Radiation therapy in the management of pancreatic adenocarcinoma: review of current evidence and future opportunities
Introduction
Pancreatic cancer remains one of the most challenging diseases in the field of oncology. Surgical resection is the only potentially curative therapy, yet only about 15% of patients have disease amenable to curative resection (1) and even in this small subset of patients, the 5-year overall survival (OS) is approximately 20% (2). Pancreatic cancer is practically broken into the following staging categories: resectable disease, borderline resectable disease, locally advanced (unresectable) disease, and metastatic disease. The role of radiation therapy in each of these disease states is evolving and this review summarizes the established evidence for radiation therapy in pancreatic cancer as well as highlights recent studies that may point to future roles for radiation in each stage of disease.
Resectable pancreatic cancer
Unfortunately, it is the minority of patients that present with resectable pancreatic cancer (1), and due to the complex anatomy of the pancreas in relation to adjacent organs and vasculature, even in upfront resectable patients there is a very high rate of margin positive resections, especially the retroperitoneal margin. Reported rates of margin positivity vary considerably between studies, but are reported to be between about 25% and 50% (2-5) and one study that defined an R1 resection to include margins negative by less than 1 mm, and used a standard pathologic protocol for tissue processing, found an R1 resection rate of 76% (6). These studies highlight the rationale for consideration of neoadjuvant therapy even in the setting of upfront resectable disease.
Yet, the only randomized clinical trial comparing neoadjuvant chemoradiation to immediate surgery closed early due to poor accrual, nevertheless the results were published (7). The study was designed to compare neoadjuvant chemoradiation utilizing 3D conformal techniques to 55.8 Gy to the primary tumor and 50.4 Gy to the regional lymph nodes with concurrent gemcitabine and cisplatin, to upfront surgery and adjuvant gemcitabine. The study did not demonstrate a difference in OS but was grossly underpowered (7).
Despite a lack of randomized data, there are notable single arm studies of neoadjuvant therapy that are worth highlighting. In a multi-institutional phase II study of neoadjuvant chemoradiation to 36 Gy in 15 fractions utilizing 3D conformal techniques with full dose concurrent gemcitabine, 17 of 20 patients underwent surgical resection with a 94% rate of R0 resection and pN0 in 65% of patients (8). A phase II study of neoadjuvant chemoradiation to 30 Gy in 10 fractions with concurrent gemcitabine prior to surgery for resectable disease was performed at MD Anderson Cancer Center (9). Radiotherapy was delivered using 3D conformal techniques and the pancreaticoduodenal, portahepatic, superior mesenteric, and celiac lymph nodes, were electively included in the treatment volume. A total of 86 patients enrolled on the study, 73 ended up undergoing surgical resection, with surgery aborted in 6 patients due to extrapancreatic disease identified at the time of surgery, resulting in 64 patients completing pancreaticoduodenectomy. There was an 11% positive margin rate with median OS of 34 months and 5-year OS of 36%. Thirty-eight percent of patients had pN1 disease (9). Investigators at Massachusetts General Hospital conducted a phase I/II study of neoadjuvant short course proton beam radiation therapy to 25 Gy in 5 fractions with concurrent capecitabine (10). There were no significant toxicities. Of 48 eligible patients, 37 underwent pancreaticoduodenectomy with 16% positive margin rate but 81% of patients had pN1 disease. And 16.2% of resected patients experienced a locoregional failure (10). Although the above studies are compelling, upfront surgical resection remains standard of care for resectable patients, nonetheless, novel studies are needed to improve outcomes for this patient cohort.
With upfront surgery as the standard management of resectable tumors, the potential role for radiation therapy in the adjuvant setting remains an important question. The existing data are conflicting and many of the studies used outdated radiation techniques. In 1985, the Gastrointestinal Tumor Study Group (GITSG) reported a small trial of 49 patients that showed a statistically significant OS benefit for patients receiving adjuvant chemoradiation with 5-FU versus patients that were treated with surgery alone (11,12). A follow-up trial by the European Organization for Research and Treatment of Cancer (EORTC) showed a trend towards improvement in OS with postoperative chemoradiation but this finding did not reach statistical significance (2-year OS 26% in observation arm and 34% in adjuvant chemoradiation arm, P=0.099) (13). The European Study Group for Pancreatic Cancer-1 (ESPAC-1) trial subsequently showed an advantage to adjuvant chemotherapy but worse outcomes for patients receiving chemoradiation (14), however the flawed methodology in this study has been highly criticized (15-17). To further evaluate this question using more modern radiotherapy techniques, a combined retrospective analysis from the Johns Hopkins Hospital and Mayo Clinic of 1,092 patients treated with adjuvant chemoradiation versus observation alone utilizing matched pair analyses demonstrated a significant improvement in survival for patients receiving adjuvant chemoradiation (21.1 vs. 15.5 months, P<0.001) (18). RTOG 0848 is an actively enrolling phase III trial attempting to answer this question in the modern era with current chemotherapy and advanced radiation techniques, and incorporating high quality radiation quality assurance.
