Allogeneic hematopoietic stem cell transplantation in advanced stage mycosis fungoides and Sézary syndrome: a concise review
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of non-Hodgkin lymphoma (NHL) that originate from skin-homing or skin-resident T-cells. Mycosis fungoides (MF) and Sézary syndrome (SS) comprise the majority of cases accounting for 70–75% of all newly diagnosed CTCL with the median age at diagnosis of 55–60 years (1,2). Staging of MF and SS through the TNMB (tumor, node, metastasis, blood) staging system continues to be the most important prognostic tool (3,4), and approximately 70% of cases present with early stage (IA–IIA) disease (4,5). Stage IA and IB disease are associated with 5-year overall survival (OS) rates of 96–99% and 75–86%, respectively (4,5). Treatment options for early stage disease include expectant management or skin directed therapies such as topical steroids, topical nitrogen mustards, light therapy and radiation (6). Advanced stage CTCL carries with it a much less favorable prognosis and often warrants systemic therapy (6). In a recently published series of 1,275 patients with advanced disease (stage IIB or greater), the median OS was just 63 months, with 2 and 5-year survival rates of 77% and 52%, respectively. Median survival for stage IIB disease was 68 months, 48 months for stage IVA, and 33 months for stage IVB (7). Treatment options include, but are not limited to bexarotene, vorinostat, romidepsin and most recently, brentuximab vedotin (for CD30+ MF) and mogamulizumab-kpkc. Unfortunately, for these agents and others the overall response rates (ORRs) are averaging only 30–40% with limited durations of response. Excellent reviews on systemic therapies are widely available (6,8,9), but beyond the scope of this review (it is discussed in detail in this issue’s review of systemic chemotherapy of CTCL by Dr. Alpdogan). Several other systemic therapies are recommended by the National Comprehensive Cancer Network (NCCN), the European Organization for Research and Treatment of Cancer (EORTC), and other cutaneous lymphoma consortiums. Similarly, the NCCN and the EORTC as well as the American Society of Blood and Marrow Transplantation (ASBMT) list allogeneic hematopoietic stem cell transplant (HSCT) as an option for advanced stage CTCL, which as of this writing remains the only potential curative treatment option (9-11).
In this review we first discuss the advancements HSCT transplantation has made in recent years. We then focus on the role of HSCT in advanced CTCL. To date there remains no randomized published control trials of HSCT versus systemic treatment; thus, this review is limited to published data consisting of small case series and retrospective analyses. We will conclude this review with our recommendations.
HSCT: advancements
High-dose chemoradiotherapy followed by HSCT is a potentially curative modality for a variety of hematologic disorder that are incurable with conventional dose chemotherapy (12). We consider that using less intensive conditioning regimens, progress in alternative donor transplantation including haploidentical and cord blood stem cell transplantation, development of better T-cell depletion methods including CD34+ cell purification and progress in post-transplant immunosuppressive therapy the major advancements in the last two decades.
Development of less intensive conditioning regimens
These approaches do not use dose intensity of chemotherapy/radiation therapy to eradicate malignancy. Rather they use immunosuppressive agents, irrespective of their anti-neoplastic properties, to facilitate donor lymphoid and stem cell engraftment. The donor lymphoid elements then destroy the residual normal and malignant lymphohematopoietic elements allowing the transition to complete donor chimerism (13,14). This type of transplant has been dramatically effective in chronic myeloid leukemia, chronic lymphocytic leukemia, and follicular lymphoma in its original application and may have utility in other diseases as well (13-16). This approach disproved the earlier dogma in the transplant community that engraftment required the administration of high dose, highly toxic, and lethally ablative conditioning regimens to sufficiently destroy the host immune system to avoid graft rejection. Although used for various patient populations, reduced intensity transplant has broadened the applicability of HSCT to older patients and to patients with comorbidities who otherwise would not tolerate the rigors of a fully myeloablative HSCT (17,18).
Regimens that are not lethally myeloablative have generated consistent engraftment using immunosuppressive drugs such as fludarabine in combination with other chemotherapeutic agents such as melphalan, busulfan or low dose total body irradiation (TBI) (16-18). Alternative reduced intensity approaches using low dose irradiation and a synergistic immunosuppressive combination of cyclosporine and mycophenolate mofetil have produced comparable results (19). Although graft-versus-host disease (GVHD) was particularly problematic, this regimen provides proof of principle that reduced intensity HSCT is a promising treatment for older patients and those patients with significant comorbidities.
Approaches to alternate donors and progress in haploidentical HSCT
A barrier to the application of reduced intensity transplantation in hematologic malignancies is the availability of donors. Only 30% of patients in North America who may benefit from HSCT will have a human leukocyte antigens (HLA)-matched sibling donor.
