis to rule out extracutaneous disease by imaging studies (PET-CT scans or CT scans) and blood work that may include flow cytometry (5,9,12). Although the need for and extent of staging work-up can be individualized as imaging may not be necessary for patients with normal blood work and physical exam. There is no standard of care for SMPTC-LPD; however, most cases are successfully treated with local treatments including surgical excision, local radiotherapy (RT), combined excision and RT, and topical/intralesional corticosteroids, specifically in cases with multiple lesions (4,5,7,9,12). Relapse rates are overall low, however patients with multiple lesions showed a higher incidence of relapses (4,10). Spontaneous resolution after a biopsy has been reported in some cases (14).
Case number 2
A 42-year-old male presented with a single red nodule on the helix of his right ear that had persisted for a year with no significant change in size (Figure 2). The patient underwent a biopsy of the lesion, which demonstrated a dense infiltrate of small to intermediate-sized T lymphocytes which were localized to the dermis. The lymphocytes were CD3+, CD5+, CD7+, CD8+ and CD2+ and were negative for CD4, CD20, and CD30. TCR beta and TCR gamma genes showed monoclonal rearrangements. PET-CT was performed which showed no hypermetabolic lesions. Blood work showed no abnormalities. The patient was diagnosed with a primary cutaneous acral CD8+ T-cell lymphoma and received treatment with local radiation therapy (30 Gy over 15 sessions). Complete remission was achieved and no recurrence was observed after two years of follow-up.
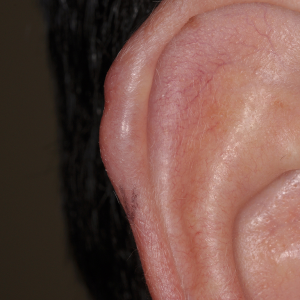
Primary cutaneous acral CD8+ T-cell lymphoma
Primary cutaneous acral CD8+ T-cell lymphoma (acral CD8+ TCL) is a rare recently described (1) indolent clonal CD8+ T-cell disorder which is by current definition localized to any acral site, although originally recognized as involving the ear (15). With clinical presentation [indolent clinical course and favorable prognosis (5)] and some histopathologic features similar to SMPTC-LPD, both entities were initially suggested to be immunophenotypic variants of the same disorder. However distinct clinicopathologic features have allowed for the separation of these entities in the newest classification schema.
Clinically, acral CD8+ TCL occurs in adult patients as a slow-growing papule or nodule that is nearly always localized to a single site, mainly the ears, nose, hands or feet (5,16). Multifocal presentation of this disorder is controversial, and at best, extremely rare (16).
The histopathologic analysis of acral CD8+ TCL lesions shows a diffuse proliferation of monomorphic medium-sized T cells in the dermis and sometimes within the subcutis, that spares the epithelium. The immunophenotype of the neoplastic T-cells is CD3+, CD8+, and βF1+ with non-activated cytotoxic proteins. The lesional cells are negative for CD4, CD30, CD56, and CD20. Monoclonal TCR gene rearrangements are found in most of the cases analyzed (15,16).
Acral CD8+ TCL is almost always confined to the skin and tends to follow an indolent clinical course despite its somewhat high-grade morphologic features and cytotoxic immunophenotype. A single recent case thought to be acral CD8+ TCL that underwent transformation to a more aggressive disease, has been reported (17).
Workup may include imaging and bloodwork as suggested for SMPTC-LPD. Acral CD8+ TCL should be managed conservatively and surgical excision or local RT are usually sufficient to obtain complete remission (5,15).