Borderline resectable pancreatic cancer (BRPC)
Pancreatic head tumors are classified as borderline resectable by the National Comprehensive Cancer Network (NCCN) criteria if there is solid tumor contact with the common hepatic artery without extension to the celiac axis or hepatic artery bifurcation allowing for safe and complete resection and reconstruction, or solid tumor contact with the superior mesenteric artery <180°. Regarding venous involvement, borderline resectable tumors have solid tumor contact with the superior mesenteric vein or portal vein of >180°, or contact of ≤180° with contour irregularity of the vein or thrombosis of the vein but with suitable vessel proximal and distal to the site of involvement allowing for safe and complete resection and vein reconstruction, or solid tumor contact with the inferior vena cava. For body and tail tumors, borderline resectable arterial involvement is defined as solid tumor contact with the celiac artery of ≤180° and solid tumor contact with the celiac artery of >180° without involvement of the aorta and with an intact and uninvolved gastroduodenal artery (19).
Positive margins are associated with a significant decrement in disease-free and OS (20-23). There is a growing body of evidence to support neoadjuvant radiation therapy for patients with BRPC with the goal of downstaging tumors and allowing for a margin negative resection. The MD Anderson Cancer Center has published their single institution experience of 160 patients treated with neoadjuvant radiation (24). Patients received either 50.4 Gy in 28 fractions or 30 Gy in 10 fractions. Sixty-six patients ultimately went to surgery with 94% achieving a negative margin and a median OS of 40 months for those that went to surgery and 13 months for those that did not (24). Recently, results of the prospective multi-center single arm Alliance trial, A021101, were published. Twenty-two patients received modified FOLFIRINOX for four cycles followed by chemoradiation to 50.4 Gy in 28 fractions with concurrent capecitabine. Fifteen patients underwent surgical resection with 14 achieving negative margins. Five patients had less than 5% residual tumor cells and 2 patients had a pathologic complete response (pCR). Median OS was 21.7 months, but 64% of patients experienced grade 3 or greater toxicity (25). Additionally, although preoperative radiation did not appear to result in improved toxicity or survival compared to postoperative radiation in a retrospective analysis from the MD Anderson Cancer Center, prolonged surgical recovery prevented delivery of adjuvant therapy in 24% of patients (26). These data suggest a benefit to neoadjuvant radiation in BRPC, but there are disadvantages to long course chemoradiation, including delaying delivery of full dose chemotherapy and surgical resection, as well as the inconvenience for patients of five and a half weeks of daily therapy.
