Future of therapy for rectal cancer
Chemoradiation is the standard treatment for locally advanced, clinically resectable (T3 and/or N+) rectal cancer (1). When 5-FU is used concurrently with radiation, continuous infusion (CI) is the conventional regimen (2,3). The NSABP R-04 trial compared preoperative chemoradiation with CI 5-FU vs. capecitabine (with or without oxaliplatin). Compared with CI 5-FU, capecitabine had similar rates of pCR (22% vs. 19%), sphincter-sparing surgery (63% vs. 61%), and grade 3+ diarrhea (11%) (4). Hofheinz et al. randomized 401 patients with CI 5-FU-based chemoradiation vs. capecitabine-based chemoradiation. Patients who received capecitabine had equivalent pCR rates (6% vs. 7%) and their 5-year survival was non-inferior (76% vs. 66%, P=0.0004) compared with CI 5-FU (5). Therefore, CI 5-FU and capecitabine-based chemoradiation regimens are equivalent. The addition of oxaliplatin to preoperative radiation is not standard of care and the results of the randomized trials are discussed below.
Advances in clinical staging and the selection of patients for preoperative therapy
Transrectal ultrasound and high resolution MRI are the most common methods to determine T stage. Historically, ultrasound was used most commonly in North America and most European investigators have preferred high resolution MRI. The advantage of MRI is its ability to identify patients likely to have close or positive radial (circumferential) margins if they underwent initial surgery and therefore, would be better treated with preoperative therapy (6). It is gaining wider acceptance in North America.
The overall accuracy in predicting T stage is approximately 50-90% with ultrasound (7) or high resolution MRI (8) and 50-70% with CT or conventional MRI (9). Although FDG-PET may be more accurate compared with CT for identification of metastatic disease (10), its use to restage patients following preoperative chemoradiation remains controversial (11-14).
Overstaging is common, especially when there is a fibrotic thickening of the rectal wall following preoperative chemoradiation. A reasonably high level of accuracy has been observed by phased array MRI for differentiating ypT0-2 vs. ypT3 disease (15). Both diffusion-weighted MRI and FDG-PET have been used to monitor therapy response and to predict outcome to preoperative therapy. With FDG-PET there is a decrease in SUV on post-radiation in responders compared to non-responders, but the clinical value of this information remains unclear (16).
Identification of positive lymph nodes is more difficult. Overall, the accuracy in detecting positive pelvic lymph nodes with the above techniques is approximately 50-75%. The accuracy of MRI is similar to CT, however, it is improved with the use of external and/or endorectal coils. Both CT and MRI can identify lymph nodes measuring >1 cm, although enlarged lymph nodes are not pathognomonic of tumor involvement. The accuracy of ultrasound for the detection of involved perirectal lymph nodes may be augmented when combined with fine needle aspiration (17). Despite these advances, the ability to accurately predict the pathologic stage following preoperative chemoradiation with MRI (18,19), ultrasound (20), FDG-PET (11-13,21) or physical exam (22) remains suboptimal.
Advances in radiation fractionation: 5 Gy ×5 vs. chemoradiation
Adjuvant preoperative therapy for rectal cancer is delivered by two fractionation schedules: short course radiation and long course chemoradiation. Patients selected for treatment with short course radiation included those with cT1-3 disease, whereas those selected for chemoradiation include T3 and/or N+ disease. Therefore, retrospective comparisons of trials are not feasible. There are two randomized trials which have included patients with cT3 and/or N+ disease also delivered sequential or postoperative chemotherapy, thereby allowing a more relevant comparison with chemoradiation. New trials of short course radiation have included patients with stages cT3 and/or N+ also delivered sequential or chemotherapy.
Short course radiation: standard approaches
There are 12 modern randomized trials of preoperative short course radiation (23). The only trial which mandated total mesorectal excision (TME) was the Dutch CKVO 95-04 trial. Patients with cT1-3 disease were randomized to TME alone or 25 Gy in 5 fractions followed by TME (24). With a 12-year median follow-up, 5-year local failure was higher with TME (11%); however, it was significantly decreased to 5% with preoperative radiation (25). The acute toxicity in the Dutch CKVO 95-04 trial was substantial, including 10% neurotoxicity, 29% perineal wound complications, and 12% postoperative leaks (26). In the patients who developed postoperative leaks, 80% required surgery resulting in 11% mortality. In contrast to the earlier randomized trials of short course radiation, multiple field radiation techniques were used. Whether the increases in morbidity and mortality were due to the learning curve associated with a new surgical technique, the 1 week interval between the completion of radiation and surgery, or both is not known.
Short course radiation is used in the SCRIPTS (Simply Capecitabine in Rectal cancer after Irradiation Plus TME surgery) trial from the Dutch Colorectal Group (CKTO 2003-16). The trial opened in 2007. Patients with clinical stage II (T3-T4, N0) or III (any T, N+) rectal adenocarcinoma (below the level of S1/S2 or inf. margin within 15 cm of the anal verge) received preoperative 5 Gy ×5 followed by TME. Patients are then randomized postoperatively to either capecitabine or observation. This trial tests the role of adjuvant chemotherapy after short course radiation.
