Stereotactic body radiation therapy for prostate cancer—a review
Introduction
Prostate cancer is the most commonly arising cancer in men, with an estimated incidence of 180,890 in 2016 within the United States, and, with an estimated 26,120 deaths, third to only lung cancer and colon cancer in absolute annual mortality (1). Broadly, treatment options for prostate cancer include prostatectomy, radiation therapy, either using external beam radiation therapy (EBRT) or brachytherapy, and active surveillance, dependent upon both the underlying comorbid conditions of the patient and the pathologic characteristics of the tumor (2). While there have been no randomized trials comparing the outcomes for patients with low-risk prostate cancer treated with prostatectomy, EBRT, and brachytherapy, retrospective data suggests medical equipoise between the three treatment modalities (3,4). Consequently, the decision regarding the management for each patient is made following an in-depth conversation between urologists, radiation oncologists, and patients regarding the goals of care and the side effect profile most acceptable to the patient.
Rationale for hypofractionation
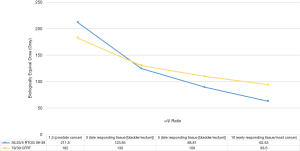
While conventionally fractionated (CF) EBRT offers the advantages of being completely non-invasive with a minimal side effect profile, a full course of treatment of 39–44 fractions can take up to 9 weeks, causing significant logistical challenges for patients (5). Decreasing the number of fractions, or hypofractionating, delivered during a course of EBRT would increase convenience for patients by decreasing the time spent during treatment. In addition to the logistical benefits of hypofractionation, there is radiobiologic data to suggest that hypofractionated radiation treatment could improve the relationship between prostate cancer cells killed and normal organ toxicity, or the therapeutic ratio (6). CF-EBRT breaks up a total course of radiation into multiple daily radiation treatments [typically 1.8–2 Gray (Gy) per fraction] because of radiobiological benefits to having a long, protracted course of treatment. Specific benefits of fractionating treatment include the repair of sublethal damage to normal tissue, reoxygenation of hypoxic tumor cells, and redistribution of tumor cells to radiosensitive phases of the cell cycle that occurs between fractions (6). The alpha-beta ratio is a parameter that describes the response of organs to radiation. Most tumors and early-responding tissue, such as skin, typically have alpha-beta ratios >10, whereas late-responding tissue such as the bladder and rectum have alpha-beta values between 3–5 (6). The smaller the alpha-beta ratio, the more responsive the organ is to a larger fraction size while conversely, the larger the alpha-beta ratio, the more sensitive the organ is to fractionation. Recent data suggests that the alpha-beta ratio for prostate cancer is 1.5 (7), suggesting that for patients with prostate cancer, hypofractionation would have a greater increase of biologically equivalent dose (BED) to the tumor than the normal tissue, improving the therapeutic ratio (8,9). The equation for BED = (nd[1+d/{alpha-beta}]), where n is # fx and d is dose/fx, can be used to illustrate this concept, and is graphically demonstrated in Figure 1. Advances in imaging techniques and treatment delivery have allowed radiation oncologists to deliver ultrahypofractionated, high-dose treatments, using a method called SBRT, which may provide additional pathways to cell kill not offered by CF-EBRT, including indirect tumor death by mediating vascular damage via ceramide-mediated apoptosis of endothelial cells and increased cellular expression of inflammatory mediators, immunomodulatory cytokines, and death receptors (10).
Hypofractionated EBRT was first used for management of prostate cancer in the United Kingdom in the 1960s. From 1964–1984, 233 patients were treated to 36 Gy in 6 fractions using a 2-dimenstional technique with acceptable clinical outcomes and toxicity (11). More moderate hypofractionation, defined as a daily fractional dose of >2 Gy, was explored in the 1990s by the Cleveland Clinic and Christie Hospital, and later by the multi-institution Radiation Therapy Oncology Group (RTOG) 04−15 trial, which investigated a regimen of 70 Gy in 28 fractions, and described biochemical control rates comparable to those achieved with CF-EBRT (12-14).
Overview of SBRT for prostate cancer
SBRT is defined by the American Society of Radiation Oncology (ASTRO) as “an external beam radiation therapy (EBRT) method used to precisely deliver a high dose of radiation to an extracranial target within the body, using either a single dose or a small number of fractions. Specialized treatment planning results in high target dose and steep dose gradients beyond the target” (15). SBRT can be delivered using non-coplanar, non-opposing arcs with either a conventional linear accelerator or with a robotic-based radiosurgery system, as used in the Cyberknife® (Accuray; CA, USA) device (6). While most studies involving SBRT for prostate cancer to date have been conducted using a robotic-based radiosurgery system, there are no differences in outcomes for patients treated with either a gantry linear accelerator or a robotic-based radiosurgery system. Dosimetric studies demonstrate that isocentric RapidArc treatment using a gantry linear accelerator can provide superior coverage to the planning target volume (PTV) with better rectum sparing, while delivering the treatment in a shorter period of time than SBRT delivered with a robotic-based radiosurgery system (16).