Stereotactic body radiation therapy (SBRT) which uses higher doses per fraction over fewer fractions to deliver very focal, ablative doses to the tumor itself has an emerging role in the neoadjuvant treatment of patients with BRPC (Table 1). The Moffitt Cancer Center published an initial review of their experience with this technique as well as a subsequent update. They first reported on 73 patients with locally advanced pancreatic cancer (LAPC) or BRPC treated in 5 fractions at 5–6 Gy per fraction to the GTV with a simultaneous integrated boost (SIB) of 7–10 Gy per fraction in the region of vessel abutment. Just over half of BRPC patients went to surgery with an R0 resection achieved in 96.9%. There was no acute grade 2 or greater toxicity and only 5.3% of patients experience late grade 3 or greater toxicity (27). In an updated analysis that included 110 BRPC patients, 51% underwent resection with a 96% R0 resection rate. Median OS was 19.2 months, and 34.2 months in patients that underwent surgical resection. There was only a 7% rate of grade 3 or greater toxicity (28). Rajagopalan et al. published on their experience of 105 patients treated with SBRT for LAPC or BRPC. Twelve patients underwent surgical resection, and of these, seven presented with BRPC and five presented with LAPC. Five of the surgical patients received 24 Gy in a single fraction, six patients received 36 Gy in 3 fractions, and one patient received 30 Gy in 3 fractions, prior to surgery. An R0 resection was achieved in 92% of patients with a pCR or <10% of viable tumor cells in 41.7% of patients. One-, 2-, and 3-year OS were 92%, 64%, and 51%, respectively. There were no grade 3 or greater toxicities attributable to SBRT (29). Recently, the group at Emory completed a phase I dose escalation trial of SBRT in BRPC. Thirteen patients were treated in 3 fraction SBRT regimens. All dose levels included an SIB to the posterior margin. Dose level 1 was 30 Gy with an SIB to 36 Gy, dose level 2 was 36 Gy with an SIB to 42 Gy, dose level 3 was 36 Gy with an SIB to 43.5 Gy, and dose level 4 was 36 Gy with an SIB to 45 Gy. Dose level 4 was reached without grade 3 or greater toxicities. Five patients experienced progressive disease before resection but the eight patients that went to surgery all had R0 resections. Four patients were alive and three were disease-free at a median follow-up of 18 months. Median OS was not reached in resected patients (30). Importantly, the studies outlined above highlight the benefits of SBRT for neoadjuvant radiation. In addition to the convenient short course, SBRT appears to be far better tolerated than neoadjuvant chemoradiation. In Alliance A021101, 64% of patients experienced grade 3 or greater toxicity, whereas minimal grade 3 toxicity was reported in the SBRT experiences outlined above. To further explore the neoadjuvant SBRT paradigm, an Alliance trial, Alliance A021501, is currently enrolling patients and is comparing neoadjuvant chemotherapy to neoadjuvant chemotherapy and SBRT prior to surgery for BRPC patients.
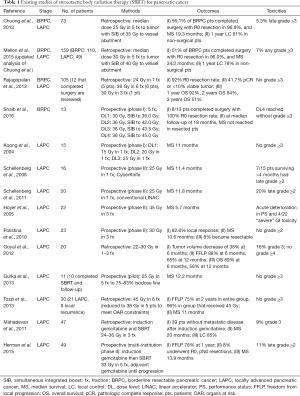
Full table
The institutional studies described above show SBRT is a well-tolerated and effective neoadjuvant modality for BRPC. The ongoing Alliance trial will help to determine if the single institution successes utilizing neoadjuvant SBRT for BRPC can be replicated in a multi-institutional cooperative group format.
LAPC (unresectable)
Locally advanced (unresectable) pancreatic cancer presents a unique challenge. Despite the high propensity for patients with pancreatic cancer to develop distant metastatic disease, local disease remains not only a significant cause of patient morbidity, but in an autopsy series about 30% of patients died of locally destructive disease (31). However, recent data from the LAP07 trial did not demonstrate a survival advantage with the addition of chemoradiation over chemotherapy alone for these LAPC patients (32). Prior to publication of the results of LAP07, chemoradiation for LAPC was already controversial, with multiple conflicting studies and concerns regarding methodology and antiquated chemotherapy and radiation techniques (33-38). Two retrospective studies then showed a benefit to induction chemotherapy followed by chemoradiation (39,40), similar to the design of LAP07. LAP07 was an international phase III trial with a two-step randomization, with the first randomization between induction gemcitabine and induction gemcitabine and erlotinib. The second randomization was to continued chemotherapy alone or chemoradiation. The study showed no difference in OS or progression-free survival (PFS) for patients that received chemotherapy alone or chemoradiation. However, there were differences in pattern of failure and chemotherapy-free interval, with 32% local progression in the chemoradiation arm and 46% in the chemotherapy alone arm, and 6.1 months until initiation of second line chemotherapy in the chemoradiation arm versus 3.7 months in the chemotherapy alone arm (32). These data do leave open the possibility that local therapy still has a role in LAPC, and there is a growing body of evidence supporting altered fractionation to improve the therapeutic ratio.
