Management of breast cancer brain metastases
Introduction
Breast cancer brain metastasis (BCBM) is more commonly found in the advancement of cancer treatment and is found in the frequency of 10% to 20% of metastatic breast cancer. Control of brain metastasis in metastatic or recurrent breast cancer is a major theme for cancer treatment. Promising targeted therapy for epidermal growth factor receptor (EGFR) gene mutation-positive lung adenocarcinoma is effective systemic treatment also for brain metastasis. On the other hand, standard treatment for BCBMs is still local treatment for brain. Systemic therapy for BCBMs may be allowed to be used to suppress disease progression after initial standard local therapy Of course, treatment modalities for brain metastasis are limited, such as local treatment with surgical treatment and radiotherapy. The prognosis of breast cancer is about 6 months according to whole brain irradiation treatment and only 2 months without treatment (1).
However, considering breast cancer divided into subtypes depending on the presence or absence of estrogen receptor (ER) and HER2 receptor, the prognosis is different between luminal type, HER2 type and triple negative (TN) type, so treatment strategy needs to be changed depending on subtype (2). On the other hand, brain metastases of breast cancer are reported to be frequent in posterior circulation such as the posterior cranial fossa and occipital lobe, differences in spatial metastasis are different from other cancers. In addition, metastases occurring in special sites such as meningeal carcinomatosis and dural metastasis are often observed in breast cancer (3).
In this review, treatment options for brain parenchymal metastasis (BPM), leptomeningeal carcinomatosis (LMC) and intracranial dural metastasis (IDM) will be explained, taking into consideration the subtype of breast cancer.
Brain parenchymal metastases
BPM is the most common complication in breast cancer. In women under the age of 40 and lung metastasis cases, BPM is considered to be particularly common (4-6). From the shape of the BPM, it is divided into nodular cases, solid cases, cystic cases and it is often accompanied with brain edema (Figure 1).
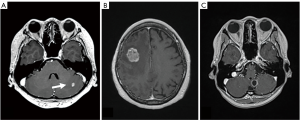
Neurological symptoms tend to be expressed depending on the size of the tumor, but in small tumors with edema there may be symptoms. Occasionally, many BPMs are highly sensitive to radiotherapy, and even if there are symptoms of increased intracranial pressure (IICP) at the time of discovery and palliation of neurological symptoms are also expected.
BCBM occurs in about 5 years after diagnosis of breast cancer. There was a difference in the timing of its expression in the subtype of breast cancer, and in the author’s experience it was 100 months for the ER positive case, 50 months for the HER2 positive case, and 30 months for the TN case (Takahashi H, unpublished data 2018). The prognostic assessment of breast cancer is clearly shown by Graded Prognostic Assessment (GPA) scores, and the prognosis after radiation is clarified by subtype (7). Even in author’s cases, there is a difference in prognosis between 14months for the ER cases, 22 months for the HER2 cases and 7 months for the TN cases (unpublished data).
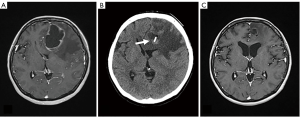
In the treatment selection of BPM, it will be considered based on the above. Unfortunately, there is no next-line chemotherapy after BPM of breast cancer. There are a few reports that it can significantly control BPM with targeted therapy such as Lapatinib (8). From the viewpoint of brain tumor, Bevacizumab can be realized to be useful for palliating remarkable brain edema (Figure 2), but the effect to suppress proliferation is limited. Treatment of BPM of breast cancer eventually becomes a choice of surgical treatment or radiation therapy that is a local therapy (9,10).