In the last two decades, new developments in haploidentical HSCT have made it a viable alternative donor option. In selected centers, haploidentical HSCT has produced excellent results in patients with hematological malignancies. Murine and human hematopoietic stem cells are able to decrease alloreactivity of cytotoxic T-cells, which is called “veto cell activity” (20,21) and immune tolerance can be induced by using high doses of stem cells especially in major histocompatibility complex (MHC)-mismatched donor/host combinations (22). Aversa et al. successfully used a mega-dose of CD34+ stem cells with an enhanced myeloablative and immunosuppressive protocol in haploidentical transplantation, which resulted in high-level engraftment of MHC disparate stem cells (23). Although almost all patients engrafted well, transplant related mortality still remained high because of increased risks of infection (24,25). On the other hand, missing MHC class I molecules in recipients of haploidentical HSCT induced donor NK cell activity, which is directly linked to “killer cell immunoglobulin like receptors (KIRs)”. Ruggeri et al. showed donor-versus-recipient NK cell activity could eliminate leukemic relapse and graft rejection (26). Transplantation from NK-alloreactive donors was associated with significantly lower relapse rate and survival in patients with acute myeloblastic leukemia (27). Using the cytokines during stem cell collection would also improve outcome of the transplant. Huang et al. reported a large group of patients with successful engraftment and relatively low treatment related mortality (TRM) by using granulocyte colony stimulating factor (G-CSF) primed bone marrow and peripheral blood stem cells (28). The Peking University group recently reported the largest haplo-HSCT study using unmanipulated G-CSF primed bone marrow and peripheral blood stem cells in 756 acute leukemia patients with successful outcomes (29).
Strategies to induce lymphocyte tolerance in HSCT
The removal of lymphocytes from the donor product has been critical to avoid the lethal GVHD that would occur due to the high degree of HLA disparity in partially-matched related HSCT. If there was a mechanism to add donor lymphocytes to the transplant process without causing significant GVHD, morbidity from graft rejection, infection, and relapse (three significant contributors to morbidity in this type of transplant) would likely decrease. If donor-host tolerance could be established at the time of the transplant, the removal of T- cells would not be necessary. In this scenario, haploidentical HSCT could be infused into the recipient as long as tolerance was established. There is data regarding the immune modulating effects of many drugs, such as cyclophosphamide (CTX) which may be exploited to create immune tolerance and immune modulation of donor lymphocytes in the transplant inoculum (30-32).
Utilizing CTX
CTX is a well-known alkylating chemotherapeutic medication and is widely used as a part of myeloablative protocols in combination with TBI. Luznik et al. showed in the preclinical murine HSCT experiments that post-transplant CTX (PT-CTX) administration resulted in a durable engraftment in the recipients of MHC-matched and -mismatched donors after non-myeloablative regimens (32-35). O’Donnell and colleagues reported that partially HLA-mismatched bone marrow could provide rapid and stable engraftment after PT-CTX (36). Recently, the Bone Marrow Transplant Clinical Trials Network (BMT-CTN) evaluated the efficacy of haploidentical HSCT with PT-CTX and double cord blood transplantation. They found similar efficacy between these alternative donor-transplants (37). Ciurea et al. with data from the Center for the International Blood and Marrow Transplant (CIBMTR) showed that survival for patients with acute myeloid leukemia after haploidentical transplantation with PT-CTX is comparable with matched unrelated donor transplantation (38). Grosso et al. modified this approach to give cyclophosphamide after administration of a fixed dose of donor T-cells to induce tolerance prior to stem cell transplantation (39).
The utility of stem cell transplantation in patients with CTCL
Autologous HSCT in CTCL
Retrospective data showed autologous stem cell transplant had excellent ORR with the majority of cases achieving a complete response (CR) (37-39). However, 75% of these cases relapsed with the median time to disease progression of a mere 2.3 months (40). Graft T-cell depletion prior to autologous transplant unfortunately also showed high relapse rates attributed to compromised cytotoxic response post-transplant (41). In a meta-analysis by Wu et al., superior OS rates and event free survival rates were observed with allogeneic transplant over autologous (42). Given this data, autologous transplant is no longer sought as a treatment modality for these patients.
Allogeneic HSCT in CTCL
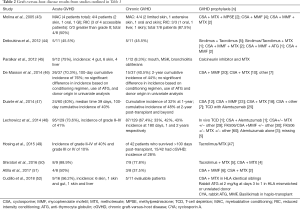
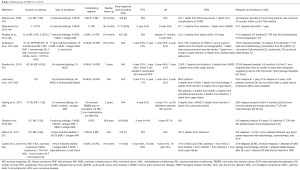
Allogeneic HSCT for advanced stage CTCL dates back as early as the 1980s (42) and several publications have since indicated that HSCT could provide a cure in patients with a previously incurable disease (Table 1). We will now review in detail 6 of the largest and most recently published case series followed by a brief summary of what we have learned about HSCT for CTCL to date.