Randomized trials
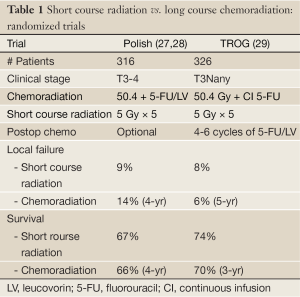
There are 2 randomized trials of short course radiation vs. chemoradiation. The Polish trial from Bujko et al. and the Intergroup TROG, AGITG, CSSANZ, RACS Trial reported by Ngan et al. (Table 1).
Full Table
Polish trial
Bujko and colleagues randomized 316 patients with cT3 rectal cancer (27,28). All tumors were above the anorectal ring, TME was performed for distal tumors only. Postoperative chemotherapy was at the discretion of the investigator. There was no radiation quality control review. Compared with short course radiation patients who received chemoradiation had a higher pCR rate (16% vs. 1%) and a lower incidence of CRM+ (4% vs. 13%, P=0.017). There were no significant differences in sphincter preservation (58% vs. 61%), crude local recurrence (14% vs. 9%), disease-free survival (56% vs. 58%) and 4-year survival (66% vs. 67%). Although acute toxicity was significantly higher with chemoradiation (18% vs. 3%, P<0.001) there was no difference in postoperative complications.
TROG, AGITG, CSSANZ, RACS intergroup trial
A similar trial from Australia and New Zealand was reported from the Tasman Radiation Oncology Group (TROG) (29). A total of 326 patients with ultrasound or MRI staged cT3Nany adenocarcinoma located in the lower 2/3rds of the rectum were randomized to short course radiation versus long course chemoradiation. All patients received 6 months of postoperative adjuvant chemotherapy. The median potential follow-up time was 5.9 years and the primary endpoint was 3-year local recurrence.
Compared with short course radiation, patients who received long course chemoradiation had a 3% lower cumulative local recurrence rate at 3 years (4.4% vs. 7.5%), and 2% at 5-year (5.7% vs. 7.5%). Neither was statistically significant. Likewise, there were no significant differences in distant failure, overall survival, or late radiation toxicity. A subset analysis of the 79 patients with distal tumors revealed a cumulative incidence of local recurrence of 12.5% for short course radiation and there were no failures with long course chemoradiation.
Although a well-designed and performed trial, there were two criticisms of the trial. First, the numbers of patients were relatively small. Second, rather than powered to show equivalence it was designed to have an 80% power to detect a difference in the projected 3-year local recurrence rate of 15% for short course radiation compared with 5% for long course chemoradiation. Although the 3% lower incidence of local recurrence with long course chemoradiation vs. short course radiation did not reach statistical significance (P=0.24) the 95% CI for the difference included an 8% difference in favor of long course (i.e. 10% vs. 2%). Overall, the data suggest a small local control advantage for long course chemoradiation, especially for distal tumors. There are two additional considerations. Neither trial was limited to patients with N+ disease and both require longer follow-up.
Late local recurrences can occur in patients with rectal cancer. The incidence of local recurrence for all patients in the preoperative radiation arm of the Dutch CKVO trial increased from 3% at a median follow-up of 3.5 years to 6% at a median follow-up of 6 years (30). In the German CAO/ARO/AIO 94 trial, patients who received preoperative chemoradiation had an increase in local recurrence (7% vs. 5%) and decrease in survival (60% vs. 74%) at 10 vs. 5 years, respectively (31). Therefore, long term follow-up, regardless of which preoperative approach is used, is needed to determine the ultimate local control rates.
Advances in chemoradiation regimens
Four randomized trials examined the role of adding oxaliplatin to 5-FU or capecitabine based preoperative chemoradiation (4,32-35). Three reported higher acute toxicity and no significant benefit in the pCR rate (4,32-34), The German trial reported the opposite results (35). The ACCORD 12 trial revealed no improvement with the addition of oxaliplatin in 3-year local control (4% vs. 5%) or survival (88% vs. 85%) (34).
Targeted biological agents are being added to preoperative chemoradiation regimens. In the adjuvant setting, preliminary results from the EXPERT-C phase II trial (50.4 Gy/CAPOX/Cetuximab) suggest a survival benefit in patients whose tumors were KRAS wild type vs. mutant (36). Early trials using preoperative chemoradiation with CAPOX + bevacizumab revealed pCR rates of 18-24% (37,38). Unfortunately, more recent trials report increased acute toxicity and have been closed early (39,40).
Selective use of radiation
Node negative rectal cancer
In general, the risks of chemoradiation in patients with pT3N0 disease outweigh the potential benefits (41,42). Patients who undergo a TME, have at least 12 nodes examined, and have stage pT3N0 disease likely do not need the radiation component of chemoradiation. The approximately 3-4% benefit in local control with radiation is not be worth the risks, especially in women of reproductive age. However, patients with pT3N0 tumors with adverse pathologic features, resected without a TME, or when fewer than 12 nodes are examined should still receive postoperative chemoradiation.