Surgical treatment of brain metastasis consists of two procedures: surgical removal under general anesthesia, Ommaya reservoir placement (ORP) under local anesthesia to aspirate cystic fluid. Since the former is carried out under general anesthesia, the renal function and respiratory function must be good, in the case of continuing chemotherapy of breast cancer or in the case of metastatic cancer state such as lung metastasis, there will be few patients who will be indicated for this surgery. The advantage of removal operation is that it can improve mass effect and brain edema quickly, that it can control IICP in the tumor with 3 centimeters or larger in diameter and that control of epilepsy improves by removing the tumor near the motor cortex. The operation of ORP is performed using CT-guided or stereotactic apparatus. It can be done with convenient technique and under local anesthesia. The aim of ORP is to reduce the cystic fluid volume for control of IICP and to reduce cyst volume for performing stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) (Figure 3) (11). The question of radiation therapy for BCBM is whether the whole brain radiotherapy (WBRT), SRT or stereotactic radiation surgery should be used. From the viewpoint of the difference of prognosis in the subtype, it is better to select SRS or SRT because early selection of WBRT in HER 2 cases may later associate with leukoencephalopathy (12,13). On the other hand, in the case of the TN case, it is likely that prognosis can be used to perform WBRT because there are many cases of multiple metastasis and complications of LMC as described later. Radiation necrosis or radiation reaction with brain edema after SRS or SRT is a common occurrence. In spite of steroid therapy, neurological symptom is progression in some these cases. Chemotherapy regimens including Bevacizumab, anti-vascular endothelial growth factor (VEGF) antibody, are scheduled in the case of radiation necrosis. Those treatments can result in dramatic decrease in brain edema. Although it is natural that SRS and SRT tend to be the first choice for treatment of current breast cancer BPM, there is no doubt that WBRT is preferred treatment for patients with poor KPS, LMC or dural metastasis. Then, if SRS or SRT is not available for patients, WBRT should be administered.
LMC
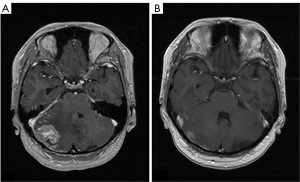
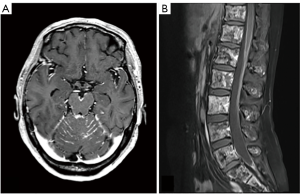
LMC is poor prognostic metastasis and occurs at a complication rate of 5% of brain metastasis (14). LMC is metastasized to hematogenous or direct invasion from skull metastasis. When brain metastasis of the first time shows LMC, it is called primary or de novo LMC. Secondary LMC occurs due to BPM, skull metastasis or dural infiltration. The latter is refractory to treatment and has a short prognosis. Diagnosis is often caught as inflammatory findings around the brainstem and cerebellar folia in contrast-enhanced brain MRI or FLAIR images (Figure 4). It may also be diagnosed as partial lesions of the cerebral sulci or cerebral falx. As a rare situation, internal acoustic canals or pituitary stalk on brain MRI is enhanced (Figure 5). However, even if it is not contrasted at all or even if there is no abnormality in FLAIR images, it may be seen as slightly changed hydrocephalus (Figure 6) (15).
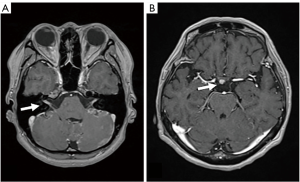
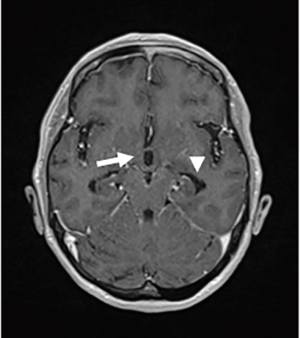
Although confirmed diagnosis is cerebrospinal fluid (CSF) cytology by lumbar puncture, increase of CSF lymphocytosis and increase of intrathecal fluid protein concentration level are also effective information. Even if CSF cytology is negative due to sampling error, class V in cytology may be obtained by repeated examination (16,17).
Symptoms are classically meningeal signs showing headache, vomiting, and stiff neck, but the author divide the disease state into five neurological symptoms: (I) meningeal signs such as headache, nausea and stiff neck; (II) IICP signs such as headache, nausea, blurred vision, loss of consciousness; (III) neurological focal signs such as cerebellar ataxia, higher brain dysfunction and epilepsy; (IV) cranial nerve disorder such as double vision, hearing loss and multiple cranial neuropathy; (V) spinal cord symptoms such as numbness, gait disturbance and incontinence (Table 1). In some reports, it is regarded as a symptom of LMC with emphasis on headache, but in the author’s experience, loss of appetite without chemotherapy is the most important key word.