Full table
In 2010, two of the largest reports to date were published in the Journal of Clinical Oncology just four months apart. The first by Duvic et al. at the MD Anderson Cancer Center details the outcomes of 19 patients over a 7-year span with CTCL prospectively treated with HSCT using a reduced intensity-conditioning (RIC) regimen (53). Subsequently in 2015, Hosing et al. published the updated results and the results of 28 additionally treated patients (47 total) (49). Forty-two of these patients received tumor debulking in the form of total body skin electron beam radiation (TBSEB) with 36 Gy over an 8-week course just prior to transplant. All patients had advanced CTCL (stage IIB or higher) with a median age of 51.5 years. Only patients who received unrelated (24) or mismatched (2) grafts received in vivo T-cell depletion using anti-thymocyte globulin (ATG) as a part of their conditioning. Most of the patients were in either CR (15%) or partial response (PR) (60%) prior to transplant.
The Kaplan-Meier estimated OS at 4 years was 51% and the estimated 4-year progression-free survival (PFS) was 26%. PFS was superior in patients who had SS vs. those with MF (72.7% vs. 11.5%; P=0.04), but there were no OS differences. The 4-year PFS in patients with MF with large cell transformation (LCT) was dismal at 8.6%. The cumulative non-relapse mortality (NRM) rate was 10.4% and 16.7% at 1 and 2 years, respectively. Contrary to other studies, the authors were not able to show an increased risk of relapse/progression (REL) with the use of ATG. The cumulative incidence grade III–IV acute GVHD was 10%. The cumulative incidence of chronic GVHD was 28%. The most common organ involved was the skin. At the time of their 2015 updated results with a median follow-up after HSCT of 2 years, 27/47 patients were alive; of these patients, 20 had CR, 1 had a PR, 3 had stable disease, and 3 had progressive disease. A total of 22 patients received additional therapy of which 8 achieved a second CR.
The second major publication in 2010 came from Duarte et al. from the European Society for Blood and Marrow Transplantation (EBMT) when they published one of the largest multi-center retrospective analyses of 60 patients with advanced stage CTCL with a median age of 46.5 years (54). Only ten patients had <10% residual disease at time of HSCT, and 16 patients (27%) received myeloablative condition regimens (MAC). The initial publication was of limited follow-up of 36 months reporting an estimated OS of 66% at 1-year and 54% at 3 years, but in 2014 they published their extended analysis reporting on OS rates of 46% and 44% at 5 and 7 years, respectively (47). PFS was reported at 32% and 30% at 5 and 7 years, respectively. A total of 27 patients (45%) experienced REL at a median of 3.8 months after HSCT and the 7-year NRM was 22%. Only 2 of these 27 patients had relapsed after 2 years suggesting that HSCT provides long-term disease control. MAC was associated with poorer NRM [hazard ratio (HR), 4.5; 95% CI, 1.43–14.15; P=0.0101] and OS (HR, 2.99; 95% CI, 1.40–6.36; P=0.0046) while RIC was not associated with higher incidence of REL. Forty (67%) of these patients were classified as having an “advanced phase” of disease course (defined as third or later CR, PR or REL, and those whom were primary refractory to systemic therapy). Patients with this defined “advanced phase” or a higher disease burden prior to HSCT and patients receiving T-cell depletion carried a higher risk of REL. Out of 17 patients whom received donor lymphocyte infusion (DLI) for REL, 10 responded with 8 CR. Twenty-seven patients were alive (45%) and 26 of 27 patients were in CR at the last follow-up visit.
In 2012, Paralkar et al. published their experience on 12 patients with advanced stage CTCL whom were HSCT recipients between 2004 and 2010 at The University of Pennsylvania (45). Seven of the patients had HLA-identical sibling donors and 10 received RIC. The median age at transplant was 53 years. Two patients (1 RIC) died within the first 100 days of transplant and of the other 10 patients who survived to 100 days, 8 reached CR. Four out of these 8 CR patients relapsed between 8–13 months after transplant of which 2 achieved a second CR by tapering immunosuppression and DLI. At the time of publication with a median follow-up of 24 months, five patients remained in CR. The Kaplan-Maier estimated 2-year OS was 58%, and the estimated median OS was 37 months. Acute GVHD developed in 9 of 12 patients with four patients developing grade III–IV GVHD. Six patents developed GVHD of the skin half of which were grade III. GVHD was the cause of death in one patient.