During treatment with SBRT, a highly conformal dose is delivered to the target while sparing the surrounding normal tissue, with steep dose fall off. In addition to sophisticated treatment planning and treatment delivery systems, image guided radiation therapy (IGRT) is an essential component of ensuring accurate treatment. SBRT procedures require images be taken of the prostate prior to each treatment, which can performed using the gantry linear accelerator using a gantry-mounted cone beam computed tomography (CBCT) scan as on devices made by Varian, Elekta, or Novalis TxTM, CT on rails, or radiofrequency transponders, such as the Calypso® (Varian; CA, USA) real-time tracking device, that are additionally able to provide intrafraction image guidance (17-20). The robotic-based radiosurgery system is able to track implanted fiducial markers using intrafractional orthogonal kilovoltage images taken every 15–30 seconds, and can make adjustments for any intrafraction motion during treatment (21).
While images taken prior to treatment account for interfraction motion of the prostate, radiation oncologists must also account for intrafraction motion, as one study using intraprostatic fiducial markers demonstrated that after 18 minutes, there is a 10% chance of displacement of the prostate of 5 mm or more (22). Consequently, when real-time imaging is not available, some authors advocate repeating an IGRT procedure every 5 minutes (23,24).
Clinical outcomes for prostate SBRT
There have, to date, been no completed phase III randomized trials comparing the outcomes of patients with prostate cancer treated with SBRT to those receiving treatment CF- EBRT. However, there have been several phase I−II trials, prospective single institution reports, and retrospective single institution studies that provide detailed outcomes for patients with localized prostate cancer treated with SBRT. The majority of data for treatment of prostate cancer with SBRT is composed of patients treated to the prostate alone without any treatment to the pelvic lymph nodes. The largest study of patients with prostate cancer treated with SBRT comes from a pooled analysis of 1,100 patients with localized prostate cancer enrolled in separate prospective phase II trials from 8 institutions between 2003–2011 and treated to 35–40 Gy over 5 fractions (25). After a median follow up of 36 months, the actuarial 5-year freedom from biochemical failure (FFBF) was 95.2%, 84.1%, and 81.2% for patients with low-, intermediate-, and high- risk disease, respectively. The study with the longest follow up time comes from a single-institution retrospective review which reported the outcomes of 477 patients with localized prostate cancer treated between 2006 and 2010 who were treated to 35–36.25 Gy in 5 fractions using a robotic-based radiosurgery system device. After a median follow up time of 72 months, the actuarial 7-year FFBF was 95.6% and 89.6% for low- and intermediate-risk group patients, respectively (26). For a complete list of reports describing outcomes for patients treated with definitive SBRT using photons for localized prostate cancer, please see Table 1. As shown in Table 1, SBRT is highly effective as definitive treatment for low-risk (90–100% FFBF) prostate cancer, while its efficacy for patients with or intermediate-risk (83.9–100% FFBF) or high-risk (33.3–90.8% FFBF) prostate cancer may be sub-optimal.

Full table
A randomized trial has demonstrated that a low-dose-rate brachytherapy boost added to whole pelvis EBRT to 46 Gy in 23 fractions provides superior FFBF when compared to dose-escalated EBRT for intermediate and high risk prostate cancer (45). Following a similar treatment paradigm, some investigators have suggested that SBRT can be used as a minimally-invasive alternative to brachytherapy as a method of delivering conformal radiation therapy and have demonstrated the efficacy of using a SBRT boost along with CF- EBRT for intermediate- and high-risk prostate cancer. The largest of these reports comes from Georgetown University, where 108 patients with prostate cancer (4 with low-, 45 with intermediate-, and 59 with high-risk disease) were treated with SBRT to 19.5 Gy in 3 fractions followed by EBRT to the prostate, proximal seminal vesicles, and areas of extracapsular extension to 45–50.4 Gy in 25–28 fractions (46). The 3-year actuarial FFBF was 100% for intermediate- and 89.8% for high-risk patients. For a complete list of studies describing the outcomes of SBRT boost given in conjunction with EBRT, please see Table 2.
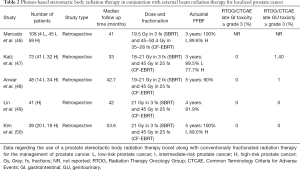
Full table
Androgen deprivation therapy (ADT) with prostate SBRT
The addition of ADT to EBRT has been shown to improve overall survival for patients with intermediate- and high-risk prostate cancer, though these studies were conducted before the era of dose-escalated EBRT (51-58). In a modern trial by Bolla et al. demonstrating the superiority of ADT with EBRT compared to patients treated with EBRT alone, only 197/819 patients received dose-escalated EBRT, and though the study did demonstrate improved clinical outcomes for these patients, the study was not powered to show improvements for the patients receiving dose-escalation (59). Whether or not ADT has a role in the era of dose-escalated EBRT for patients with intermediate risk prostate cancer is currently an active research question, and is being investigated by RTOG 08−15. The indications of ADT for patients undergoing prostate SBRT are similarly unclear. In a multi-institutional pooled data set of patients undergoing SBRT for prostate cancer, 147 patients underwent ADT in conjunction with SBRT (25). There was no difference in 5-year FFBF (92.6% vs. 91.3%, P=0.71) between patients receiving ADT and those not receiving ADT, though there was no uniform criteria for ADT use. The relatively poor results with SBRT alone for patients with high-risk prostate cancer suggest that some additional form of systemic therapy may prove beneficial. However, there are at this time no clear indications for the use of ADT with SBRT for patients with prostate cancer.