Full table
There is no effective treatment because breast cancer does not have targeted treatment like EGFR-TKI in lung cancer. Radiotherapy such as WBRT/ whole spinal cord irradiation and intrathecal chemotherapy, spinal drainage and shunt operation for IICP control are performed for the purpose of symptom palliation, but the effect is limited (18). There are many reports using Trastuzumab for intrathecal chemotherapy, but the author has no experience (19,20). However, it is also experienced that treatment intervention may allow survival for more than one year after treatment (21). In the report of LMC by the subtype of breast cancer, it is characterized by a large number of LMC in TN cases and LMC caused by progress of skull invasion directly to the subarachnoid space in the ER positive cases. Considering that the TN case has a short time from breast cancer onset to brain metastasis and the prognosis after brain metastasis is poor, it is necessary to always observe with the occurrence of LMC in mind (22-24).
Intracranial dural metastases
IDM is not frequent, but it is one of cancer metastatic complications often seen in breast cancer and prostate cancer. The spread-mode of IDM is usually direct infiltration from skull metastasis and occasionally hematogenous spread (25). Image findings are useful for diagnosis, and gadolinium-enhanced brain MRI scans is mainly used. In the head CT scans, the contrast of the dura mater in the brain surface has low resolution and cannot be diagnosed. It is easy to understand when classified by dural enhancement, and it is divided into diffuse dural enhanced-case (type DDE) and focal-type. Focal type can be classified into convexity-case (type CO), falx-case (type F) and cerebellar tentorial-case (type CT) depending on tumor localization (Figure 7) (15).
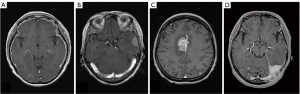
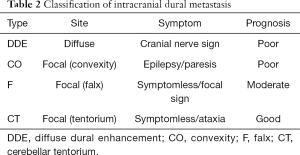
Type DDE is a contrast-enhancing lesion of homogeneous or irregular dural thickening with bilateral or unilateral spread enhancement (26,27). Neurological symptoms are also characteristic for each type of IDM and need to be known in management. In type DDE, it often shows cranial neuropathy such as peripheral facial nerve palsy, and progression will result in Garcin syndrome exhibiting unilateral poly-cranial neuropathy. In the case of type CO, brain edema is locally accompanied, and it exhibits cerebral focal sign such as hemi paresis and epilepsy. In type F and type CT, diagnosis may be delayed because it will become asymptomatic (Table 2). Because dura mater is outside of blood brain barrier, in HER2 positive case, Trastuzumab is also effective, but if IDM occurs during Trastuzumab administration, the treatment option is only radiotherapy. SRT is also effective if it is a focal type, but the effect will be limited considering the spread of dural infiltration. From the viewpoint of radiation susceptibility, WBRT might still play a role to relive neurological symptom.
Full table
The treatment of IDM, however, is not standardized now, further studies are needed to clarify IDM in the patient with breast cancer.
Conclusions
If the control of neurologic symptoms is poor for BCBM, it means an end of the cancer therapy. In the present conditions without effective treatment, the therapeutic purpose of brain metastases is to improve or palliate the patient’s neurologic symptoms. Brain metastasis from breast cancer is characterized by subtype, and the prognosis changes by it, too.
Metastatic central nervous system complication of breast cancer has three types; brain parenchymal metastases, leptomeningeal carcinomatoses and intracranial dural metastases. It is important to distinguish them to consider their management.