Recently, Lechowicz et al. analyzed the CIBMTR data, which included outcomes of 129 patients, reported between 2000–2009 (48). Most of the patients had relapsed/refractory disease. Sixty-four percent received non-myeloablative/RIC conditioning regimens. NRM at 1 and 5 years was 19%. Risk of disease progression was 50% (95% CI, 41–60%) at 1-year and 61% (95% CI, 50–71%) at 5 years. OS at 1 and 5 years was 54% (95% CI, 45–63%) and 32% (95% CI, 22–44%), respectively.
de Masson and colleagues from the French Society of Bone Marrow Transplantation (FSBMT) and French Study Group on Cutaneous Lymphomas published their experience on 37 cases of HSCT for advanced stage CTCL. One caveat was the fact that many patients (54%) had LCT-MF (46). The median age was 44 years and 24 patients (66%) had stage IV disease. Seventeen patients (46%) had sibling donors, and 20 (54%) received their transplant from unrelated donors (2 cord blood). The use of ATG in 16 (43%) patients was driven by local protocol. No regimens constituted depletion of T cells in vitro. RIC was used in 25 (68%) of patients.
After a median follow-up of 29 months, 23 patients were alive (62%) and 14 had died (38%); 8 (22%) from disease REL and 6 (16%) from NRM. The type of conditioning regimen had no significant impact on NRM in univariate analysis. The estimated 1 and 2-year OS rates were 65% and 57%, respectively. The estimated PFS was 39% at 1 year and 31% at 2 years. For LCT-MF (n=20), 1 and 2-year PFS were 39% and 26%, respectively. In total, 19 (51%) patients experienced REL after transplantation with a median time to progression of 10 weeks. Ninety percent of all relapses occurred within the first year after HSCT. While 8 of these 19 patients died from REL, 6 patients went on to achieve a second CR and 5 patients experienced at least PR with additional therapy after their REL. Pre-transplant CR or very good PRs was associated with a decreased risk of REL in univariate analysis. The absence of ATG was the only factor significantly associated with NRM in univariate analysis. Interestingly, also in multivariate analysis the use of ATG was the only factor significantly associated with decreased PFS suggesting that intact immune system is required for the graft-versus-lymphoma activity.
The most recently published study provided by Cudillo et al. details a retrospective cohort of 16 patients treated with HSCT with a 76-month median follow-up (52). With a median age of 54 years, 10/16 patients received RIC conditioning regimens, and ATG was used in mismatched or matched unrelated donors. Only 11 patients were evaluable for efficacy as 5 patients had died early in their treatment course including 3 patients treated with RIC and 4 patients dying from complications of GVHD. However, for those alive the REL rate was just 20% at 1-year and 27% at 10 years. Remarkably, 9/16 patients were alive while 8 remained in CR at the 76-month median follow-up. For all patients the probability of OS was 61% (95% CI, 40–91%) and 54% (95% CI, 33–86%), at 1- and 10-year post-transplant, respectively. At relapse, DLI was incorporated in four patients treated with RIC. Two of these 4 patients responded with minimal and no GVHD, respectively. Of the 2 patients whom did not respond to DLI, 1 died from GVHD complications.
One very interesting finding described by the authors was the clinical outcomes measured at “an interval time from diagnosis to transplant less than or greater than 46 months”, which was their median time observed in their 16-patient cohort. The OS at both 1 and 10 years was 88% (95% CI, 67–100%) for patients transplanted less than 46-months from diagnosis. The OS was 37% (95% CI, 15–92%) and 25% (95% CI, 8–83%) at 1 and 10 years, respectively, for patients transplanted greater than 46 months from diagnosis (log-rank P<0.04) [HR 7.26; 95% CI, 0.86–60.95; P<0.068]. Similarly, they found the probability of disease-free survival (DFS) at 1 year to be 73% (95% CI, 47–100%) and at 10 years to be 58% (95% CI, 32–100%), for patients undergoing transplant less than 46 months from diagnosis which was significantly worse than the DFS of 13% at both 1 and 10 years (95% CI, 2–78%) for patients transplanted at greater than 46 months (log-rank P<0.05), [HR 3.68; 95% CI, 0.91–14.87; P<0.067].