Ben-Josef et al. conducted a phase I/II study of dose escalated intensity modulated radiation therapy (IMRT) with gemcitabine in patients with unresectable pancreatic cancer (41). Fifty patients were enrolled with 2-year freedom from local progression and OS of 59% and 30%, respectively. The authors suggested a dose of 55 Gy in 25 fractions which was associated with a probability of dose limiting toxicity of 0.24 (41). Krishnan et al. recently published their experience utilizing dose escalated hypofractionated radiation in this setting (42). Upon review of 200 patients with LAPC, 47 patients had tumors >1 cm from luminal organs and received dose escalated IMRT to a biologically equivalent dose (BED) >70 Gy. The most common regimens were 63–70 Gy in 28 fractions (25 patients) and 67.5 Gy in 15 fractions (7 patients). Improved OS was seen with these regimens compared with patients receiving fractionation schedules with BED <70 Gy, and interestingly the degree of GTV coverage did not appear to affect outcome, advocating for integrated boost at least to a partial GTV as tolerated. There were no differences in toxicity between the groups receiving BED <70 or >70 Gy (42).
The use of SBRT in the setting of LAPC has been investigated in several prospective studies (Table 1). In 2004, Koong et al. at Stanford University first published a single fraction phase I dose escalation trial of 15, 20, and 25 Gy, in 15 patients (43). Median OS for all patients was 11 months and there were no grade 3 or greater toxicities (43). A phase II study of 25 Gy in a single fraction utilizing CyberKnife resulted in significant late toxicities. Seven of 15 patients surviving greater than 4 months experienced late grade 2 or greater late GI toxicity (5 grade 2 ulcers, 1 grade 3 duodenal stenosis, 1 grade 4 duodenal perforation), and median OS was 11.4 months (44). Using the same approach of 25 Gy in a single fraction delivered on a standard linear accelerator at Stanford had a similar median OS of 11.8 months and a 20% rate of late grade 2 or greater GI toxicity (45).
Simultaneously in Denmark, investigators were evaluating the use of a multifraction regimen. They treated 22 patients to 45 Gy in 3 fractions, but large volumes were treated and toxicities were unacceptable with a median OS of 5.7 months (46). More recently, Polistina et al. treated 23 patients to 30 Gy in 3 fractions with an 82.6% local response rate and median OS of 10.6 months (47). Eight percent of patients became resectable and there were no grade 2 or greater toxicities (47). Goyal et al. published a study of 20 patients treated with SBRT to 22–30 Gy in 1–3 fractions with a mean tumor volume reduction of 38% 6 months after SBRT and freedom from local progression of 88% at 6 months and 65% at 12 months (48). OS was 89% at 6 months and 56% at 12 months. 16% of patients experienced grade 3 toxicities and there were no grade 4 or 5 toxicities (48). The Georgetown group published their series of 11 patients (10 completed SBRT and follow-up) treated to 25 Gy in 5 fractions to the 75–83% isodose line (49). Median OS was 12.2 months and there were no grade 3 or higher toxicities (49). Tozzi et al. published a series of 30 patients (21 with LAPC and 9 with local recurrence after surgery) treated to 36–45 Gy in 6 fractions (45 Gy was the planned dose but it was reduced to 36 Gy in 5 patients due to an inability to meet organ at risk constraints) (50). Freedom from local progression was 75% at 2 years in the entire group and 96% in the group that received 45 Gy. Median OS was 11 months and there were no grade 3 or greater toxicities (50). Mahadevan et al. treated patients with induction gemcitabine followed by SBRT if they did not develop distant metastases (51). Of 47 patients treated with induction gemcitabine, 39 were without metastatic disease after 2 cycles and completed SBRT. Patients received 24 to 36 Gy in 3 fractions between cycles 3 and 4 of gemcitabine with median OS of 20 months, 85% local control, and 9% grade 3 toxicities (51).
Finally, Herman et al. conducted a multi-institution phase II trial demonstrating that this approach is feasible and practical outside of the single institution experiences (52). Patients received gemcitabine (1,000 mg/m2) for 3 doses and then SBRT to 33 Gy in 5 fractions after a 1 week break. Gemcitabine was continued until disease progression or toxicity. Median OS was 13.9 months and freedom from local progression at one year was 78%. Importantly, 8% of patients ultimately underwent margin negative, node negative resections. The rate of late grade 2 or higher toxicity was 11% (this included 3 grade 5 toxicities: C. difficile infection, sepsis, and GI bleed associated with direct tumor extension, and 2 grade 4 toxicities: fistula and ulcer) (52). Figure 1 shows a typical SBRT treatment plan to deliver 33 Gy to a locally advanced pancreas tumor and demonstrates the proximity of the normal tissues, particularly the duodenum, to the high dose region. Additional protocols are ongoing to establish if higher doses in fractionated SBRT regimens can be delivered safely, and integrate aims to better elucidate the mechanisms of SBRT-mediated tumor destruction and normal tissue injury.