The risk/benefit ratio in patients seen preoperatively with cT3N0 disease is more complex. Neither preoperative imaging, molecular markers, or clinicopathologic factors can reproducibly identify patients with LN+ disease (43). The development of more accurate methods to identify LN+ disease is essential.
In the German CAO/ARO/AIO-94 trial, 18% of patients clinically staged as cT3N0 and underwent initial surgery without preoperative therapy had pT1-2N0 disease. Therefore, those patients would have been over-treated if they had received preoperative therapy. Although not ideal, preoperative therapy is still preferred to performing surgery first in this subset of patients. Even after preoperative chemoradiation which downstages tumors, Guillem et al. reported that 22% of patients have ypN+ disease at the time of surgery (44). In patients who undergo surgery alone this number is as high as 40% (45). These patients will then require postoperative chemoradiation which, compared with preoperative chemoradiation, has higher local recurrence, acute and chronic toxicity and, if a low anastomosis is performed, inferior functional results.
Furthermore, the incidence of positive nodes is not dependent on the distance from the anal verge (44). In his series, of the 103 patients with tumors from 0-5 cm from the anal verge, 23% were ypN+, whereas of the 85 with tumors 6-12 cm from the anal verge the incidence was 20%. These data suggest that up to 12 cm from the anal verge, the risk of positive nodes, and likely local recurrence, is similar.
Node positive rectal cancer
Given the improvements in systemic chemotherapy there may be an opportunity to use preoperative radiation more selectively. In a prospective trial reported in abstract form, Cercek et al. treated 32 patients with uT2N1 or uT3N0-1 rectal cancer who by preoperative assessment with neoadjuvant FOLFOX + bevacizumab (46). Only patients who did not require an abdominoperineal resection were eligible. Pelvic radiation was reserved for patients who progressed preoperatively or, following surgery had either pT4, pN2, or positive margins. Of the 30 patients who underwent surgery none required radiation, the pCR rate was 27%, and 2 required postoperative radiation. This approach remains investigational and is being prospectively tested in the phase II/III Alliance N1048 trial.
Upper rectal cancer
The limited data examining the impact of the distance from the anal verge on local recurrence are subset analysis not stratified by distance. There are no prospective randomized data. Furthermore, there are additional variables which may have contributed to differences in local recurrence. For example, TME was standard in the Dutch CVKO and German trials and not in the Swedish trial. All 3 trials included patients with tumors >12 cm from the anal verge in the “upper or high” category. Since the peritoneal reflection varies from 12-16 cm some patients with tumors above the peritoneal reflection (colon cancer) were included in the 3 trials. Most investigators now limit preoperative treatment to tumors <12 cm from the anal verge (44). Lastly, distance measurements using a flexible proctoscope are less accurate than a straight proctoscope. Flexible scopes were used in the Dutch CKVO trial. The German trial used a straight scope. In the Swedish trial proctoscopic information was not mentioned and eligibility was limited to tumors “below the promontory as identified by barium enema.” The Polish trial is not included since all tumors were within reach by digital examination (28).
Tumors defined as “high” in both the Dutch CKVO and Swedish trials (defined as >10.1 cm and 11 cm, respectively) had a lower incidence of local recurrence compared with mid and lower tumors. Short course radiation did not significantly decrease local recurrence. By multivariate analysis, tumor location was an independent prognostic variable in the Dutch CKVO trial. It is interesting to note that radiation did significantly decrease local recurrence for mid tumors in both trials whereas for lower tumors it was helpful in the Swedish trial.
In contrast, there was no significant difference in local recurrence between mid and upper tumors in the German trial (47). However, data were not provided. In a subset analysis, patients with tumors above 6 cm had a lower local recurrence rate.
Nash and colleagues reported that in a retrospective analysis of 627 patients with stage I-IV rectal cancer treated with either surgery alone or chemoradiation, the pelvic recurrence rate was lower in tumors 7-12 cm from the anal verge vs. 0-6 cm (3% vs. 7%, P=0.009) (48). However, mucosal, distant, and overall recurrences were not significantly different.
Given the conflicting data combined with the report from Guillem et al. confirming that the incidence of positive nodes is the same from 0-12 cm from the anal verge, treatment decisions based on the current definitions of low vs. mid vs. high should not be used.
Advances in chemoradiation plus conservative surgery
Experience with preoperative chemoradiation followed by local excision is limited. Borschitz et al. reported local recurrence rates by pathologic stage: ypT1: 2%; ypT2: 6-20% (49). The incidence was 43% in ypT3 tumors which did not respond. Kundel et al. examined 320 patients with T2-4N0-1 rectal cancer and reported a subset of 14 patients who underwent a local excision for ypT0 disease (50). With a median follow-up of 48 months none developed local recurrence or distant failure. In a compilation of 100 patients reported in 11 series, 7% had local recurrence and 8% had distant failure.