Dose/fractionation, expansion, and patient eligibility
There are currently no consensus guidelines regarding the optimal dose, volumetric expansions, or patient-eligibility for prostate SBRT. A majority of the data thus far reporting the outcomes for patients undergoing prostate SBRT have received 35–36.25 Gy in 5 daily fractions, a dose that provides good clinical outcomes for low- risk disease, while its efficacy in intermediate- or high-risk disease is less clear (25-44). A multi-institutional phase I-II trial treating patients to 50 Gy in 5 fractions described patients suffering from a high rate of GI toxicity, with 9.9% suffering from Common Toxicity Criteria for Adverse Events (CTCAE) v 3.0 ≥ grade 3 toxicity (32). Notably, in the cohort receiving 50 Gy in 5 fractions, 6/61 patients required diverting colostomy to heal from the high-grade rectal toxicity, leading the authors to conclude this dose regimen resulted in an unacceptable level of late toxicity. In order to safely deliver the high doses offered by SBRT to the prostate while minimizing normal organ toxicity, the volumetric expansions surrounding the prostate must be minimal. The randomized RTOG 09−38 trial placed a 5 mm PTV expansion around the prostate in all directions except posteriorly, where a 3 mm expansion was applied in order to minimize dose to the anterior rectal wall (23). Some authors using a robotic-based radiosurgery system have placed a 2 mm volumetric PTV expansion around the prostate, while reducing the posterior expansion to 0 mm (25). Prospective phase I–II Trials have included low-, intermediate-, and high-risk prostate cancer patients for treatment with prostate SBRT (25,26,32-34,37,40). The FFBF for patients with high-risk disease have been between 33.3–90.8%, suggesting that SBRT may not be the optimal treatment modality for this group of patients, and that the optimal candidates for SBRT may be solely low- or select intermediate-risk patients, though even some studies report FFBF rates for intermediate-risk patients as low as 83.9% (42). One possible solution to managing higher risk disease may be with dose escalation. A single institution review from the Cleveland Clinic demonstrated that treating intermediate and high risk prostate cancer with a dose of 50 Gy in 5 fractions while limiting a high-dose avoidance zone (HDAZ), which was a volume created by placing a 3 mm expansion on the urethra, rectum, and bladder, to 36.25 Gy in 5 fractions, was effective, with a 95.8% FFBF rate at two years (44). However, this result should be interpreted with caution, as this is a single-institution review with a small patient volume and limited follow up.
PSA kinetics following prostate SBRT
The PSA kinetics following prostate SBRT are distinct and may suggest a greater degree of efficacy when compared to those following treatment with CF-EBRT. One study from UCLA compared patterns of PSA response for 439 patients with low- or intermediate-risk prostate cancer following treatment with SBRT, high-dose-rate (HDR) brachytherapy, or CF-intensity modulated radiation therapy (IMRT) (60). The authors found that significantly more patients treated with SBRT or HDR brachytherapy had PSA nadirs of <0.5 ng/mL than those treated with IMRT (76.2% and 75.9% vs. 44.9%, respectively, P<0.0001) and that overall, SBRT and HDR brachytherapy caused significantly larger PSA decay rates (P<0.001) than IMRT, leading the authors to conclude that this difference in PSA kinetics may present a distinct radiobiological effect, and may be predictive of superior clinical outcomes. Other studies have confirmed that after one year of treatment, when compared to patients receiving treatment with CF-EBRT, the median PSA slope and nadir are lower for patients treated with SBRT (61,62).
Prostate SBRT vs. EBRT and brachytherapy
As stated previously, there have been no completed randomized trials comparing outcomes for patients treated with SBRT or CF-EBRT. RTOG 09−38 was a phase II trial that randomized 240 patients with low-risk prostate cancer to one of two hypofractionated regimens: 36.25 Gy in 5 fractions (SBRT) or 51.6 Gy in 12 fractions (23). Patient-reported outcomes were similar between the two arms, though efficacy endpoints have yet to be reported. A group in Philadelphia conducted a propensity score matched comparison of 263 patients with localized prostate cancer treated with SBRT or IMRT, and found no difference in 5-year FFBF between matched SBRT and IMRT groups (88.7% vs. 95.5%, respectively; P=0.1720) and no difference in toxicity, leading the authors to conclude that given the lower cost and convenience for patients, SBRT may be a suitable alternative treatment for patients with prostate cancer (63). Another group conducted a multi-institutional analysis of 437 patients with intermediate risk prostate cancer treated with either SBRT (n=300) and HDR brachytherapy (n=137) and at 4 years, found no differences in FFBF in either treatment arm (95.3% vs. 98.5%, respectively, P=0.17), and concluded that both SBRT and HDR brachytherapy were appropriate treatments for patients with intermediate risk prostate cancer.