Case number 3
A 69-year-old woman presented with asymptomatic subcutaneous nodules on her upper arm and lower back that had developed within the course of several months. She reported no systemic symptoms such as night sweats, fevers, chills, unintentional weight loss, joint pain (other than osteoarthritis of her knee joint), sun-sensitivity or mucosal ulcerations. On physical examination hyperpigmented plaques overlying subcutaneous nodules were noticed on the right upper arm, buttocks and lower back (Figure 3A). Skin biopsy showed a predominantly lobular panniculitic infiltrate of atypical medium to large sized T lymphocytes rimming adipocytes in a background of karyorrhectic debris and cytophagocytosis. Additional pathologic changes noted included a vacuolar interface alteration and dermal mucin with clusters of CD20+ B-cells. The atypical T cells were CD3+, with an admixture of CD4+ and CD8+ subsets and a predominance of TCR beta labeling. Approximately 15% of the atypical lymphocytes expressed TCR Delta+. A number of them expressed CD56 and granzyme-B. EBER-ISH was negative. On molecular studies, a prominent peak was identified in TCR gamma which did not meet criteria for clonality. PET-CT scan showed multiple hypermetabolic subcutaneous nodules in the skin and the breast (Figure 3B), thus an ultrasound-guided biopsy from the breast was performed. This biopsy showed a similar atypical T cell infiltrate involving fibroadipose tissue. The immunophenotype matched that seen in the skin biopsies. CBC showed low white blood counts (3.1 K/µL) and mild normocytic normochromic anemia (hemoglobin 10.8 g/dL) with no other abnormalities and no evidence of hemophagocytic lymphohistiocytosis (HLH). Anti-nuclear antibody titers were positive (1:320 homogenous pattern and 1:80 nucleolar titer). Blood flow cytometry showed no abnormal lymphocytic population. Bone marrow biopsy showed no evidence of lymphoma.
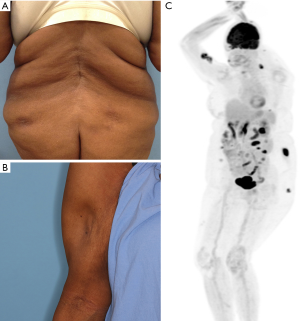
A diagnosis of SPTCL was favored and treatment with methotrexate (25 mg weekly) was recommended. The patient was seen by a rheumatologist who agreed with this plan. The skin lesions dramatically decreased within few months of treatment and lipoatrophy with rigid subcutaneous foci suggesting calcifications was noticed in some of the previously involved areas (Figure 3C). Methotrexate dose was decreased to 10 mg/week with no evidence of disease recurrence.
SPTCL
SPTCL is a T-cell lymphoma with clinicopathologic features simulating panniculitis. The 2016 WHO classification of lymphoid neoplasms now distinguishes between SPTCL characterized by a CD8+ αβ T-cell phenotype and PCGDTCL, characterized by γδ T-cell phenotype (1,3). Both entities are rare cytotoxic panniculitis-like lymphomas; however, while SPTCL often follows an indolent clinical course (18), PCGDTCL is typically aggressive and associated with poor prognosis (19) (as discussed in detail later in this review).
SPTCL may occur in both adults and children and is more common in females (18,20). It presents usually as multiple subcutaneous nodules involving the trunk and the extremities. Solitary lesions are less frequently seen, and extracutaneous involvement is uncommon. The subcutaneous nodules may regress, leaving lipoatrophy and calcifications. Fatigue, fever and weight loss may be reported. Cytopenias and elevated liver functions may be seen on bloodwork; HLH is seen in 15–20% of patients and may be fatal (18). Associated autoimmune diseases, mainly systemic lupus erythematosus, are seen in up to 20% of the patients (18,21,22).
Histopathologically, SPTCL cases demonstrate a dense subcutaneous lymphocytic infiltrate in a nodular or diffuse pattern that appears as a lobular panniculitis with rimming of adipocytes. Fat necrosis, karyorrhexis and cytophagocytosis are common, and reactive cells may be present as well. The overlying dermis and epidermis are usually spared by the lymphoma, and angioinvasion and angio-destruction are uncommon.
The immunophenotype of the neoplastic lymphocytes is CD3+, CD8+, and βF1+ with cytotoxic protein expression (TIA1, granzyme-B and perforin). CD4, CD30 and CD56 are usually negative; there may be loss of CD2, CD5, and CD7 (18). SPTCL is not associated with Epstein-Barr virus (EBV) infection. Monoclonal rearrangement of TCR genes is seen in most cases.