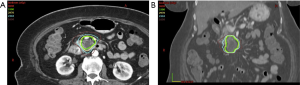
As further dose escalation is carefully pursued, through SBRT or longer course hypofractionation, and we incorporate these techniques with more aggressive systemic therapy, further improvements in survival for patients with LAPC may be achieved.
Emerging technologies
Protons have a favorable depth-dose distribution compared to photons and were first utilized clinically decades ago (53), with a significant increase in the number of active proton therapy programs over the last several years (54). For pancreatic cancer there are several dosimetric studies suggesting potential for reduction in dose to organs at risk with proton therapy compared to photon therapy (55-60). Further, there are a handful of published clinical experiences utilizing proton beam radiotherapy for pancreatic cancer. As described above in the section “resectable pancreatic cancer”, investigators at Massachusetts General Hospital conducted a phase I/II study of neoadjuvant short course proton beam radiation therapy to 25 Gy in 5 fractions with concurrent capecitabine, and treatment was well tolerated. Researchers at the University of Florida published their experience of 22 patients with resectable (n=5), borderline resectable (n=5), and unresectable (n=12) pancreatic cancer treated with proton beam radiotherapy to 50.4–59.4 cobalt gray equivalent (CGE) with concurrent capecitabine, also reporting a favorable toxicity profile (61). One theoretical advantage of proton therapy in this setting is increased normal tissue sparing potentially allowing further dose escalation and possibly increased efficacy. However, a Japanese study treated patients with LAPC with proton beam radiation to 67.5 Gy (RBE) in 25 fractions with concurrent gemcitabine and approximately half of patients were found to have gastric and duodenal ulcers on endoscopic examination (62,63). Therefore, although proton beam radiotherapy remains a promising technology for improving outcomes for pancreatic cancer patients, additional investigation is needed.
Another technological advancement in radiotherapy that has the potential to impact pancreatic cancer management are magnetic resonance image guided radiotherapy (MR-IGRT) systems. Onboard MRI improves soft tissue anatomy visualization (64), which in the treatment of pancreatic cancer, has the potential to improve delineation of both the treatment target and nearby organs at risk. The proximity of sensitive gastrointestinal organs to the treatment target in pancreatic cancer can be limiting in escalating radiation dose. Interestingly, a recent publication exploring online-adaptive radiation therapy with MR-IGRT for abdominal and thoracic tumors demonstrated that online-adaptive MRI-guided SBRT is feasible and may allow PTV dose escalation and improved organ at risk sparing (65).
Immunotherapy and radiotherapy
Immunotherapy is a rapidly evolving area of cancer medicine with immune checkpoint inhibitors receiving FDA approval in multiple malignancies, including melanoma, lung cancer, bladder cancer, kidney cancer, lymphoma, and Merkel cell carcinoma. However, pancreatic cancer is less immunogenic than many of the histologies that have responded well to immune checkpoint inhibition (66), and studies of single agent immune checkpoint inhibitors have not shown activity against pancreatic cancer (67,68). Royal et al. published a phase II study of single agent ipilimumab, an anti-CTLA-4 antibody, for patients with locally advanced or metastatic pancreatic cancer. Twenty-seven patients were enrolled (20 with metastatic disease and 7 with locally advanced disease) and of these 27 patients, none experienced an objective response, although one patient did exhibit a delayed response after initial progressive disease (68). Brahmer et al. conducted a multicenter phase I study of an anti-PD-L1 antibody against multiple advanced cancer histologies that included 14 patients with pancreatic cancer, and although responses were observed in patients with melanoma, renal cell cancer, non-small cell lung cancer, and ovarian cancer, no patients with pancreatic cancer were found to have an objective response to anti-PD-L1 therapy (67). Despite these early setbacks for immunotherapy in the management of pancreatic cancer, there is a growing interest in the utilization of immune checkpoint inhibition in combination with other therapies, including chemotherapy, vaccine therapies, and radiotherapy, as a mechanism to enhance the efficacy of systemic immunotherapeutics in non-immunogenic tumors. Numerous preclinical studies have shown that radiation induces the release of tumor antigens potentiating anti-tumor immunity (69-74), and in pancreatic cancer, specifically, radiation has also been shown to upregulate PD-L1 expression and synergize with ant-PD-L1 therapy in preclinical models (75).