Toxicities
The primary toxicities associated with prostate SBRT are genitourinary (GU), gastrointestinal (GI), and sexual dysfunction. Most studies of toxicities due to prostate SBRT report RTOG/CTCAE ≥ grade 3 toxicity is typically <5%, with the notable exception being a phase I/II trial that treated patients to a dose of 50 Gy in 5 fractions, a dose which the authors conclude caused an unacceptably high level of rectal toxicity (25-44,46-50). A multi-institutional pooled analysis from 864 patients enrolled in phase II clinical trials and prospective protocols of prostate SBRT examined quality of life (QOL) outcomes for patients using the Expanded Prostate Cancer Index Composite (EPIC) questionnaire for the urinary, bowel, and sexual domains (64). The results showed that while there was a transient decline in the EPIC score for the urinary and bowel domains, at 24 months the EPIC scores for these domains had nearly returned to baseline. The scores for the sexual domain, however, declined steadily following treatment, with those patients interviewed at 72 months follow-up reporting a 13.7 point decrease from baseline. These results are supported by another single institution review of patient reported outcomes from 228 patients treated with prostate SBRT, who reported transient declined in urinary and bowel function that nearly returned to baseline within 2 years, but continued to report a steady decrease in sexual function without recovery (65).
A concern amongst physicians with the use of SBRT is that the large dose per fraction may lead to worse toxicity, particularly late toxicity, amongst patients (66,67). However, patient reported outcomes suggest similar toxicity rates and QOL decreases following SBRT when compared with other treatment modalities. In a multi-institutional pooled cohort analysis of 803 patients treated with IMRT, brachytherapy, or SBRT, patient-reported QOL outcomes using the EPIC questionnaire were assessed and compared across treatment modalities. On multivariate analysis, comparing SBRT to IMRT revealed treatment with SBRT was associated with superior bowel function scores (P=0.00014) but similar urinary irritation scores (P=0.55) (68). The same analysis, when comparing SBRT to brachytherapy, revealed that patients treated with SBRT reported both superior bowel function scores (P=0.001) and superior urinary irritation scores (P=0.00051). A recent survey queried 329 patients who had received treatment with either IMRT, HDR brachytherapy, or SBRT for localized prostate cancer at UCLA regarding treatment regret and expectations versus reality of toxicities experienced (69). The incidence of regret was least for patients treated with SBRT (19% for IMRT, 18% for HDR, and 5% for SBRT, P<0.01), and a greater proportion of patients receiving treatment with SBRT rated that their actual toxicities were less than expected (43% for SBRT, 20% for IMRT, and 10% for HDR, P<0.01).
Various dosimetric and treatment-related factors can be adjusted to minimize the likelihood of toxicity. Authors have reported that keeping 35% of the circumference of the rectal wall <39 Gy, 50% of the rectal wall <24 Gy, the urethral point dose <47 Gy, and the bladder volume receiving 19 Gy <15 mL may decrease the incidence of Grade 2+ GI/ GU toxicity (70,71). Prolongation of treatment, and an extended period of time between treatments, can also decrease the rate of toxicities in these patients. In an early phase II trial evaluating the safety and efficacy of SBRT for prostate cancer, investigators noted a reduced rate of urinary rectal toxicity with treatment administered every other day when compared to treatment delivered on 5 consecutive days (0% vs. 38%, P=0.0035) (72). A Canadian randomized trial sought to investigate the potential benefit of prolonging time between SBRT fractions, and compared SBRT treatments delivered every other day and to SBRT treatments delivered once a week, and demonstrated superior acute bowel toxicity (P<0.01) and urinary QOL (P<0.01) with the treatment delivered once per week (73). The placement of a rectal spacer may also decrease the rate of rectal toxicity in prostate SBRT patients. Rectal spacers increase the distance between the anterior rectal wall and the prostate from 1.6 to 12.6 mm, and the insertion of a spacer has, in a randomized trial, reduced the severity of rectal toxicity and improved bowel related QOL in patients undergoing CF-EBRT for prostate cancer (74). Dosimetric studies have demonstrated that the presence of a rectal spacer can decrease the volume of the rectal wall receiving the 90% isodose line up to 90%, and a randomized phase III trial has demonstrated that the use of a rectal spacer for patients with prostate cancer undergoing CF IMRT reduces both rectal and GU toxicities, with patients receiving a spacer during treatment reporting superior bowel and urinary QOL scores (75). A phase II trial is currently being conducted to determine the rate of rectal toxicities for patients undergoing SBRT with the injection of a rectal spacer, which is expected confirm the benefits of a rectal spacer for prostate cancer patients undergoing SBRT (76).