This approach has been examined prospectively in the ACOSOG Z6031 trial (51). Patients with uT2N0 disease received preoperative CAPOX and radiation therapy. Those with stage ypT0-2 and negative margins following a local excision had observation only. Patients with stage ypT3 and/or positive margins underwent radical surgery. A total of 77 patients were enrolled and the pCR rate was 43%. Local recurrence and survival results are pending. A similar trial (GRECCAR 2) will accrue 300 patients with cT2-3 disease.
Advances in the non-operative approach (watch and wait)
Although the conventional adjuvant treatment for rectal cancer is preoperative chemoradiation there are clinical settings where surgery has not been performed. These include patients early stage tumors, those with medically inoperable disease, and patients who have refused surgery following a favorable response to preoperative chemoradiation. In these settings, radiation has been delivered by a variety of techniques including endocavitary (contact) treatment, brachytherapy, and pelvic external beam.
Retrospective series
Treatment of rectal cancer without surgery is not a new concept. Although patients can be cured, the results are inferior to surgery. A number of modern retrospective series report the use of radiation alone or chemoradiation, most commonly for patients who are medically inoperable or refuse surgery.
In general, patients received pelvic radiation followed by a boost with either external beam and/or brachytherapy. Brierley et al. from the Princess Margaret Hospital reported the results of pelvic radiation alone (40-60 Gy) in patients who refused surgery or had unresectable or medically inoperable disease (52). The overall 5-year survival was 27% and by the mobility of the primary tumor was: mobile, 47%; partially fixed, 27%; and fixed, 4%.
Gerard and associates reported the combination of pelvic radiation, endocavitary, and brachytherapy in 63 patients with uT2-3 tumors (53). For patients with uT3 disease the 5-year local failure and survival rates were 20% and 35%, respectively.
A total of 48 patients with cT3 disease who received radiation or chemoradiation alone due to medical inoperability or patient refusal was reported by Lim et al. (54). The clinical CR rate was 56% and, with a median follow-up of 49 months, 37% had progression of disease.
Prospective series
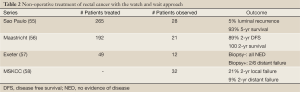
There are four series which advocate the watch and wait approach following preoperative chemoradiation (Table 2). The first was initially reported by Habr-Gama and colleagues in 2004 (59). A total of 265 patients were treated with preoperative 50.4 Gy plus 5-FU and leucovorin. Of those, 27% achieved a clinical CR and were selected for observation only. Close follow-up was required including frequent exams, biopsy, and abdominal/pelvic CT scans every 6 months.
Full Table
With a mean follow-up of 57 months there was a 3% luminal recurrence rate, 4% distant metastasis rate, and 100% 5-year survival. In an update of 361 patients, the local recurrence rate was 5% and 5-year overall survival was 93% in the 28% who achieved a clinical CR (55). It should be emphasized that patients with cT1-3 disease were included and those who developed a local recurrence in the first year were excluded from analysis.
Mass et al. reported their experience of 192 patients with locally advanced rectal cancer (60). Patients were staged with diffusion weighted MRI and had either cT4, cT3 with threatened margins, or node positive disease. They received 50.4 Gy plus capecitabine and 6-8 weeks later underwent MRI restaging. A strict definition of clinical CR was used which included meeting all of the following criteria: (I) substantial downsizing with no residual tumor or residual fibrosis only, (II) no lymph nodes, and (III) endoscopy revealing no tumor or a small residual erythematous ulcer or scar, (IV) negative biopsy, and (V) no palpable tumor.
A subset of 21 patients had a clinical CR and, based on patient preference, were included in a wait-and-see policy. Of the 21, 10 had distal tumors which would have required an abdominoperineal resection. The mean follow-up was 25 months. One patient developed an endoluminal local recurrence without nodal recurrence at 22 months and was salvaged with TEM surgery. The cumulative probability of 2-year disease free survival was 89% and overall survival was 100%.
Dalton and colleagues from Exeter treated 49 patients with 45 Gy plus capecitabine (57). Twelve achieved a cCR by MRI and underwent biopsy 6-8 weeks later. They were then followed closely by PET, CT, MRI, and endoscopy. The 6 who were biopsy negative were all without evidence of disease with a median follow-up of 26 months and 2 of the 6 who were biopsy positive developed distant failure.
The series from Smith et al. reported 32 patients with uT2-4 and/or N+ disease who received 50.4 Gy (45-56 Gy) + 5-FU or Capecitabine (58). A clinical CR was defined as a negative endoscopy at 4-10 weeks. Biopsy and imaging were optional. The local failure rate was 19% crude and 21% 2-yr actuarial. The median time to local failure was 11 months and all were salvaged and without evidence of disease at 17 months. Of note, 5 of the 6 local failures were endoluminal. The incidence of distant failure was 9%.
Their current recommendation for follow-up following chemoradiation is a 2-stage process. At 6-7 weeks patients undergo and exam and endoscopy. If they have a cCR they are followed. If < cCR then it is repeated at 10-12 wks. Patients who have a cCR at 14 months will likely remain in cCR.
