Meningioma research—status quo and quo vadis
Meningiomas are the most common intracranial tumors. Most meningiomas can be cured by surgical resection and are commonly considered “an easy win” by neurooncologists, who are oftentimes confronted with more difficult tumor types such as gliomas and brain metastases (1). However, up to 20–25% of meningioma cases are not curable by surgical resection due to their location in surgically inaccessible areas such as the skull base, multifocality, or aggressive behaviour associated with higher grade histology or molecular features. Such “difficult meningiomas” have recently moved into the focus of interdisciplinary basic, translational and clinical research. With the advent of next generation sequencing technologies, our understanding of the molecular drivers of meningiomas has rapidly expanded and new clinically actionable mutations have recently been discovered (2-4). Fascinating insights have emerged and paved the way for the development of prognostically relevant classifications based on molecular data (5-11), improved application of established treatments and novel therapeutic approaches with targeted drugs. In this issue of Chinese Clinical Oncology (CCO) international experts provide an overview of the current knowledge and emerging perspectives in meningioma research. The topics covered include advances in histopathological and molecular classification, radiotherapy, surgery, and animal models of meningioma. Without doubt, meningioma research has become one of the most dynamically evolving fields of neurooncology and is producing insights that benefit our patients. Importantly, several prospective international clinical trials enrolling patients with aggressive or recurrent meningiomas are ongoing and will likely provide us with more effective therapies (Table 1). As we continue to explore novel therapeutic approaches, and expand and validate molecular classifications of meningiomas, the paradigm of meningioma diagnosis and management will change.
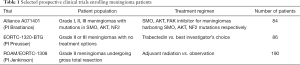
Full table
Acknowledgements
None.
References
- Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 2016;17:e383-91. [Crossref] [PubMed]
- Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 2013;45:285-9. [Crossref] [PubMed]
- Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013;339:1077-80. [Crossref] [PubMed]
- Sahm F, Bissel J, Koelsche C, et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol 2013;126:757-62. [Crossref] [PubMed]
- Abedalthagafi M, Bi WL, Aizer AA, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol 2016;18:649-55. [Crossref] [PubMed]
- Sahm F, Schrimpf D, Olar A, et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J Natl Cancer Inst 2015.108. [PubMed]
- Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 2017;18:682-94. [Crossref] [PubMed]
- Shankar GM, Abedalthagafi M, Vaubel RA, et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol 2017;19:535-45. [PubMed]
- Strickland MR, Gill CM, Nayyar N, et al. Targeted sequencing of SMO and AKT1 in anterior skull base meningiomas. J Neurosurg 2016.1-7. [Crossref] [PubMed]
- Du Z, Abedalthagafi M, Aizer AA, et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget 2015;6:4704-16. [Crossref] [PubMed]
- Reuss DE, Piro RM, Jones DT, et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol 2013;125:351-8. [Crossref] [PubMed]