Interestingly, there have also been case reports in the literature of radiation synergizing with immunotherapy in the form of the abscopal effect (response in tumors away from the irradiated site) (76,77), and early clinical trials exploring this concept have demonstrated promising results (78,79), Further, there are numerous ongoing clinical trials testing radiation therapy combined with immunotherapy for pancreatic cancer patients with resectable to metastatic disease (80,81). Incorporating radiation with immunotherapy has the potential to greatly influence how we manage pancreatic cancer patients in the future.
Finally, as our understanding of the role of the immune system in cancer control increases, it is also important that we aim to minimize the negative effect our therapies may have on a patient’s immune system. Specifically in the context of radiation therapy, one other potential advantage to smaller treatment fields utilized in pancreas SBRT, is that one would project this treatment to be less immunosuppressive than conventional radiation fields. In fact, Wild et al. compared the rates of radiation-induced lymphopenia (RIL) in LAPC patients receiving SBRT versus conventional chemoradiation (CRT), and found that SBRT was associated with significantly less severe RIL than CRT (82). Importantly, higher post-treatment total lymphocyte count was also associated with improved survival.
Conclusions
Pancreatic cancer is a devastating disease with historically poor outcomes, but there is extensive active research aimed to improve outcomes for pancreatic cancer patients. In all stages of disease, the role of radiation is evolving. Neoadjuvant SBRT appears to be a promising strategy to improve surgical outcomes and patient survival. Locally advanced unresectable disease presents a uniquely challenging situation, and approaches to safely administer dose-escalated SBRT for these patients may ultimately improve outcomes as well. Finally, immunotherapy in combination with radiation is an area of active research that may also impact the management of pancreatic cancer patients in future years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control 2006;17:403-9. [Crossref] [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10:1199-210; discussion 210-1. [Crossref] [PubMed]
- Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg 2006;10:1338-45; discussion 45-6. [Crossref] [PubMed]
- Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94. [Crossref] [PubMed]
- Willett CG, Lewandrowski K, Warshaw AL, et al. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993;217:144-8. [Crossref] [PubMed]
- Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651-60. [Crossref] [PubMed]
- Golcher H, Brunner TB, Witzigmann H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol 2015;191:7-16. [Crossref] [PubMed]
- Talamonti MS, Small W Jr, Mulcahy MF, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol 2006;13:150-8. [Crossref] [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [Crossref] [PubMed]
- Hong TS, Ryan DP, Borger DR, et al. A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma. Int J Radiat Oncol Biol Phys 2014;89:830-8. [Crossref] [PubMed]
- Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer 1987;59:2006-10. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 82-4. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet 2001;358:1565-6. [Crossref] [PubMed]
- Choti MA. Adjuvant therapy for pancreatic cancer--the debate continues. N Engl J Med 2004;350:1249-51. [Crossref] [PubMed]
- Koshy MC, Landry JC, Cavanaugh SX, et al. A challenge to the therapeutic nihilism of ESPAC-1. Int J Radiat Oncol Biol Phys 2005;61:965-6. [Crossref] [PubMed]
- Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol 2010;17:981-90. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 2.2017. Available online: www.nccn.org
- Campbell F, Smith RA, Whelan P, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009;55:277-83. [Crossref] [PubMed]
- Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855-62. [Crossref] [PubMed]
- Kinsella TJ, Seo Y, Willis J, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol 2008;31:446-53. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001;234:758-68. [Crossref] [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 46-8. [Crossref] [PubMed]
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151:e161137. [Crossref] [PubMed]
- Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997;15:928-37. [Crossref] [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Rajagopalan MS, Heron DE, Wegner RE, et al. Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer. Radiat Oncol 2013;8:254. [Crossref] [PubMed]
- Shaib WL, Hawk N, Cassidy RJ, et al. A Phase 1 Study of Stereotactic Body Radiation Therapy Dose Escalation for Borderline Resectable Pancreatic Cancer After Modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol Biol Phys 2016;96:296-303. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst 1988;80:751-5. [Crossref] [PubMed]
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592-9. [Crossref] [PubMed]