Most series suggest an improved outcome in patients who achieve a pathologic CR following preoperative chemoradiation. Patients who are downstaged to ypT0 following chemoradiation have a 5% incidence of positive nodes and a corresponding low nodal recurrence rate (50). Mass and colleagues reported a pooled analysis of 27 series reporting patients who underwent preoperative chemoradiation and achieved a pathologic CR (61). There was a significant increase in 5-year disease free survival compared to those who did not achieve a pCR (83% vs. 63%, P=0.0001).
Selecting patients for a non-operative approach based on response is reasonable. The challenge is identifying a surrogate method to surgery for the identification of a pathologic CR. Current methods include endoscopy and physical exam, ultrasound, CT, MRI, and PET either alone or in combination. With the exception of the rigorous approach used by Mass et al. in their wait- and-see series (60), most have not been consistently successful. Glynne-Jones and associates reviewed 218 phase II and 28 phase III trials of preoperative radiation or chemoradiation and confirmed that clinical and/or radiologic response does not sufficiently correlate with pathologic response and do not recommend a “wait and see’ approach to surgery following preoperative therapy (62). However, further refinements in imaging may improve the selection process.
In summary, surgery remains a standard component of the treatment of rectal cancer. The wait-and-see approach is encouraging but remains investigational. More accurate methods for post-chemoradiation assessment are needed.
Advances in chemoradiation regimens
Both cytotoxic and targeted chemotherapeutic agents have been incorporated into preoperative chemoradiation regimens. Most of the phase I/II regimens report higher pCR rates compared with historical rates seen with 5-FU alone. The RTOG 0247 randomized phase II trial of CAPEIRI vs. CAPOX based chemoradiation revealed a higher pCR with the irinotecan based regimen (21% vs. 10%) with no difference in grade 3+ acute toxicity (63).
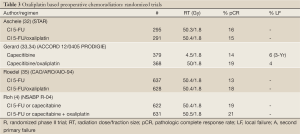
Four randomized trials have examined the impact of addition of oxaliplatin to 5-FU or capecitabine based chemoradiation on response rates and acute toxicity in patients with cT3-4 and/N+ rectal cancer (Table 3). The STAR-01 trial randomized 747 patients to preoperative chemoradiation with 50.4 Gy + CI 5-FU +/- oxaliplatin (60 mg/m2 weekly) (32). There was a significant increase in grade 3+ toxicity with oxaliplatin (24% vs. 8%, P<0.001) with no improvement in the pCR rate (15% vs. 16%). In the ACCORD 12/0405 PRODIGIE trial, 598 patients were randomized to preoperative chemoradiation with 50 Gy plus capecitabine + oxaliplatin (CAPOX) vs. 45 Gy plus capecitabine (33). There was a similar significant increase in grade 3+ toxicity with oxaliplatin (25% vs. 11%, P<0.001) with no improvement in the pCR rate (19% vs. 14%). Oxaliplatin did not improve 3-year local recurrence (6% vs. 4%) or survival (88% vs. 83%) (34).
Full Table
The NSABP R-04 trial was a 4 arm trial (2×2 comparison) of CI 5-FU vs. capecitabine based preoperative chemoradiation (50.4 Gy) with or without oxaliplatin (4). A total of 1,606 patients with cT3 and or N+ disease were randomized. The addition of oxaliplatin (to either 5-FU or capecitabine) was associated with a significantly higher incidence of grade 3+ diarrhea (15% vs. 7%, P=0.0001) with no improvement in the incidence of pCR (21% vs. 19%) or sphincter-sparing surgery (60% vs. 64%).
The German CAO/ARO/AIO-04 randomized 1,265 patients with cT3-4 and or N+ disease to the preoperative arm of CAO/ARO/AIO-94 (50.4 Gy + 5-FU) versus 50.4 Gy/5-FU+ oxaliplatin (50 mg/m2 weekly) (35). In contrast with the STAR-01, ACCORD, and NSABP R-04 trials, patient who received oxaliplatin based CMT had a significant improvement in pCR (17% vs. 13%, P=0.045) with no corresponding increase in acute grade 3+ toxicity (23% vs. 22%). The results of the 5th trial (PETACC-6) are pending.
Since 3 of 4 randomized trials reveal an increase in acute toxicity with no benefit in the pCR rate the current standard is not to include oxaliplatin to preoperative chemoradiation regimens. However, local control and survival data are not available and this recommendation may need to be modified once these data are reported.
The role of targeted biological agents such as bevacizumab and cetuximab are being tested. Phase I/II trials using preoperative chemoradiation with CAPOX + bevacizumab reveal pCR rates of 18-24% (37,38). Two recent trials combining bevacizumab to preoperative FOLFOX (56) or capecitabine (40) based chemoradiation were stopped early due to excessive toxicity.
Although the report from Heidelberg of CAPEIRI based chemoradiation reported a pCR rate of 25% (64) other trials with 5-FU, capecitabine, or CAPOX have more limited rates of 5-12% (65,66).