Diagnosis of SPTCL requires good representation of the subcutaneous fat tissue in the skin biopsy and repeat biopsies may be needed to confirm this diagnosis. The overlapping clinical and histological characteristic features of SPTCL and lupus erythematosus panniculitis (LEP) can lead to misdiagnosis between the two entities. The coexistence of SPTCL with clinical, serologic, and histopathologic findings characteristic of collagen vascular disease such as LEP may occur, which, along with a sometimes indeterminate histopathology has caused theorization that the inflammatory disease and the lymphoma lie along a biologic spectrum (23,24).
SPTCL usually follows an indolent course and lesions may even resolve spontaneously. SPTCL often carries a favorable prognosis. Treatment approaches that are commonly used for indolent CTCLs have proven effective in SPTCL as well, including bexarotene (25), and methotrexate (26). Cyclosporine (20,22) has been reported as an effective treatment (20,22,26). RT may be considered for focal skin disease. Observation alone may be appropriate in some cases (26). Aggressive therapeutic approaches that include multi-agent chemotherapy and hematopoietic stem-cell transplantation (HSCT) are reserved for refractory cases or cases with HLH (26).
Case number 4
A 51-year-old male presented with numerous diffuse ulcerated plaques and tumors that rapidly developed within four months. On physical examination, scattered hyperpigmented plaques and nodules, some ulcerated, were noticed on the face, trunk, genitalia and extremities (Figure 4). Skin biopsies showed an epidermotropic CD3+, predominantly CD8+ lymphoid infiltrate. CD30 and CD25 were negative. Monoclonal rearrangements of the beta and gamma TCR genes were detected. Other than slightly elevated LDH and alanine aminotransferase, blood work was normal and no abnormal lymphocytic population was found in the blood. HIV and HTLV-1 serologies were negative. A PET-CT scan showed multiple hypermetabolic cutaneous nodules throughout the body consistent with lymphoma, and axillary, pelvic and inguinal nodes were suspicious for malignancy. A differential diagnosis of primary cutaneous CD8+ PCAETL versus CD8+ MF was suggested (based on the pathology alone). Systemic chemotherapy with gemcitabine plus liposomal doxorubicin resulted in initial response but later the disease progressed. The patient was then treated with pralatrexate with a mixed response, followed by total body electron beam (TSEB) with improvement. He later had significant progression of extracutaneous disease and was treated with dexamethasone, cyclophosphamide, etoposide and vincristine, however unfortunately expired one month later, nine months after he was initially diagnosed. An autopsy revealed visceral involvement of mediastinal lymph nodes, liver, spleen, pancreas, lungs and thyroid by lymphoma.
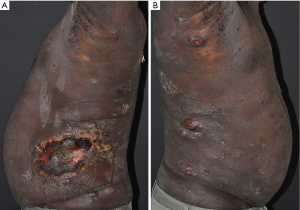
Primary cutaneous CD8+ PCAETL
Primary cutaneous CD8+ PCAETL comprises <1% of all cutaneous lymphoma cases (2). It is characterized by a proliferation of cytotoxic CD8+ T cells that are primarily localized to the epidermis and which result in extensive epidermal necrosis and skin ulceration. CD8+ PCAETL appears mostly in adults and has an aggressive clinical behavior with rapid progression. It is associated with a poor prognosis, and the median overall survival ranges from 12 to 32 months (27-29). Some controversial cases may lack the characteristic markers CD8 or TCR-β/βF1+ and the wider term “primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma” has been suggested to better account for such cases (27).
Clinically, CD8+ PCAETL is characterized by the abrupt onset of rapidly progressing multiple, usually widespread, ulcerating and hemorrhagic plaques, papulo-nodules or tumors with possible extracutaneous involvement of the oral mucosa, lungs, adrenal glands, testis, and the central nervous system (27,28). Lymph node or bone marrow involvement is uncommon (27,29). A preceding poorly-defined eczematous or papulosquamous rash can be observed in some of the patients (27,30).