What we have learned from these and prior publications utilizing HSCT for CTCL
Choice of conditioning regimen
As previously described RIC has significantly decreased the NRM in NHL (17,18). Duarte et al. showed higher NRM and poorer OS with MAC with no impact on relapse-free survival. Using only RIC in their cohort of 47 patients, Hosing et al. (49) demonstrated an estimated 4-year OS of 51%. According to de Masson et al., the choice of conditioning regimen had no statistically significant impact on TRM or efficacy. Ten out of the 12 patients described by Paralkar et al. (45) received RIC with 42% alive with sustained clinical responses at a median 22-month follow-up. At a median follow-up of 32 months Shiratori et al. reported an estimated 3-year OS of 85.7% and PFS of 44.4% in which all nine patients received RIC (50). Lechowicz et al. (48) reported the data from the CIBMT from 2000–2009. In total 83 patients received RIC (64%). OS was 56% and 41% at 1 and 3 years, respectively for RIC and 51% and 31%, respectively, for MAC. NRM at 1 year was 19% and at 5 years was 22%. NRM did not differ significantly between RIC and MAC cohorts. These data suggest that MAC has not improved outcomes and is not necessary for patients with advanced stage CTCL.
T-cell depletion
de Masson and colleagues from the FSBMT group reported the use of ATG in 43% of their patients (which was driven by local protocol and was not associated with any specific donor type) was the only factor significantly associated with a decreased TRM (HR 1.10−7; 95% CI, 4.10−8 to 2.10−7; P<0.001) as all 6 of the patients whom died during autologous stem cell transplantation (ASCT) did not receive ATG. On the contrary ATG was the only factor associated with increased risk of REL (HR 4.8; 95% CI, 1.8–12.9; P=0.002). The EBMT groups also showed that receiving T-cell depletion carried a higher risk of REL (HR 2.48; 95% CI, 1.15–5.35; P=0.0207) (54). However, Hosing et al. (49) were not able to show a similar increased risk of REL with ATG. Yet, in their cohort 42 (89%) patients received TBSEB radiation therapy just prior to HSCT, of whom 25 (60%) achieved a CR in the skin. We conclude with the EBMT and French group data that in vivo T-cell depletion may affect the outcome of the disease.
Disease status prior to transplant
Very good PR or CR prior to HSCT was the most important prognostic factor for increased PFS in univariate analysis (HR 0.3; 95% CI, 0.1–0.8; P=0.01) in the report by de Masson. Duarte et al. (47) reported only 10/60 patients had <10% residual disease at time of HSCT. However, they did report patients with a lower disease burden prior to HSCT had a lower cumulative incidence of REL compared with those with a higher disease burden (P=0.04). TBSEB was administered prior to transplantation in their prospective protocol in the reports by Duvic et al. (53) and Hosing et al. (49) Although they did not comment on the statistical significance of having a CR prior to transplant, they did report a post-HSCT CR rate of 58%. With this data we may conclude that minimal disease prior to transplant is most ideal, but not a strict requirement to harness the graft-versus-lymphoma effect.
Timing of transplant
It is important to note again the additional observations made by Duarte et al. (47) in that patients with what they defined as “advanced disease” phase (the definition of which is detailed above) at HSCT had an increased risk of REL [HR 3.07; 95% CI, 1.15–8.20; P=0.0249], lower PFS (HR 3.26; 95% CI, 1.43–7.47; P=0.0051) and worse OS [HR 3.72; 95% CI, 1.49–9.30; P=0.0049] compared to “early disease” phase. Additionally, Cudillo et al. (52) found benefit with earlier transplants. The OS at both 1 and 10 years was 88% (95% CI, 67–100%) for patients transplanted less than 46 months from diagnosis vs. 37% (95% CI, 15–92%) and 25% (95% CI, 8–83%), respectively for patients transplanted greater than 46-months from diagnosis (log-rank P<0.04) [HR 7.26; 95% CI, 0.86–60.95; P<0.068)]. Likewise, they also found DFS at 1 year and 10 years benefited transplanting at an “early disease” phase. In the meta-analysis by Lechowicz et al. (48) of 129 HSCT recipients reported to the CIBMTR, 49% of patients were transplanted >36 months from their diagnosis. They did not comment on the significance. We believe that early transplant consultation may improve outcome of disease in this specific patient population.
Graft vs. lymphoma
Paralkar et al. (45) detailed their experience on two relapses in their cohort and the argument of graft-versus-lymphoma (Table 2). The first patient whom relapsed 10 months after HSCT received DLI (2.2×108 nucleated cells/kg) at first relapse and achieved CR that persisted for 26 months. The second patient achieved second CR with discontinuation of immunosuppression and remained in CR. None of these patients had received additional treatments for their CTCL. In the EBMT report by Duarte et al. (47), 10/17 patients whom received DLI responded including 8 CR, confirming allogeneic response would provide anti-lymphoma activity. Cudillo et al. (52) had a 50% success rate with REL treated with DLI. Hosing et al. (49) observed secondary responses in 8 of 22 relapses with immunomodulation post-transplant. Herbert et al. published their successes in three patients whom relapsed after HSCT with decreasing immunosuppression and the use of DLI (55). The authors made an important observation in that all their patients relapsed with the high grade LCT-MF disease they had prior to transplant. This observation had not been described previously, and the authors suggest the graft-versus-lymphoma benefits are greatest pre-LCT. This theory is supported by the poor 4-year PFS in patients with MF with LCT of 8.6% in the Hosing prospective cohort, and supports the literature arguing for earlier transplant. Certainly, the use of DLI can be fraught with balancing GVHD in many cases, but this literature is quite clear that immunomodulation can be very successful and should be utilized in patients with REL post-HSCT with no to minimal evidence of GVHD.