Patient selection based on KRAS expression is useful in patients with metastatic disease (38). Preliminary results from the phase II EXPERT-C trial (50.4 Gy/CAPOX/Cetuximab) suggest a survival benefit in patients whose tumors were KRAS wild type vs. mutant (36).
Advances in chemotherapy sequencing
The Spanish GCR-3 randomized phase II trial compared neoadjuvant chemotherapy followed by chemoradiation with conventional preoperative chemoradiation followed by surgery and postoperative chemotherapy (67). A total of 108 patients received preoperative 50.4 Gy plus CAPOX and were randomized to receive 4 months of CAPOX either by induction or adjuvant (postoperative). Although the pCR rates were not different (14% vs. 13%) both grade 3+ toxicity was lower (17% vs. 51%, P=0.00004) and the ability to receive all 4 chemotherapy cycles was higher (93% vs. 51%, P=0.0001) with the induction approach.
Advances in predictors of response
Most series suggest that there is improved outcome with increasing pathologic response to preoperative therapy (68-70). A retrospective review of 566 patients who achieved a pCR after receiving a variety of preoperative chemoradiation regimens at multiple European centers was reported by Capirci and associates (69). With a median follow-up of 46 months the local recurrence rate was only 1.6% and the 5-year disease-free and overall survival rates were 85% and 90% respectively. A pooled analysis 3,105 patients from 14 studies confirmed a significant improvement in local recurrence, distant failure, disease free, and overall survival for the 16% of patients who achieved a pCR (ypT0N0M0) compared to those without a pCR (61). Acellular mucin pools are seen in 15% of tumors following chemoradiation and do not have a significant impact on outcome (71).
Although a number of molecular markers are predictive of outcome in colorectal cancer (38,72,73), they have had varying success in identifying patients who may respond to preoperative therapy (74-76). Kuremsky et al. reviewed 1,204 studies examining a total of 36 molecular biomarkers which may have predictive value (77). Restricting the analysis to patients treated with preoperative chemoradiation and to gene products examined by 5 or more studies, only p53, epidermal growth factor receptor (EGFR), thymidylate synthase, Ki-67, p21, and bax/bcl-2 met these criteria. Of these, quantitatively evaluated EGFR or EGFR polymorphisms, thymidylate synthase polymorphisms, and p21 have been identified as promising candidates that should be evaluated in larger prospective trials for their ability to guide preoperative therapy. Since the studies are limited retrospective trials and most do not examined multiple markers, the need for adjuvant therapy should still be based on T and N stage.
Konski and associates performed pre and post-treatment 18-FDG PET scans on 53 patients receiving preoperative chemoradiation (78). By multivariate analysis the percent decrease in SUV trended marginally in predicting pCR (P=0.07).
Advances in radiation techniques and dose
The clinical utility of routine 3-D and IMRT treatment planning techniques are being investigated (79,80). The most important contributions of 3-D treatment planning are the ability to plan and localize the target and normal tissues at all levels of the treatment volume and to obtain dose volume histogram data. An analysis of 3-D treatment planning techniques suggests that the volume of small bowel in the radiation field is decreased with protons as compared with photons (81). IMRT treatment planning techniques can further decrease the volume of small bowel in the field (82). However, the clinical benefit of IMRT compared to 3-D or conventional treatment delivery for rectal cancer remains to be determined (80). Guidelines for the definition and delineation of the clinical target volumes (CTV) are available from a number of investigators (83,84).
The RTOG R-0012 phase II randomized trial compared twice a day preoperative chemoradiation up to 60 Gy (1.2 Gy to 45.6 Gy, with a boost of 9.6-14.4 Gy) with conventional fractionation (1.8 Gy to 45 Gy, with a boost of 5.4-9.0 Gy) plus 5-FU/irinotecan (85). Both regimens resulted in a 28% pCR rate, but were also associated witha >40% rate of grade 3-4 acute toxicity.
Summary
The therapy of rectal cancer continues to evolve. Both diagnostic and therapeutic advances are challenging historical approaches and have opened new directions for the future and are areas of clinical investigation.
Acknowledgements
Disclosure: The author declares no conflicts of interest.
References
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324:709-15. [PubMed]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [PubMed]
- Roh MS, Yothers GA, O’Connell MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol 2011;29:abstr 3503.
- Hofheinz R, Wenz FK, Post S, et al. Capecitabine (Cape) versus 5-fluorouracil (5-FU)–based (neo)adjuvant chemoradiotherapy (CRT) for locally advanced rectal cancer (LARC): Long-term results of a randomized, phase III trial. J Clin Oncol 2011;29:abstr 3504.
- Salerno GV, Daniels IR, Moran BJ, et al. Magnetic resonance imaging prediction of an involved surgical resection margin in low rectal cancer. Dis Colon Rectum 2009;52:632-9. [PubMed]
- Barbaro B, Valentini V, Coco C, et al. Tumor vascularity evaluated by transrectal color Doppler US in predicting therapy outcome for low-lying rectal cancer. Int J Radiat Oncol Biol Phys 2005;63:1304-8. [PubMed]
- Valentini V, DePaoli A, Gambacorta MA, et al. Chemoradiation with infusional 5-FU and ZD1839 (Gefitinib-Iressa) in patients with locally advanced rectal cancer: a phase II trial. Int J Radiat Oncol Biol Phys 2006;66:s168.