- Hazel JJ, Thirlwell MP, Huggins M, et al. Multi-drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: a prospective randomized trial. J Can Assoc Radiol 1981;32:164-5. [PubMed]
- Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol 1985;3:373-8. [Crossref] [PubMed]
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12. [Crossref] [PubMed]
- Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981;48:1705-10. [Crossref] [PubMed]
- Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326-31. [Crossref] [PubMed]
- Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007;110:47-55. [Crossref] [PubMed]
- Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71. [Crossref] [PubMed]
- Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys 2016;94:755-65. [Crossref] [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [Crossref] [PubMed]
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86. [Crossref] [PubMed]
- Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:181-8. [Crossref] [PubMed]
- Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005;76:48-53. [Crossref] [PubMed]
- Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol 2010;17:2092-101. [Crossref] [PubMed]
- Goyal K, Einstein D, Ibarra RA, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res 2012;174:319-25. [Crossref] [PubMed]
- Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol 2013;8:44. [Crossref] [PubMed]
- Tozzi A, Comito T, Alongi F, et al. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol 2013;8:148. [Crossref] [PubMed]
- Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011;81:e615-22. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol 2010;7:37-43. [Crossref] [PubMed]
- Foote RL, Stafford SL, Petersen IA, et al. The clinical case for proton beam therapy. Radiat Oncol 2012;7:174. [Crossref] [PubMed]
- Bouchard M, Amos RA, Briere TM, et al. Dose escalation with proton or photon radiation treatment for pancreatic cancer. Radiother Oncol 2009;92:238-43. [Crossref] [PubMed]
- Ding X, Dionisi F, Tang S, et al. A comprehensive dosimetric study of pancreatic cancer treatment using three-dimensional conformal radiation therapy (3DCRT), intensity-modulated radiation therapy (IMRT), volumetric-modulated radiation therapy (VMAT), and passive-scattering and modulated-scanning proton therapy (PT). Med Dosim 2014;39:139-45. [Crossref] [PubMed]
- Hsiung-Stripp DC, McDonough J, Masters HM, et al. Comparative treatment planning between proton and X-ray therapy in pancreatic cancer. Med Dosim 2001;26:255-9. [Crossref] [PubMed]
- Kozak KR, Kachnic LA, Adams J, et al. Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys 2007;68:1557-66. [Crossref] [PubMed]
- Lee RY, Nichols RC Jr, Huh SN, et al. Proton therapy may allow for comprehensive elective nodal coverage for patients receiving neoadjuvant radiotherapy for localized pancreatic head cancers. J Gastrointest Oncol 2013;4:374-9. [PubMed]
- Thompson RF, Mayekar SU, Zhai H, et al. A dosimetric comparison of proton and photon therapy in unresectable cancers of the head of pancreas. Med Phys 2014;41:081711. [Crossref] [PubMed]
- Nichols RC Jr, George TJ, Zaiden RA Jr, et al. Proton therapy with concomitant capecitabine for pancreatic and ampullary cancers is associated with a low incidence of gastrointestinal toxicity. Acta Oncol 2013;52:498-505. [Crossref] [PubMed]
- Takatori K, Terashima K, Yoshida R, et al. Upper gastrointestinal complications associated with gemcitabine-concurrent proton radiotherapy for inoperable pancreatic cancer. J Gastroenterol 2014;49:1074-80. [Crossref] [PubMed]
- Terashima K, Demizu Y, Hashimoto N, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol 2012;103:25-31. [Crossref] [PubMed]
- Noel CE, Parikh PJ, Spencer CR, et al. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol 2015;54:1474-82. [Crossref] [PubMed]
- Henke L, Kashani R, Yang D, et al. Simulated Online Adaptive Magnetic Resonance-Guided Stereotactic Body Radiation Therapy for the Treatment of Oligometastatic Disease of the Abdomen and Central Thorax: Characterization of Potential Advantages. Int J Radiat Oncol Biol Phys 2016;96:1078-86. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005;11:728-34. [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer 2004;109:685-90. [Crossref] [PubMed]
- Newcomb EW, Lukyanov Y, Kawashima N, et al. Radiotherapy enhances antitumor effect of anti-CD137 therapy in a mouse Glioma model. Radiat Res 2010;173:426-32. [Crossref] [PubMed]
- Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res 2003;63:8466-75. [PubMed]
- Azad A, Yin Lim S, D'Costa Z, et al. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med 2017;9:167-80. [Crossref] [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795-803. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Crittenden M, Kohrt H, Levy R, et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol 2015;25:54-64. [Crossref] [PubMed]
- Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016;4:51. [Crossref] [PubMed]
- Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2016;94:571-9. [Crossref] [PubMed]