Cost effectiveness of SBRT
Prostate cancer SBRT is typically 4–5 fractions, significantly less than the 39–44 fraction course of CF- EBRT, increasing patient convenience and potentially increasing patient satisfaction with treatment, as patients report length of treatment as the biggest drawback of CF-EBRT (77). Additionally, without taking into account the cost of treatment, a 5-fraction SBRT course was estimated to save the patient $1,522 during the course of treatment due to decreases in travel time, time off work, and parking costs when compared with a typical 39-fraction course of EBRT (34). SBRT may potentially offer cost savings to payers. In one cost-effectiveness analysis between SBRT and CF- IMRT using Markov modeling, and assuming equal efficacy and equal toxicity rates, the mean cost and quality adjusted life years (QALYS) of SBRT was $22,152 and 7.9 years, compared to $35,431 and 7.9 years for patients treated with IMRT (78). The authors calculated that if 50% of patients eligible for SBRT but treated with IMRT were instead treated with SBRT, this would save payers $250 million per year. A second cost-effectiveness study compared treated with SBRT, CF- IMRT, and proton-based radiation (PBT) for patients from both a payer perspective and from a societal perspective (by accounting for the age-specific cost per hour of time lost in treatment) using published reports to calculate and toxicity rates and assuming equal efficacy of each treatment (79). The authors found that SBRT was the least expensive treatment option when predicting lifetime costs from both a payer perspective ($24,873, $33,068, and $69,412 for SBRT, CF-IMRT, and PBT, respectively) and a societal perspective ($25,097, $35,088, and $71,657 for SBRT, CF-IMRT, and PBT respectively), and concluded that SBRT was the most cost-effective treatment strategy. A study from Canada sought to compare the required resources for provision of SBRT with a robotic-based radiosurgery system or different linear accelerator based radiation techniques to CF-IMRT using administrative data (80). The costs of SBRT were determined to be $4,368/patient (arc-based), $4,443/patient (fixed gantry treatment), and $6,333/patient (robotic-based radiosurgery system) while the costs for 39-fraction IMRT were $5,935/patient (arc-based) and $7,992/patient (fixed gantry). The authors concluded that given equal safety and efficacy, arc-based SBRT was the most cost-effective management option.
Future directions
While the National Comprehensive Cancer Network has listed SBRT for prostate cancer as an appropriate treatment regimen under select circumstances, the gold standard of evidence in medicine, phase III randomized trials, is currently lacking (2). Two such trials are currently accruing patients, the UK-based Prostate Advanced in Comparative Evidence (PACE) trial, which is comparing SBRT (36.25 Gy in 5 fractions) to surgery for operable patients and SBRT to CF-EBRT for nonsurgical patients (NCT01584258), and the Scandinavian HYPO trial, which is comparing SBRT (42.7 Gy in 7 fractions) to CF- IMRT for intermediate risk prostate cancer patient (ISRCTN45905321).
Novel uses and treatment techniques utilizing prostate SBRT technology are promising. One such method is the use of SBRT to deliver a selective intraprostatic boost for visualized focal prostate cancer lesions. Researchers have demonstrated the safety and efficacy of treating Magnetic Resonance Imaging (MRI)-visible prostate lesions with an integrated boost to 11 Gy per fraction while the remaining prostate received 38 Gy in 4 fractions (81). While visualization of intraprostatic lesions remain challenging, newer prostate cancer imaging techniques including 11C-choline positron emission tomography/CT may further elucidate occult prostate lesions, potentially aiding in the discovery of lesions that may be amenable to a selective SBRT boost (82). SBRT may also be used in the management of prostate cancer recurrence following EBRT. Fuller et al. have described the successful treatment of 29 patients with locally recurrent prostate cancer who had previously undergone radiation to 34 Gy in 5 fractions, and described a 2-year FFBF of 82% with a 7% of patients experiencing grade 3 GU toxicity and no patients experiencing GI toxicity > grade 1 (83). Another group of investigators has also described re-irradiation with SBRT to 21 patients to a dose of 36.25 Gy in 5 fractions, and described a 1-year FFBF of 83.3% with no reported ≥ grade 3 toxicities (84). The increased experience with SBRT coupled with improvements in image guidance and treatment delivery will likely increase the applications of SBRT for patients with prostate cancer that will both improve the patient experience during and after treatment while maintaining or improving clinical outcomes.
Conclusions
Prostate SBRT, while not having been compared to CF-EBRT in a randomized trial, has been shown in multiple prospective phase I–II trials and single institution reviews to be both safe and effective. It is a cost-effective treatment offering increased convenience to patients, and has the potential to offer great savings to both payers. It is likely that SBRT will continue to achieve increased acceptance amongst the medical community and will become a widely- adopted standard of care for treatment of localized prostate cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cancer Stat Facts:Cancer of Any Site:Number of New Cases and Deaths. Available online: https://seer.cancer.gov/statfacts/html/all.html, accessed March 3, 2017.