Graft vs. host disease
Paralkar et al. (45) reported GVHD developing in 9 of 12 patients with 4 developing grade III–IV GVHD (Table 2). Six patents developed GVHD of the skin half of which were grade III, and GVHD was the cause of death in one patient. de Masson reported a 70% occurrence rate of acute GVHD of which nearly half developed grade II or higher disease and chronic GVHD developed in 15 patients. Seven out of the 8 patients described by Molina et al. developed GVHD (43). Shiratori’s group document acute GVHD in 8/9 transplants and chronic GVHD in 7/9 patients. Meanwhile, Hosing et al. (49) reported much less rates of GVHD with an incidence of grade II–IV acute GVHD of 40% with an incidence of chronic GVHD of 28%. The cumulative incidence of grade III–IV acute GVHD was 10%. The cumulative incidence of chronic GVHD was 28%. They hypothesized the routine use of TBSEB may lessen the development of cutaneous GVHD.
Relapse rates
In the CIBMTR meta-analysis by Lechowicz et al. (48) of the REL rate was 50% at 1-year and 61% at 5 years, suggesting that the majority of REL occur within the first year post-transplant. Similarly, Hosing et al. (49) reported 50% REL with the majority occurring within 6 months of transplant. Duarte et al. (47) showed a 45% REL at a median of 3.8 months post-transplant, but only two relapses occurred beyond 2 years. de Masson et al. (46) totaled 19 (51%) relapses with a median time to progression of 10 weeks, and 90% of all REL occurred within the first year. Relapses occurred in 5/9 patients described in the Shiratori cohort at a median of 45 days (50). We conclude that as much as half of the patients with CTCL may have a relapse after HSCT in the first year after transplant and a decreased tumor burden prior to the transplant may improve the post-transplant relapse risk.
OS
There are no randomized trials comparing HSCT vs. systemic therapies. We must then assess meaningful OS, PFS, and REL in comparison with historical controls, and few of these retrospective analysis report beyond 5 years. Duarte et al. (47) in their extended analysis reported OS of 46% and 44% at 5 and 7 years, respectively. The Kaplan-Meier estimate of OS at 4 years was 51% reported by Hosing et al. (49) In the CIBMTR analysis by Lechowicz et al. (48) the OS was 56% and 41% at 1 and 3 years respectively for RIC and 51% and 31% respectively for MAC. de Masson et al. (46) estimated 1- and 2-year OS rates were 65% and 57%, respectively. For Cudillo et al. (52) the probability of OS was 61% (95% CI, 40–91%) and 54% (95% CI, 33–86%) at 1 and 10 years post-transplant, respectively. Compared to the most recently published data on 1,275 patients with advanced stage disease (IIB–IV) where the median projected 5-year OS rates was 52%, the results of HSCT with a curative intent are encouraging (7).
Our recommendations for HSCT for advanced stage CTCL
- We agree with the recommendations from the NCCN, EORTC and the ASBMT of including HSCT as a treatment option for advanced stage, relapsed or refractory CTCL;
- We agree with their recommendations to not offer HSCT in early stage (IA–IIA) CTCL and we also agree the strong recommendation against autologous stem cell transplant for CTCL in any clinical setting;
- While the optimal timing of HSCT transplant (1st line, 2nd line or greater) has yet to be determined, we recommend early consultation in transplant eligible patients. This recommendation is based on the data showing increased OS and decreased REL when HSCT is utilized as an earlier treatment option and before progression to LCT;
- We have shown convincing data supporting the use of RIC over MAC in improving NRM with equivocal efficacy, and we recommend a RIC conditioning regimen in all eligible patients. Minimal disease burden at time of transplant, while showing better ORR rates in some studies, has not been consistent in the literature. Additionally, the now well recognized graft-versus-lymphoma effect negates the need for strict CR prior to transplant;
- The use of TBSEBT has been successful in the MD Anderson cohort, but others have reported severe cutaneous toxicities. In patients with significant skin involvement we do recommend this as an option;
- Keeping with the graft-versus-lymphoma effect, we consider that T-cell depletion is not necessary in this patient population. On the other hand, the incidence and severity of GVHD has been decreased with T-cell depletion. GVHD is all but universal; however, with better understanding of the GVHD process and better treatments, this complication has become more manageable.