- Kim NK, Kim MJ, Park JK, et al. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol 2000;7:732-7. [PubMed]
- Nahas CS, Akhurst T, Yeung H, et al. Positron emission tomography detection of distant metastatic or synchronous disease in patients with locally advanced rectal cancer receiving preoperative chemoradiation. Ann Surg Oncol 2008;15:704-11. [PubMed]
- Buijsen J, van den Bogaard J, Janssen MH, et al. FDG-PET provides the best correlation with the tumor specimen compared to MRI and CT in rectal cancer. Radiother Oncol 2011;98:270-6. [PubMed]
- Everaert H, Hoorens A, Vanhove C, et al. Prediction of response to neoadjuvant radiotherapy in patients with locally advanced rectal cancer by means of sequential 18FDG-PET. Int J Radiat Oncol Biol Phys 2011;80:91-6. [PubMed]
- Mak D, Joon DL, Chao M, et al. The use of PET in assessing tumor response after neoadjuvant chemoradiation for rectal cancer. Radiother Oncol 2010;97:205-11. [PubMed]
- Shanmugan S, Arrangoiz R, Nitzkorski JR, et al. Predicting pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer using 18FDG-PET/CT. Ann Surg Oncol 2012;19:2178-85. [PubMed]
- Barbaro B, Fiorucci C, Tebala C, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 2009;250:730-9. [PubMed]
- Calvo FA, Domper M, Matute R, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 2004;58:528-35. [PubMed]
- Shami VM, Parmar KS, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum 2004;47:59-65. [PubMed]
- Kim YH, Kim DY, Kim TH, et al. Usefulness of magnetic resonance volumetric evaluation in predicting response to preoperative concurrent chemoradiotherapy in patients with resectable rectal cancer. Int J Radiat Oncol Biol Phys 2005;62:761-8. [PubMed]
- Kuo LJ, Chern MC, Tsou MH, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum 2005;48:23-8. [PubMed]
- Barbaro B, Schulsinger A, Valentini V, et al. The accuracy of transrectal ultrasound in predicting the pathological stage of low-lying rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 1999;43:1043-7. [PubMed]
- Calvo FA, Cabezón L, González C, et al. (18)F-FDG PET bio-metabolic monitoring of neoadjuvant therapy effects in rectal cancer: focus on nodal disease characteristics. Radiother Oncol 2010;97:212-6. [PubMed]
- Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol 2005;23:3475-9. [PubMed]
- Skibber JM, Hoff PM, Minsky BD. Cancer of the Rectum. In: DeVita VT, Hellman S, Rosenberg SA. eds. Cancer: Principles and Practice of Oncology (ed 6). Philadelphia: Lippincott, Williams and Wilkens, 2001:1271-318.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [PubMed]
- van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-82. [PubMed]
- Marijnen CA, Kapiteijn E, van de Velde CJ, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol 2002;20:817-25. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72:15-24. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [PubMed]
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [PubMed]
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [PubMed]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638-44. [PubMed]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 2012;30:4558-65. [PubMed]
- Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012;13:679-87. [PubMed]
- Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol 2012;30:1620-7. [PubMed]
- Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 2009;27:3020-6. [PubMed]
- van Cutsem E, Lang I, D’haens G, et al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience. J Clin Oncol 2008;26: abstr 2.
- Dipetrillo T, Pricolo V, Lagares-Garcia J, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:124-9. [PubMed]
- Resch G, De Vries A, Öfner D, et al. Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer--a two stage phase II clinical trial. Radiother Oncol 2012;102:10-3. [PubMed]
- Nissan A, Stojadinovic A, Shia J, et al. Predictors of recurrence in patients with T2 and early T3, N0 adenocarcinoma of the rectum treated by surgery alone. J Clin Oncol 2006;24:4078-84. [PubMed]
- Greene FL, Stewart AK, Norton HJ. New tumor-node-metastasis staging strategy for node-positive (stage III) rectal cancer: an analysis. J Clin Oncol 2004;22:1778-84. [PubMed]
- Kim JH, Beets GL, Kim MJ, et al. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004;52:78-83. [PubMed]
- Guillem JG, Díaz-González JA, Minsky BD, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol 2008;26:368-73. [PubMed]
- Mendenhall WM, Bland KI, Rout WR, et al. Clinically resectable adenocarcinoma of the rectum treated with preoperative irradiation and surgery. Dis Colon Rectum 1988;31:287-90. [PubMed]
- Cercek A, Weiser MR, Goodman KA, et al. Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation. J Clin Oncol 2010;28:abstr 3649.
- Sauer R, Roedel C. The author’s reply. New Engl J Med 2005;352:510-1.