- National Comprehensive Cancer Network (NCCN). Prostate cancer. Version 1.2017. Available online: https://nccn.org/professionals/physician_gls/pdf/prostate.pdf, February 20, 2017.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. [Crossref] [PubMed]
- Kupelian PA, Potters L, Khuntia D, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:25-33. [Crossref] [PubMed]
- Dandapani SV, Sanda MG. Measuring health-related quality of life consequences from primary treatment for early-stage prostate cancer. Semin Radiat Oncol 2008;18:67-72. [Crossref] [PubMed]
- Avkshtol V, Dong Y, Hayes SB, et al. A comparison of robotic arm versus gantry linear accelerator stereotactic body radiation therapy for prostate cancer. Res Rep Urol 2016;8:145-8. [Crossref] [PubMed]
- Miralbell R, Roberts SA, Zubizarreta E, et al. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets:α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys 2012;82:e17-e24. [Crossref] [PubMed]
- Zaorsky NG, Harrison AS, Trabulsi EJ, et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat Rev Urol 2013;10:565-79. [Crossref] [PubMed]
- Kim DN, Straka C, Cho LC, et al. Early and multiple PSA bounces can occur following high-dose prostate stereotactic body radiation therapy:subset analysis of a phase 1/ 2 trial. Pract Radiat Oncol 2017;7:e43-e49. [Crossref] [PubMed]
- Kim HJ, Phak JH, Kim WC, et al. Prostate-specific antigen kinetics after stereotactic body radiotherapy as monotherapy or boost after whole pelvic radiotherapy for localized prostate cancer. Prostate Int 2015;3:118-22. [Crossref] [PubMed]
- Collins CD, Lloyd-Davies RW, Swan AV. Radical external beam radiotherapy for localised carcinoma of the prostate using a hypofractionation technique. Clin Oncol (R Coll Radiol) 1991;3:127-32. [Crossref] [PubMed]
- Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localizedprostate cancer:Cleveland Clinic experience. Int J Radiat Oncol Biol Phys 2007;68:1424-30. [Crossref] [PubMed]
- Livsey JE, Cowan RA, Wylie JP, et al. Hypofractionated conformal radiotherapy in carcinoma of the prostate:five-year outcome analysis. Int J Radiat Oncol Biol Phys 2003;57:1254-9. [Crossref] [PubMed]
- Lee WR, Dignam JJ, Amin M, et al. NRG Oncology RTOG 0415: a randomized phase 3 noninferiority study comparing 2 fractionation schedules in patients with low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2016;94:3-4. [Crossref]
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [Crossref] [PubMed]
- Lin YW, Lin KH, Ho HW, et al. Treatment plan comparison between stereotactic body radiation therapy techniques for prostate cancer: non-isocentric CyberKnife versus isocentric RapidArc. Phys Med 2014;30:654-61. [Crossref] [PubMed]
- Wierzbicki M, Schaly B, Peters T, et al. Automatic image guidance for prostate IMRT using low dose CBCT. Med Phys 2010;37:3677-86. [Crossref] [PubMed]
- Klayton T, Price R, Buyyounouski MK, et al. Prostate bed motion during intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys 2012;84:130-6. [Crossref] [PubMed]
- Cavalieri R, Gay HA, Liu J, et al. Total error shift patterns for daily CT on rails image-guided radiotherapy to the prostate bed. Radiat Oncol 2011;6:142. [Crossref] [PubMed]
- Ishiyama H, Teh BS, Lo SS, et al. Stereotactic body radiation therapy for prostate cancer. Future Oncol 2011;7:1077-86. [Crossref] [PubMed]
- Munoz F, Fiandra C, Franco P, et al. Tracking target position variability using intraprostatic fiducial markers and electronic portal imaging in prostate cancer radiotherapy. Radiol Med 2012;117:1057-70. [Crossref] [PubMed]
- Kron T, Thomas J, Fox C, et al. Intra-fraction prostate displacement in radiotherapy estimated from pre- and post-treatment imaging of patients with implanted fiducial markers. Radiother Oncol 2010;95:191-7. [Crossref] [PubMed]
- Lukka H, Stephanie P, Bruner D, et al. Patient-reported outcomes in NRG Oncology/RTOG 0938, a randomized phase 2 study evaluating 2 ultrahypofractionated regimens (UHRs) for prostate cancer. Int J Radiat Oncol Biol Phys 2016;94:2. [Crossref]
- Xie Y, Djajaputra D, King CR, et al. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:236-46. [Crossref] [PubMed]
- King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013;109:217-21. [Crossref] [PubMed]
- Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol 2014;4:240. [Crossref] [PubMed]
- Meier R, Beckman A, Henning G, et al. Five-year outcomes from a multicenter trial of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2016;96:S33-S34. [Crossref]
- Bernetich M, Oliai C, Lanciano R, et al. SBRT for the primary treatment of localized prostate cancer: the effect of gleason score, dose and heterogeneity of intermediate risk on outcome utilizing 2.2014 NCCN risk stratification guidelines. Front Oncol 2014;4:312. [Crossref] [PubMed]
- Friedland JL, Freeman DE, Masterson-McGary ME, et al. Stereotactic body radiotherapy:an emerging treatment approach for localized prostate cancer. Technol Cancer Res Treat 2009;8:387-92. [Crossref] [PubMed]