On histopathology, an extensive epidermotropic proliferation of atypical lymphocytes arranged in a pagetoid pattern is frequently accompanied by focal to confluent keratinocyte necrosis and frank ulceration. Infiltration of the skin adnexal structures (29) and upper dermal red cell extravasation are common. Dermal and subcutaneous infiltration can be seen in tumoral lesions (29) and the presence of such lesions at diagnosis is associated with a poor outcome (4). Immunophenotyping typically shows that the cells are CD3+, CD8+, βF1+ and CD45RA+, with cytotoxic granules (TIA1, granzyme B, perforin). CD7 is often positive, while CD2, CD4, CD5 and CD45RO are negative (28,29). Cases of CD4/CD8 double-negativity have been described (4). The neoplastic cells show monoclonal TCR gene rearrangements (3,31). There is no association with EBV. Difficulty in distinguishing CD8+ PCAETL from more indolent CTCLs (32) and inflammatory imitators (33) can delay the diagnosis and affect the outcome.
In cases with a diagnosis or suspicion of CD8+ PCAETL we complete the following workup: CBC with differential, LDH, and comprehensive metabolic panel, chest/abdominal/pelvic CT with contrast of diagnostic quality or PET-CT scan. HIV serology should be completed as CD8+ T-cells skin infiltrates have been reported with advanced HIV infection (34).
The rapid progression and aggressive course of CD8+ PCAETL requires a rapid therapeutic approach that includes aggressive multiagent chemotherapy regimens and HSCT if possible. In many patients the disease is resistant to multiple chemotherapies. A retrospective study of 34 patients who were treated with various modalities [oral bexarotene, romidepsin, etoposide, gemcitabine, liposomal doxorubicin, local radiation and total skin electron beam therapy (TSEBT)] failed to show significant survival advantage for any of these therapies, and HSCT was the sole therapy that resulted in sustained response in 5 of 6 patients (27). Implementation of an aggressive strategy early in the course of treatment, including considering allogeneic HSCT at the time of diagnosis, has been recently suggested based on additional cases (35).
Case number 5
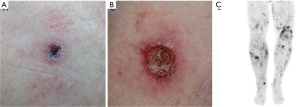
A 62-year-old male was referred for evaluation of ulcerated subcutaneous nodules on his thigh and buttock that grew larger within several months (Figure 5A). The patient reported a history of similar lesions that had resolved spontaneously several months earlier. On physical examination, an ulcerated subcutaneous nodule, 3 cm in diameter, was seen on the right thigh and a skin-colored subcutaneous 2-cm nodule was palpated on the buttock. Skin biopsies showed an ulcerated epidermotropic and perivascular nodular infiltrate of atypical lymphocytes extending through the dermis in one biopsy and atypical lymphoid infiltrate in the dermis and subcutis associated with an interface dermatitis in another biopsy. The atypical lymphoid infiltrates were strongly CD2+, CD3+, CD56+, TCRγ+, TIA1 and granzyme B positive, and negative for CD4, CD8, CD30 and βF1. Monoclonal rearrangements involving the TCR gamma gene was detected. Blood work was unremarkable. PET-CT scan showed scattered multiple hypermetabolic cutaneous thickening and subcutaneous opacities with no nodal or visceral involvement. The findings altogether were consistent with a PCGDTCL. As the patient presented with a low burden of disease that seemed to behave indolently, he was initially treated with oral bexarotene but the lesions expanded and became more ulcerated (Figure 5B). Local RT and topical mechlorethamine were added and later treatment was changed to oral methotrexate with temporary response. After several months of therapy, the patient developed widespread disease progression as demonstrated on PET-CT scan showing new multiple subcutaneous lesions (Figure 5C) accompanied by evidence of HLH. A more aggressive treatment approach was initiated with ifosfamide, carboplatin, and etoposide (ICE) chemotherapy with plan for consolidation with allogeneic HSCT; however due to further disease progression on ICE, this regimen was discontinued. The patient was subsequently enrolled on a clinical trial, however he expired within few months.