Acknowledgements
We appreciate Nobuaki Sato MD, Director of Niigata Cancer Center Hospital, for patient referral.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gaspar L, Scott C, Rotman M. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Berghoff A, Bago-Horvath Z, De Vries C, et al. Brain metastases free survival differs between breast cancer subtypes. Br J Cancer 2012;106:440-6. [Crossref] [PubMed]
- Kyeong S, Cha YJ, Ahn SG, et al. Subtypes of breast cancer show different spatial distributions of brain metastases. PLoS One 2017;12. [Crossref] [PubMed]
- Tsukada Y, Fouad A, Pickren JW. Central nervous system metastases from breast carcinoma: Autopsy study. Cancer 1983;52:2349-54. [Crossref] [PubMed]
- Evans AJ, James JJ, Cornford EJ, et al. Brain metastases from breast cancer: Identification of high-risk group. Clin Oncol (R Coll Radiol) 2004;16:345-9. [Crossref] [PubMed]
- Lin NU, Carey NU, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2008;26:1993-9. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 2009;15:1452-9. [Crossref] [PubMed]
- Gil-Gil MJ, Martinez-Garcia M, Sierra A, et al. Breast cancer brain metastases: a review of the literature and a current multidisciplinary management guideline. Clin Transl Oncol 2014;16:436-46. [Crossref] [PubMed]
- Yaeger KA, Nair MN. Surgery for brain metastases. Surg Neurol Int 2013;4:S203-8. [Crossref] [PubMed]
- Yamanaka Y, Shuto T, Kato Y, et al. J Neurosurg (Suppl) 2006;105:79-81.
- Quattrocchi CC, Errante Y, Gaudino C, et al. Spatial brain distribution of intra-axial metastatic lesions in breast and lung cancer patients. J Neurooncol 2012;110:79-87. [Crossref] [PubMed]
- Hengel K, Sidhu G, Choi J, et al. Attributes of brain metastases from breast and lung cancer. Int J Clin Oncol 2013;18:396-401. [Crossref] [PubMed]
- Scott BJ, Oberheim-Bush NA, Kesari S. Leptomeningeal metastasis in breast cancer-a systemic review. Oncotarget 2016;7:3740-7. [Crossref] [PubMed]
- Mahendru G, Chong V. Meninges in cancer imaging. Cancer Imaging 2009;9 Spec No A:S14-S21.
- Clarke JL, Perez HR, Jacks LM, et al. Leptomeningeal metastases in MRI era. Neurology 2010;74:1449-54. [Crossref] [PubMed]
- Chamberlain MC, Kormanik PA, Glantz MJ. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro Oncol 2001;3:42-5. [Crossref] [PubMed]
- Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumors. Ann Oncol 2017;28:iv84-iv99. [Crossref] [PubMed]
- Scott BJ, Kesari S. Review articles: Leptomeningeal metastases in breast cancer. Am J Cancer Res 2013;3:117-26. [PubMed]
- Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal Trastuzumab. Lancet Oncol 2006;7:778-80. [Crossref] [PubMed]
- Vincent A, Lesser G, Brown D, et al. Prolonged Regression of Metastatic Leptomeningeal Breast Cancer That Has Failed Conventional Therapy: A Case Report and Review of the Literature. J Breast Cancer 2013;16:122-6. [Crossref] [PubMed]
- Mittica G, Senneta R, Richard L, et al. Meningeal carcinomatosis under diagnosis and overestimation: incidence in a large consecutive and unselected population of breast cancer patients. BMC Cancer 2015;15:1021-8. [Crossref] [PubMed]
- Morikawa A, Jordan L, Rozner R, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer 2017;17:23-8. [Crossref] [PubMed]
- Sacco K, Muhammad A, Saleem W, et al. Leptomeningeal carcinomatosis as primary presentation of relapse in breast cancer Oncol Lett 2016;12:779-82. (Review). [Crossref] [PubMed]
- Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer 2009;115:1947-53. [Crossref] [PubMed]
- Grisold W, Grisold A. Cancer around the brain. Neuro-Oncology Practice 2014;1:13-21. [Crossref] [PubMed]
- River Y, Schwartz A, Gomori JM, et al. Clinical significance of diffuse dural enhancement detected by magnetic resonance imaging. J Neurosurg 1996;85:777-83. [Crossref] [PubMed]