- Mantz C. A phase II trial of stereotactic ablative body radiotherapy for low-risk prostate cancer using a non-robotic linear accelerator and real time target tracking:report of toxicity, quality of life, and disease control outcomes with 5-year minimum follow-up. Front Oncol 2014;4:279. [Crossref] [PubMed]
- Bolzicco G, Favretto MS, Satariano N, et al. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol 2013;13:49. [Crossref] [PubMed]
- Hannan R, Tumati V, Xie XJ, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer- Results from a multi-institutional clinical trial. Eur J Cancer 2016;59:142-51. [Crossref] [PubMed]
- D’Agostino G, Franzese C, De Rose F, et al. High-quality Linac-based Stereotactic Body Radiation Therapy with Flattening Filter Free Beams and Volumetric Modulated Arc Therapy for Low-Intermediate Risk Prostate Cancer. A Mono-institutional Experience with 90 Patients. Clin Oncol (R Coll Radiol) 2016;28:e173-e178. [Crossref] [PubMed]
- Loblaw A, Cheung P, D’Alimonte L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiother Oncol 2013;107:153-8. [Crossref] [PubMed]
- Fuller DB, Naitoh J, Mardirossian G. Virtual HDR CyberKnife SBRT for localized prostatic carcinoma: 5-year disease-free survival and toxicity observations. Front Oncol 2014;4:321. [Crossref] [PubMed]
- Rucinska M, Kieszkowska-Grudny A, Nawrocki S. SHARP hypofractionated stereotactic radiotherapy is well tolerated in prostate cancer: Toxicity and quality of life assessment. Strahlenther Onkol 2016;192:449-57. [Crossref] [PubMed]
- McBride SM, Wong DS, Dombrowski JJ, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma:preliminary results of a multi-institutional phase 1 feasibility trial. Cancer 2012;118:3681-90. [Crossref] [PubMed]
- Lee SW, Jang HS, Lee JH, et al. Stereotactic body radiation therapy for prostate cancer patients with old age or medical comorbidity: a 5-year follow-up of an investigational study. Medicine (Baltimore) 2014;93:e290. [Crossref] [PubMed]
- Kang JK, Cho CK, Choi CW, et al. Image-guided stereotactic body radiation therapy for localized prostate cancer. Tumori 2011;97:43-8. [PubMed]
- Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys 2007;67:1099-105. [Crossref] [PubMed]
- Jeong BK, Jeong H, Ha IB, et al. Stereotactic Body Radiation Therapy for Low- to Intermediate risk Prostate Adenocarcinoma. J Korean Med Sci 2015;30:710-5. [Crossref] [PubMed]
- Park YH, Choi IY, Yoon SC, et al. Prostate-specific antigen kinetics after primary stereotactic body radiation therapy using CyberKnife for localized prostate cancer. Prostate Int 2015;3:6-9. [Crossref] [PubMed]
- Kim HJ, Phak JH, Kim WC. Hypofractionated stereotactic body radiotherapy in low- and intermediate-risk prostate carcinoma. Radiat Oncol J 2016;34:260-4. [Crossref] [PubMed]
- Kotecha R, Djemil T, Tendulkar RD, et al. Dose-Escalated Stereotactic Body Radiation Therapy for Patients With Intermediate- and High-Risk Prostate Cancer:Initial Dosimetry Analysis and Patient Outcomes. Int J Radiat Oncol Biol Phys 2016;95:960-4. [Crossref] [PubMed]
- Morris WJ, Tyldesley S, Pai HH, et al. ASCENDE-RT: a multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable risk localized prostate cancer. J Clin Oncol 2015;33:3. [Crossref]
- Mercado C, Kress MA, Cyr RA, et al. Intensity-modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer:the Georgetown University experience. Front Oncol 2016;6:114. [Crossref] [PubMed]
- Katz AJ, Santoro M, Ashley R, et al. Stereotactic body radiotherapy as boost for organ-confined prostate cancer. Technol Cancer Res Treat 2010;9:575-82. [Crossref] [PubMed]
- Anwar M, Weinberg V, Seymour Z, et al. Outcomes of hypofractionated stereotactic body radiotherapy boost for intermediate and high-risk prostate cancer. Radiat Oncol 2016;11:8. [Crossref] [PubMed]
- Lin YW, Lin LC, Lin KL. The early result of whole pelvic radiotherapy and stereotactic body radiotherapy boost for high-risk localized prostate cancer. Front Oncol 2014;4:278. [Crossref] [PubMed]
- Kim HJ, Phak JH, Kim WC. Clinical outcomes of whole pelvis radiotherapy and stereotactic body radiotherapy boost for intermediate- and high-risk prostate cancer. Asia Pac J Clin Oncol 2016. [Epub ahead of print]. [PubMed]
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk:10-year results of an EORTC randomized study. Lancet Oncol 2010;11:1066-73. [Crossref] [PubMed]
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma- long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 2005;61:1285-90. [Crossref] [PubMed]
- Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer:10-year data from the TROG 96.01 randomised trial. Lancet Oncol 2011;12:451-9. [Crossref] [PubMed]
- Roach M 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 2008;26:585-91. [Crossref] [PubMed]
- Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008;26:2497-504. [Crossref] [PubMed]
- Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360:2516-27. [Crossref] [PubMed]
- D'Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 2008;299:289-95. [PubMed]
- Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 2011;365:107-18. [Crossref] [PubMed]
- Bolla M, Maingon P, Carrie C, et al. Short androgen suppression and radiation dose escalation for intermediate-and high-risk localized prostate cancer:results of EORTC Trial 22991. J Clin Oncol 2016;34:1748-56. [Crossref] [PubMed]
- Kishan AU, Wang PC, Upadhyaya SK, et al. SBRT and HDR brachytherapy produce lower PSA nadirs and different PSA decay patterns than conventionally fractionated IMRT in patients with low- or intermediate-risk prostate cancer. Pract Radiat Oncol 2016;6:268-75. [Crossref] [PubMed]
- Anwar M, Weinberg V, Chang AJ, et al. Hypofractionated SBRT versus conventionally fractionated EBRT for prostate cancer: comparison of PSA slope and nadir. Radiat Oncol 2014;9:42. [Crossref] [PubMed]
- Lee SH, Kim HJ, Kim WC. Prostate-specific antigen kinetics following hypofractionated stereotactic body radiotherapy versus conventionally fractionated external beam radiotherapy for low- and intermediate-risk prostate cancer. Asia Pac J Clin Oncol 2016;12:388-95. [Crossref] [PubMed]
- Oliai C, Bernetich M, Brady L, et al. Propensity score matched comparison of SBRT versus IMRT for the treatment of localized prostate cancer. J Radiat Oncol 2016;5:187-95. [Crossref] [PubMed]
- King CR, Collins S, Fuller D, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 2013;87:939-45. [Crossref] [PubMed]
- Bhattasali O, Chen LN, Woo J, et al. Patient-reported outcomes following stereotactic body radiation therapy for clinically localized prostate cancer. Radiat Oncol 2014;9:52. [Crossref] [PubMed]
- Yu JB, Cramer LD, Herrin J. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol 2014;32:1195-201. [Crossref] [PubMed]
- D’Amico AV. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: less cost at the expense of more genitourinary toxicity is a concerning but testable hypothesis. J Clin Oncol 2014;32:1183-5. [Crossref] [PubMed]
- Evans JR, Zhao S, Daignault S, et al. Patient-reported quality of life after stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and brachytherapy. Radiother Oncol 2015;116:179-84. [Crossref] [PubMed]
- Shaverdian N, Verruttipong D, Wang PC, et al. Exploring value from the patient’s perspective between modern radiation therapy modalities for localized prostate cancer. Int J Radiat Oncol Biol Phys 2017;97:516-25. [Crossref] [PubMed]
- Seymour ZA, Chang AJ, Zhang L, et al. Dose-volume analysis and the temporal nature of toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol 2015;5:e465-e472. [Crossref] [PubMed]
- Kim DW, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;89:509-17. [Crossref] [PubMed]
- King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009;73:1043-8. [Crossref] [PubMed]
- Quon HC, Ong A, Cheung P, et al. PATRIOT trial: randomized phase 2 study of prostate stereotactic body radiation therapy comparing 11 versus 29 days overall treatment time. Int J Radiat Oncol Biol Phys 2015;93:S198. [Crossref]
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2015;92:971-7. [Crossref] [PubMed]
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys 2017;97:976-85. [Crossref] [PubMed]
- Chapet O, Udrescu C, Tanguy R, et al. Dosimetric implications of an injection of hyaluronic acid for preserving the rectal wall in prostate stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;88:425-32. [Crossref] [PubMed]
- Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what’s important. J Gen Intern Med. 2000;15:694-701. [Crossref] [PubMed]
- Hodges JC, Lotan Y, Boike TP, et al. Cost-effectiveness analysis of SBRT versus IMRT: an emerging initial radiation treatment option for organ-confined prostate cancer. Am J Manag Care 2012;18:e186-93. [PubMed]
- Parthan A, Pruttivarasin N, Davies D, et al. Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer. Front Oncol 2012;2:81. [Crossref] [PubMed]
- Sharieff W, Grenspoon JN, Dayes I, et al. The Technique, Resources and Costs of Stereotactic Body Radiotherapy of Prostate Cancer: A Comparison of Dose Regimens and Delivery Systems. Technol Cancer Res Treat 2016;15:171-8. [Crossref] [PubMed]
- Aluwini S, van Rooij P, Hoogeman M, et al. Stereotactic body radiotherapy with a focal boost to the MRI-visible tumor as monotherapy for low- and intermediate-risk prostate cancer: early results. Radiat Oncol 2013;8:84. [Crossref] [PubMed]
- Martorana G, Schiavina R, Corti B, et al. 11C-choline positron emission tomography/computerized tomography for tumor localization of primary prostate cancer in comparison with 12-core biopsy. J Urol 2006;176:954-960. [Crossref] [PubMed]
- Fuller DB, Wurzer J, Shirazi R, et al. High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol 2015;5:e615-e623. [Crossref] [PubMed]
- Janoray G, Reynaud-Bougnoux A, Ruffier-Loubiere A, et al. Stereotactic body re-irradiation therapy for locally recurrent prostate cancer after external-beam radiation therapy: Initial report. Cancer Radiother 2016;20:275-81. [Crossref] [PubMed]