PCGDTCL
PCGDTCL is a rare and very aggressive CTCL subtype (1) composed of a clonal proliferation of mature activated T cells expressing TCRγδ chains and cytotoxic molecules (3,19,36). PCGDTCL accounts for approximately 1% of all primary cutaneous lymphomas and it manifests mainly in middle-aged to elderly patients. Five-year overall survival rates range between 11% to 33% (18,19,37,38) and the median survival is 15 months (19). The heterogeneous clinical presentations and histological patterns (39,40), the presence of reactive γδ T-cell subsets in indolent CTCLs (41,42) and inexperience in the interpretation of these infiltrates has made this a challenging diagnosis (42).
The clinical presentation is usually with multiple lesions, characterized by large and deep plaques, nodules or tumors, that may resemble panniculitis and commonly show superimposed superficial erosions or ulcerations (18,38). PCGDTCL usually appear on the lower limbs, but trunk and upper limbs can be involved (3,37,38). In some cases, MF- or psoriasis-like lesions may be initially noticed and later evolve into an aggressive disease with ulceration (38). Lymph node and bone marrow are mostly uninvolved (3,38). B symptoms, including fevers, night sweats and weight loss are not uncommon. HLH presents with fever, hepatosplenomegaly and cytopenias, and may accompany PCGDTCL, especially in patients with subcutaneous lesions (37,43,44). Although typically aggressive and associated with poor prognosis, rare cases initially presenting with indolent behavior have been reported (45); we approach any such possibility cautiously.
In skin biopsies, variable epidermotropic, dermal or subcutaneous lymphoid infiltrate patterns can be seen (37,38), and may be present synchronously in the same patient or even the same lesion (3). The subcutaneous pattern can resemble SPTCL, but is usually also associated with infiltration of the dermis and epidermis (18,19). Epidermal involvement can be comprised of an interface reaction with vacuolar degeneration, or by pagetoid lymphocytes (18,37). Angiocentricity and angiodestruction can be seen (19). Features of cytotoxicity include hemorrhage, vasculitis with fibrinoid necrosis, epidermal necrosis or ulceration, and subcutaneous fat necrosis with karyorrhexis (38). Immunohistochemical stains show that the neoplastic cells are CD3+, TCRγδ+ (42,46) and CD5- and βF1- with strong expression of at least one cytotoxic marker (38), while CD7 and CD56 may be positive or negative (38). CD4 and CD8 are often double negative though CD8 may be expressed or elevated in some cases (37). Changes in T-cell antigen expression may occur over time (immunophenotypic shift) with no clinical or morphologic correlates reported (47). EBV is generally not present in the neoplastic cells (3). Molecular studies show monoclonally rearranged TCR genes (37,38). The critical distinction from systemic GDTCL, hepatosplenic gamma/delta T-cell lymphoma, requires correlation with the clinical history, radiologic imaging and staging biopsies. A leukemic architecture at histopathologic evaluation (e.g., dense sheets with a Grenz zone) can favor the systemic lymphoma. Recently, our group showed that while high numbers of TCRδ expressing T-cells are most often diagnostic for PCGDTCL, lower numbers of TCRδ+ cells can be found in indolent CTCL and in other lymphoproliferative disorders (as described for example in case 3 above). We recommend cautious follow-up in all cases showing positive TCRδ labeling and specifically when infiltrates are comprised of greater than 25% γδ T-cells (42).
All therapies in PCGDTCL have shown modest effectiveness, and disease resistance to multi-agent chemotherapy is not uncommon (18,19,38). HSCT has been successful in some patients (18,38,48). Given the aggressive course, low response and poor prognosis we initially manage most patients with PCGDTCL similarly to other cytotoxic T-cell lymphomas such as hepatosplenic gamma/delta T-cell lymphoma with platinum-based induction chemotherapy followed by HSCT (26). Improved understanding of the molecular characteristics of the gamma/delta lymphomas through gene expression profiling has led to identification of potential therapeutic targets. In particular, similar to hepatosplenic T cell lymphoma, STAT5B and STAT3 mutations were noted to be present in 27% and 14% of cases of PCGDTCL respectively, suggesting the potential role for JAK inhibition in these diseases (49). Furthermore, additional targets identified in HSTCL, such as phosphoinositide-3-kinase (PI3K) signaling, are likely to be relevant in PCGDTCL (50). Clinical trials with ruxolitinib (JAK1/2 inhibitor), cerdulatinib (JAK 1/3 and SYK inhibitor), and duvelisib (PI3K-δ,γ inhibitor) are currently enrolling patients with T-cell lymphoma, including PCGDTCL, and thus will help characterize the therapeutic potential of these targets in the gamma/delta lymphomas (NCT02974647, NCT01994382, and NCT02783625).