- Nash GM, Weiss A, Dasgupta R, et al. Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis Colon Rectum 2010;53:1365-73. [PubMed]
- Borschitz T, Wachtlin D, Möhler M, et al. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol 2008;15:712-20. [PubMed]
- Kundel Y, Brenner R, Purim O, et al. Is local excision after complete pathological response to neoadjuvant chemoradiation for rectal cancer an acceptable treatment option? Dis Colon Rectum 2010;53:1624-31. [PubMed]
- Garcia-Aguilar J, Shi Q, Thomas CR, et al. Pathologic complete response (pCR) to neoadjuvant chemoradiation (CRT) of uT2uN0 rectal cancer (RC) treated by local excision (LE): Results of the ACOSOG Z6041 trial. J Clin Oncol 2012;28:abstr 3510.
- Brierley JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys 1995;31:255-9. [PubMed]
- Gerard JP, Chapet O, Ramaioli A, et al. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys 2002;54:142-9. [PubMed]
- Lim L, Chao M, Shapiro J, et al. Long-term outcomes of patients with localized rectal cancer treated with chemoradiation or radiotherapy alone because of medical inoperability or patient refusal. Dis Colon Rectum 2007;50:2032-9. [PubMed]
- Habr-Gama A, Perez RO, Proscurshim I, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 2006;10:1319-28. [PubMed]
- Campbell PT, Newton CC, Dehal AN, et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 2012;30:42-52. [PubMed]
- Dalton RS, Velineni R, Osborne ME, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis 2012;14:567-71. [PubMed]
- Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 2012;256:965-72. [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-7. [PubMed]
- Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633-40. [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [PubMed]
- Glynne-Jones R, Wallace M, Livingstone JI, et al. Complete clinical response after preoperative chemoradiation in rectal cancer: is a “wait and see” policy justified? Dis Colon Rectum 2008;51:10-9. [PubMed]
- Wong SJ, Winter K, Meropol NJ, et al. Radiation Therapy Oncology Group 0247: a randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:1367-75. [PubMed]
- Das P, Lin EH, Bhatia S, et al. Preoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer: a matched-pair analysis. Int J Radiat Oncol Biol Phys 2006;66:1378-83. [PubMed]
- Chung KY, Minsky B, Schrag D, et al. Phase I trial of preoperative cetuximab with concurrent continuous infusion 5-fluorouracil and pelvic radiation in patients with local-regionally advanced rectal cancer. J Clin Oncol 2006;24:abstr 3560.
- Rödel C, Arnold D, Hipp M, et al. Phase I-II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys 2008;70:1081-6. [PubMed]
- Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 2010;28:859-65. [PubMed]
- Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 2005;241:829-36; discussion 836-8. [PubMed]
- Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008;72:99-107. [PubMed]
- Yeo SG, Kim DY, Kim TH, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg 2010;252:998-1004. [PubMed]
- Shia J, McManus M, Guillem JG, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol 2011;35:127-34. [PubMed]
- Haddock MG, Miller RC, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 2011;79:143-50. [PubMed]
- Wilson PM, Labonte MJ, Lenz HJ. Molecular markers in the treatment of metastatic colorectal cancer. Cancer J 2010;16:262-72. [PubMed]
- Bertolini F, Bengala C, Losi L, et al. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1455-61. [PubMed]
- Unsal Kilic D, Uner A, Akyurek N, et al. Matrix metalloproteinase-9 expression correlated with tumor response in patients with locally advanced rectal cancer undergoing preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;67:196-203. [PubMed]
- Johnston PG. Prognostic markers of local relapse in rectal cancer: are we any further forward? J Clin Oncol 2006;24:4049-50. [PubMed]
- Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys 2009;74:673-88. [PubMed]
- Konski A, Li T, Sigurdson E, et al. Use of molecular imaging to predict clinical outcome in patients with rectal cancer after preoperative chemotherapy and radiation. Int J Radiat Oncol Biol Phys 2009;74:55-9. [PubMed]
- Meyer J, Czito B, Yin FF, et al. Advanced radiation therapy technologies in the treatment of rectal and anal cancer: intensity-modulated photon therapy and proton therapy. Clin Colorectal Cancer 2007;6:348-56. [PubMed]
- Aristu JJ, Arbea L, Rodriguez J, et al. Phase I-II trial of concurrent capecitabine and oxaliplatin with preoperative intensity-modulated radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2008;71:748-55. [PubMed]
- Tatsuzaki H, Urie MM, Willett CG. 3-D comparative study of proton vs. x-ray radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 1992;22:369-74. [PubMed]
- Callister MD, Ezzell GA, Gunderson LL. IMRT reduces the dose to small bowel and other pelvic organs in the preoperative treatment of rectal cancer. Int J Radiat Oncol Biol Phys 2006;66: abstr s290.
- Roels S, Duthoy W, Haustermans K, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys 2006;65:1129-42. [PubMed]
- Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009;74:824-30. [PubMed]
- Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol 2006;24:650-5. [PubMed]