Conclusions
HSCT for advanced CTCL has made significant strides over the past 30 years, and it remains the only curative approach. Unfortunately, our data and experience are limited to small scale retrospective analyses, case reports and meta-analyses all of which have different conditioning regimens, GVHD prophylaxis, response criteria and types of donors. Clinical trials addressing the optimal timing of HSCT prior to or vs. front-line systemic therapies as well as post-transplant maintenance studies are clearly needed. Additionally, dedicated prospective prognostication studies such as the ongoing PROCLIPI study hope to identify the subsets of patients who will have more aggressive clinical courses (7,56), some of whom may ultimately be appropriate for earlier HSCT strategies even without advanced stage disease at diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood 2009;113:5064-73. [Crossref] [PubMed]
- Imam MH, Shenoy PJ, Flowers CR, et al. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma 2013;54:752-9. [Crossref] [PubMed]
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010;28:4730-9. [Crossref] [PubMed]
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol 2003;139:857-66. [Crossref] [PubMed]
- van Doorn R, Van Haselen CW, van Voorst Vader PC, et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol 2000;136:504-10. [Crossref] [PubMed]
- Horwitz SM, Olsen EA, Duvic M, et al. Review of the treatment of mycosis fungoides and sezary syndrome: a stage-based approach. J Natl Compr Canc Netw 2008;6:436-42. [Crossref] [PubMed]
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sezary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol 2015;33:3766-73. [Crossref] [PubMed]
- Virmani P, Hwang SH, Hastings JG, et al. Systemic therapy for cutaneous T-cell lymphoma: who, when, what, and why? Expert Rev Hematol 2017;10:111-21. [Crossref] [PubMed]
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome - Update 2017. Eur J Cancer 2017;77:57-74. [Crossref] [PubMed]
- Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical Practice Recommendations on Indication and Timing of Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An International Collaborative Effort on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2017;23:1826-38. [Crossref] [PubMed]
- Horwitz SM, Ansell SM, Ai WZ, et al. NCCN Guidelines Insights: T-Cell Lymphomas, Version 2.2018. J Natl Compr Canc Netw 2018;16:123-35. [Crossref] [PubMed]
- Thomas ED. Karnofsky Memorial Lecture. Marrow transplantation for Malignant Diseases. J Clin Oncol 1983;1:517-31. [Crossref] [PubMed]
- Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 1979;300:1068-73. [Crossref] [PubMed]
- Weiden PL, Sullivan KM, Flournoy N, et al. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med 1981;304:1529-33. [Crossref] [PubMed]
- van Besien KW, de Lima M, Giralt SA, et al. Management of lymphoma recurrence after allogeneic transplantation: the relevance of graft-versus-lymphoma effect. Bone Marrow Transplant 1997;19:977-82. [Crossref] [PubMed]
- Khouri IF, Keating M, Korbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998;16:2817-24. [Crossref] [PubMed]
- McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001;97:3390-400. [Crossref] [PubMed]
- Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998;91:756-63. [PubMed]
- Hegenbart U, Niederwieser D, Sandmaier BM, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol 2006;24:444-53. [Crossref] [PubMed]
- Bachar-Lustig E, Li HW, Marcus H, et al. Tolerance induction by megadose stem cell transplants: synergism between SCA-1+ Lin- cells and nonalloreactive T cells. Transplant Proc 1998;30:4007-8. [Crossref] [PubMed]
- Gur H, Krauthgamer R, Berrebi A, et al. Tolerance induction by megadose hematopoietic progenitor cells: expansion of veto cells by short-term culture of purified human CD34(+) cells. Blood 2002;99:4174-81. [Crossref] [PubMed]
- Reisner Y, Gur H, Reich-Zeliger S, et al. Hematopoietic stem cell transplantation across major genetic barriers: tolerance induction by megadose CD34 cells and other veto cells. Ann N Y Acad Sci 2003;996:72-9. [Crossref] [PubMed]
- Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005;23:3447-54. [Crossref] [PubMed]
- Aversa F, Reisner Y, Martelli MF. The haploidentical option for high-risk haematological malignancies. Blood Cells Mol Dis 2008;40:8-12. [Crossref] [PubMed]
- Aversa F, Martelli MF, Velardi A. Haploidentical hematopoietic stem cell transplantation with a megadose T-cell-depleted graft: harnessing natural and adaptive immunity. Semin Oncol 2012;39:643-52. [Crossref] [PubMed]
- Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295:2097-100. [Crossref] [PubMed]
- Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood 2007;110:433-40. [Crossref] [PubMed]
- Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006;38:291-7. [Crossref] [PubMed]
- Wang Y, Liu DH, Liu KY, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013;119:978-85. [Crossref] [PubMed]
- Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med 1989;169:213-38. [Crossref] [PubMed]
- Mayumi H, Umesue M, Nomoto K. Cyclophosphamide-induced immunological tolerance: an overview. Immunobiology 1996;195:129-39. [Crossref] [PubMed]
- Luznik L, Engstrom LW, Iannone R, et al. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant 2002;8:131-8. [Crossref] [PubMed]
- Luznik L, Jalla S, Engstrom LW, et al. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood 2001;98:3456-64. [Crossref] [PubMed]
- Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 2010;47:65-77. [Crossref] [PubMed]
- Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 2012;39:683-93. [Crossref] [PubMed]
- O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2002;8:377-86. [Crossref] [PubMed]
- Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008;14:641-50. [Crossref] [PubMed]
- Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015;126:1033-40. [Crossref] [PubMed]
- Grosso D, Carabasi M, Filicko-O'Hara J, et al. A 2-step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T-cell dosing. Blood 2011;118:4732-9. [Crossref] [PubMed]
- Duarte RF, Schmitz N, Servitje O, et al. Haematopoietic stem cell transplantation for patients with primary cutaneous T-cell lymphoma. Bone Marrow Transplant 2008;41:597-604. [Crossref] [PubMed]
- Olavarria E, Child F, Woolford A, et al. T-cell depletion and autologous stem cell transplantation in the management of tumour stage mycosis fungoides with peripheral blood involvement. Br J Haematol 2001;114:624-31. [Crossref] [PubMed]
- Wu PA, Kim YH, Lavori PW, et al. A meta-analysis of patients receiving allogeneic or autologous hematopoietic stem cell transplant in mycosis fungoides and Sezary syndrome. Biol Blood Marrow Transplant 2009;15:982-90. [Crossref] [PubMed]
- Molina A, Zain J, Arber DA, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol 2005;23:6163-71. [Crossref] [PubMed]
- Delioukina M, Zain J, Palmer JM, et al. Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone Marrow Transplant 2012;47:65-72. [Crossref] [PubMed]
- Paralkar VR, Nasta SD, Morrissey K, et al. Allogeneic hematopoietic SCT for primary cutaneous T cell lymphomas. Bone Marrow Transplant 2012;47:940-5. [Crossref] [PubMed]
- de Masson A, Beylot-Barry M, Bouaziz JD, et al. Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French Society of Bone Marrow Transplantation and French Study Group on Cutaneous Lymphomas. Haematologica 2014;99:527-34. [Crossref] [PubMed]
- Duarte RF, Boumendil A, Onida F, et al. Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a European society for blood and marrow transplantation lymphoma working party extended analysis. J Clin Oncol 2014;32:3347-8. [Crossref] [PubMed]
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant 2014;49:1360-5. [Crossref] [PubMed]
- Hosing C, Bassett R, Dabaja B, et al. Allogeneic stem-cell transplantation in patients with cutaneous lymphoma: updated results from a single institution. Ann Oncol 2015;26:2490-5. [PubMed]
- Shiratori S, Fujimoto K, Nishimura M, et al. Allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning for mycosis fungoides and Sezary syndrome. Hematol Oncol 2016;34:9-16. [Crossref] [PubMed]
- Atilla E, Atilla PA, Bozdag SC, et al. Allogeneic hematopoietic stem cell transplantation for refractory mycosis fungoides (MF) and Sezary syndrome (SS). Int J Hematol 2017;106:426-30. [Crossref] [PubMed]
- Cudillo L, Cerretti R, Picardi A, et al. Allogeneic hematopoietic stem cell transplantation in Primary Cutaneous T Cell Lymphoma. Ann Hematol 2018;97:1041-8. [Crossref] [PubMed]
- Duvic M, Donato M, Dabaja B, et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol 2010;28:2365-72. [Crossref] [PubMed]
- Duarte RF, Canals C, Onida F, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2010;28:4492-9. [Crossref] [PubMed]
- Herbert KE, Spencer A, Grigg A, et al. Graft-versus-lymphoma effect in refractory cutaneous T-cell lymphoma after reduced-intensity HLA-matched sibling allogeneic stem cell transplantation. Bone Marrow Transplant 2004;34:521-5. [Crossref] [PubMed]
- Scarisbrick J, Quaglino P, Vermeer M, et al. Prospective cutaneous lymphoma international study (PROCLIPI) in early stage mycosis fungoides. 3rd World Congress of Cutaneous Lymphomas. Abstract C-02 Presented Oct 26, 2016.