Conclusions
Compared to MF/Sézary Syndrome and primary cutaneous CD30+ lymphoproliferative disorders, the rare CTCL are more controversial regarding characterization and classification. Due to the rarity of these disorders and clinicopathologic overlap, there is limited data to guide therapeutic approaches and treatment recommendations are primarily based upon data from small series. Moving forward, multicenter collaboration will be essential to better define diagnosis and management of these rare CTCL subtypes.
Acknowledgements
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnote
Conflicts of Interest: Dr. AJ Moskowitz has received research support from Seattle Genetics, Merck, Bristol-Myers Squibb, Incyte. She has received honorarium from Kyowa Hakko Kirin Pharma, Miragen Therapeutics, Takedea Pharmaceuticals, ADC Therapeutics, Seattle Genetics, Cell Medica, Bristol-Myers Squibb, Erytech Pharma. The other authors have no conflicts of interest to declare.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Willemze R. CD30-Negative Cutaneous T-Cell Lymphomas Other than Mycosis Fungoides. Surg Pathol Clin 2014;7:229-52. [Crossref] [PubMed]
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85. [Crossref] [PubMed]
- Alberti-Violetti S, Torres-Cabala CA, Talpur R, et al. Clinicopathological and molecular study of primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma. J Cutan Pathol 2016;43:1121-30. [Crossref] [PubMed]
- Virmani P, Jawed S, Myskowski PL, et al. Long-term follow-up and management of small and medium-sized CD4(+) T cell lymphoma and CD8(+) lymphoid proliferations of acral sites: a multicenter experience. Int J Dermatol 2016;55:1248-54. [Crossref] [PubMed]
- Beltraminelli H, Leinweber B, Kerl H, et al. Primary cutaneous CD4+ small-/medium-sized pleomorphic T-cell lymphoma: a cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance? A study of 136 cases. Am J Dermatopathol 2009;31:317-22. [Crossref] [PubMed]
- Yuan Z, Grass GD, Robinson TJ, et al. Management of primary cutaneous CD4+ small and medium pleomorphic T-cell lymphoma: A retrospective study. J Am Acad Dermatol 2018;79:772-4. [Crossref] [PubMed]
- Baum CL, Link BK, Neppalli VT, et al. Reappraisal of the provisional entity primary cutaneous CD4+ small/medium pleomorphic T-cell lymphoma: a series of 10 adult and pediatric patients and review of the literature. J Am Acad Dermatol 2011;65:739-48. [Crossref] [PubMed]
- Geller S, Markova A, Pulitzer M, et al. Acral angiokeratoma-like pseudolymphoma in a middle-aged woman. J Cutan Pathol 2017;44:878-81. [Crossref] [PubMed]
- Grogg KL, Jung S, Erickson LA, et al. Primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma: a clonal T-cell lymphoproliferative disorder with indolent behavior. Mod Pathol 2008;21:708-15. [Crossref] [PubMed]
- Garcia-Herrera A, Colomo L, Camos M, et al. Primary cutaneous small/medium CD4+ T-cell lymphomas: a heterogeneous group of tumors with different clinicopathologic features and outcome. J Clin Oncol 2008;26:3364-71. [Crossref] [PubMed]
- James E, Sokhn JG, Gibson JF, et al. CD4 + primary cutaneous small/medium-sized pleomorphic T-cell lymphoma: a retrospective case series and review of literature. Leuk Lymphoma 2015;56:951-7. [Crossref] [PubMed]
- Rodriguez Pinilla SM, Roncador G, Rodriguez-Peralto JL, et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am J Surg Pathol 2009;33:81-90. [Crossref] [PubMed]
- Cetinozman F, Jansen PM, Willemze R. Expression of programmed death-1 in primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma, cutaneous pseudo-T-cell lymphoma, and other types of cutaneous T-cell lymphoma. Am J Surg Pathol 2012;36:109-16. [Crossref] [PubMed]
- Petrella T, Maubec E, Cornillet-Lefebvre P, et al. Indolent CD8-positive lymphoid proliferation of the ear: a distinct primary cutaneous T-cell lymphoma? Am J Surg Pathol 2007;31:1887-92. [Crossref] [PubMed]
- Kluk J, Kai A, Koch D, et al. Indolent CD8-positive lymphoid proliferation of acral sites: three further cases of a rare entity and an update on a unique patient. J Cutan Pathol 2016;43:125-36. [Crossref] [PubMed]
- Alberti-Violetti S, Fanoni D, Provasi M, et al. Primary cutaneous acral CD8 positive T-cell lymphoma with extra-cutaneous involvement: A long-standing case with an unexpected progression. J Cutan Pathol 2017;44:964-8. [Crossref] [PubMed]
- Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood 2008;111:838-45. [Crossref] [PubMed]
- Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood 2003;101:3407-12. [Crossref] [PubMed]
- Huppmann AR, Xi L, Raffeld M, et al. Subcutaneous panniculitis-like T-cell lymphoma in the pediatric age group: a lymphoma of low malignant potential. Pediatr Blood Cancer 2013;60:1165-70. [Crossref] [PubMed]
- Pincus LB, LeBoit PE, McCalmont TH, et al. Subcutaneous panniculitis-like T-cell lymphoma with overlapping clinicopathologic features of lupus erythematosus: coexistence of 2 entities? Am J Dermatopathol 2009;31:520-6. [Crossref] [PubMed]
- Wu X, Subtil A, Craiglow B, et al. The coexistence of lupus erythematosus panniculitis and subcutaneous panniculitis-like T-cell lymphoma in the same patient. JAAD Case Rep 2018;4:179-84. [Crossref] [PubMed]
- Bosisio F, Boi S, Caputo V, et al. Lobular panniculitic infiltrates with overlapping histopathologic features of lupus panniculitis (lupus profundus) and subcutaneous T-cell lymphoma: a conceptual and practical dilemma. Am J Surg Pathol 2015;39:206-11. [Crossref] [PubMed]
- Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol 2001;28:235-47. [Crossref] [PubMed]
- Mehta N, Wayne AS, Kim YH, et al. Bexarotene is active against subcutaneous panniculitis-like T-cell lymphoma in adult and pediatric populations. Clin Lymphoma Myeloma Leuk 2012;12:20-5. [Crossref] [PubMed]
- Kheterpal M, Mehta-Shah N, Virmani P, et al. Managing Patients with Cutaneous B-Cell and T-Cell Lymphomas Other Than Mycosis Fungoides. Curr Hematol Malig Rep 2016;11:224-33. [Crossref] [PubMed]
- Guitart J, Martinez-Escala ME, Subtil A, et al. Primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphomas: reappraisal of a provisional entity in the 2016 WHO classification of cutaneous lymphomas. Mod Pathol 2017;30:761-72. [Crossref] [PubMed]
- Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol 1999;155:483-92. [Crossref] [PubMed]
- Robson A, Assaf C, Bagot M, et al. Aggressive epidermotropic cutaneous CD8+ lymphoma: a cutaneous lymphoma with distinct clinical and pathological features. Report of an EORTC Cutaneous Lymphoma Task Force Workshop. Histopathology 2015;67:425-41. [Crossref] [PubMed]
- Geller S, Pulitzer M, Myskowski PL. Diffuse eruptive ulcerated plaques. Int J Dermatol 2018;57:1055-7. [Crossref] [PubMed]
- Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol 2012;67:748-59. [Crossref] [PubMed]
- Martinez-Escala ME, Kantor RW, Cices A, et al. CD8+ mycosis fungoides: A low-grade lymphoproliferative disorder. J Am Acad Dermatol 2017;77:489-96. [Crossref] [PubMed]
- Sheng N, Li Z, Su W, et al. A Case of Primary Cutaneous Aggressive Epidermotropic CD8+ Cytotoxic T-cell Lymphoma Misdiagnosed as Febrile Ulceronecrotic Mucha-Habermann Disease. Acta Derm Venereol 2016;96:136-7. [Crossref] [PubMed]
- Guitart J, Variakojis D, Kuzel T, et al. Cutaneous CD8 T cell infiltrates in advanced HIV infection. J Am Acad Dermatol 1999;41:722-7. [Crossref] [PubMed]
- Cyrenne BM, Gibson JF, Subtil A, et al. Transplantation in the Treatment of Primary Cutaneous Aggressive Epidermotropic Cytotoxic CD8-Positive T-Cell Lymphoma. Clin Lymphoma Myeloma Leuk 2018;18:e85-93. [Crossref] [PubMed]
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program 2009.523-31. [Crossref] [PubMed]
- Toro JR, Beaty M, Sorbara L, et al. gamma delta T-cell lymphoma of the skin: a clinical, microscopic, and molecular study. Arch Dermatol 2000;136:1024-32. [Crossref] [PubMed]
- Guitart J, Weisenburger DD, Subtil A, et al. Cutaneous gammadelta T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol 2012;36:1656-65. [Crossref] [PubMed]
- Berti E, Cerri A, Cavicchini S, et al. Primary cutaneous gamma/delta T-cell lymphoma presenting as disseminated pagetoid reticulosis. J Invest Dermatol 1991;96:718-23. [Crossref] [PubMed]
- Burg G, Dummer R, Wilhelm M, et al. A subcutaneous delta-positive T-cell lymphoma that produces interferon gamma. N Engl J Med 1991;325:1078-81. [Crossref] [PubMed]
- Guitart J, Martinez-Escala ME. gammadelta T-cell in cutaneous and subcutaneous lymphoid infiltrates: malignant or not? J Cutan Pathol 2016;43:1242-4. [Crossref] [PubMed]
- Pulitzer M, Geller S, Kumar E, et al. T-cell receptor-δ expression and γδ+ T-cell infiltrates in primary cutaneous γδ T-cell lymphoma and other cutaneous T-cell lymphoproliferative disorders. Histopathology 2018;73:653-62. [Crossref] [PubMed]
- Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol 2009;6:707-17. [Crossref] [PubMed]
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic Lymphohistiocytosis in Cutaneous T-Cell Lymphoma. JAMA Dermatol 2018;154:828-31. [Crossref] [PubMed]
- Hosler GA, Liegeois N, Anhalt GJ, et al. Transformation of cutaneous gamma/delta T-cell lymphoma following 15 years of indolent behavior. J Cutan Pathol 2008;35:1063-7. [Crossref] [PubMed]
- Jungbluth AA, Frosina D, Fayad M, et al. Immunohistochemical Detection of γ/δ T Lymphocytes in Formalin-fixed Paraffin-embedded Tissues. Appl Immunohistochem Mol Morphol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Agbay RL, Torres-Cabala CA, Patel KP, et al. Immunophenotypic Shifts in Primary Cutaneous gammadelta T-Cell Lymphoma Suggest Antigenic Modulation: A Study of Sequential Biopsy Specimens. Am J Surg Pathol 2017;41:431-45. [Crossref] [PubMed]
- Gibson JF, Alpdogan O, Subtil A, et al. Hematopoietic stem cell transplantation for primary cutaneous gammadelta T-cell lymphoma and refractory subcutaneous panniculitis-like T-cell lymphoma. J Am Acad Dermatol 2015;72:1010-5.e5. [Crossref] [PubMed]
- Kucuk C, Jiang B, Hu X, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun 2015;6:6025. [Crossref] [PubMed]
- McKinney M, Moffitt AB, Gaulard P, et al. The Genetic Basis of Hepatosplenic T-cell Lymphoma. Cancer Discov 2017;7:369-79. [Crossref] [PubMed]


