Immunotherapy of patients with metastatic melanoma
Background
The prognosis of advanced malignant melanoma (MM) is poor in past time, accounting for 75% of skin cancer related death (1). In the past 40 years, MM treatment was a comprehensive treatment pattern, including surgical resection in early stage, surgical resection plus adjuvant high dose interferon in advanced stage, dacarbazine (DTIC, standard drug) chemotherapy-based in non-resectable patients. However, response rate was less than 20% of single-agent chemotherapy drugs, median overall survival (OS) were only 2 to 8 months, 5-year survival rate was less than 10%, and prognosis was extremely poor (2).
The development of targeted therapy and immunotherapy, especially immunotherapy, has changed the situation.
Targeted therapy, such as BRAF inhibitors, increased the objective response rate (ORR) to about 50% in MM patients, and median progression-free survival (PFS) prolonged about 7 months, but most of patients were recurrence in 1 to 2 years due to acquired resistance via MAPK pathway activation (3). The prognosis improvement of another kind of targeted drugs, MEK inhibitor, was not obvious in the general population, but the benefits in metastatic MM with BRAF mutation was more significant (4). BRAF inhibitor plus MEK inhibitor in patients carried BRAF mutation became first-line therapy based on satisfactory performance of combination (5,6).
In 1998, high-dose interleukin-2 (HD IL-2) marked the first immunotherapy to enter the field of MM treatment. But HD IL-2 therapy was only appropriate for those patients with good physical status, good organ function, and light tumor burden in strict monitoring, due to toxic reactions, but PFS (about 6% to 8%) of minority patients could be prolonged significantly (7). And adoptive T cell therapy (ACT) and the tumor vaccine because of poor clinical efficacy gradually disappeared (8). With the understanding of tumor immune monitoring and escaping mechanisms, checkpoint inhibitors, including the pathogen death factor 1 (PD-1), cytotoxic T lymphocyte antigen 4 (CTLA-4) become the hot spots and new directions of immunotherapy.
In 2011, the checkpoint inhibitor, headed by CTLA-4 inhibitor Ipilimumab (IPI), was approved by the FDA to access the market and became a milestone in the treatment of advanced melanoma. In 2014, Nivolumab (NIVO), Pembrolizumab (PEMBRO) has also been approved by the FDA for advanced MM treatment. Compared with early chemotherapy or immunotherapy vaccines, checkpoint inhibitors significantly prolong OS and PFS, and reduce the risk of recurrence and mortality (9-12). The combination of Nivo and ezetum monoclonal antibody increased the ORR to 57% to 61% and the median PFS was prolonged to 11.5 months. Therefore, on October 1, 2015, the FDA approved the combined regimen for the treatment of unresectable or metastatic the MM (13,14).
At present, immunotherapy has become the first line of MM standard treatment. MM become a leader in the application of tumor immunotherapy, perhaps due to its “hot tumor” of the immune characteristics. “Hot tumor” means the tumor microenvironment within a large number of tumor-specific T cells or killer immune cells (e.g., CD8+ T cells), studies show that these tumors may long-lasting respond to inhibitor of PD-1, and anti-tumor effect is significant. In contrast, the microenvironment without tumor-specific T cell infiltration is called “cold tumor”. Mechanisms to avoid immune monitoring are more independent of immunological checkpoint molecules, but involving dendritic cells (DC) damage, so immunotherapy there may be invalid (15).
In view of the above differences, the researchers in the consideration of applying immunotherapy to other tumors, often need to identify whether the tumor is a hot tumor at first. But immunotherapy combined treatment once again broke through this restriction. It has been shown that CTLA-4 inhibitors increase the expression of tumor-infiltrating lymphocytes and interferon (IFN)-γ-induced genes in the tumor microenvironment followed by or in combination with PD-1 inhibitors, thus increasing PD-L1 expression of tumor cells to response PD-1 inhibitors, then “cold tumor” into “hot tumor” (16-19). The combination of immunotherapy clinical data in non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), metastatic colorectal cancer (mCRC) and other tumors support for this hypothesis.
The mechanism of combination immunotherapy
The importance of the immune response and immune escape in MM development process as early as 60 years ago, has been reported (20). The incidence is higher in immunosuppressed MM patients, whereas the infiltration of lymphocytes and tumor-specific antibodies is favorable prognostic factors of MM (21,22). These two evidences further suggest the complex relationship between MM and the immune system.
There is a dynamic balance between the immune system and the tumor cells. According to the theory of immune editing 3E, this equilibrium is divided into three stages: clearance, balance and escape. In the clearance phase, tumor antigens can be treated/delivered, activated T cells proliferate and migrate with effector cells in large numbers, and effectively kill tumors; in the equilibrium phase, the immune system cannot completely kill all tumor cells, but can still control or prevent further growth of the tumor; while in the escape phase, tumor cells may use different ways to shut down the immune response, and thus evade immune destruction (23).
Immunological checkpoint molecules play a key role in the process of tumor escape immune surveillance. PD-1 can inhibit T cell activation by binding to its ligand PD-L1 or PD-L2. CTLA-4 and antigen-presenting cells (APC) surface of CD80 and CD86 binding, T cells can also be inactivated. By antibody competitive inhibition of PD-1 or CTLA-4 can block these mechanisms, thus enhancing the cytotoxic activity of T cells (24). CTLA-4 pathway mainly acts on T cells-APC, which affects the activation and effect of T cells. The PD-1 pathway mainly acts on tumor cells—activated lymphocytes, reduces the degree of activation and cytotoxicity of T cells. They are mutual influential but relatively independent (Figure 1). Therefore, the combination of the two dual inhibition may play a synergistic role for one of the ligand/receptor negative patients, combination therapy may also onset (24).
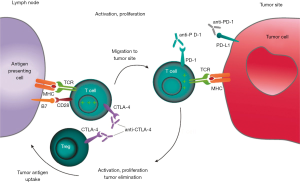
Another mechanism for the feasibility of combination therapy is the reversal of cold/heat tumors. “Hot tumors” are tumors which tumor-infiltrating lymphocytes presenting in the tumor microenvironment. Their immune escape mechanisms usually include upregulation of the molecules at the immunological checkpoint, upregulation of indoleamine 2,3-dioxygenase (IDO), the recruitment of regulatory T cells (Treg), and the absence of surface antigen expression. Therefore, such tumors often have a good response to PD-1 and CTLA-4 inhibitors. Cold tumor is the lack of lymphocyte infiltration, PD-L1 expression, in the immune escape process of the lack of innate immunity of the host recognition process, but also ineffective T cell recruitment, so the PD-1 inhibitors generally no response. But the study found, CTLA-4 inhibitor used in the "Cold tumors", by recruiting and fully activated CD3+/CD4+ T cells and CD3+/CD8+ T cells increased lymphocytes in the tumor microenvironment infiltration, increased interferon (INF)-γ induced gene expression, thereby regulated microenvironment PD-L1 expression levels of tumor cells to PD-1 inhibitors response possible (15-19).
Combination immunotherapy in melanoma
Basic research period
Animal model studies conducted in rats in 2010 showed that double blocking caused by CTLA-4 and PD-1 pathway blockade could greatly enhance their respective antitumor effects. The combination of PD-1 and CTLA-4 inhibitors reduced tumor volume in rats by 65%, whereas CTLA-4 inhibitors alone subsided only 10% of tumors. Combined therapy can enhance the tumor response, increase the number of T cells, and reduce the number of Tregs in the tumor microenvironment, so that the T cell-Treg ratio in tumor cells reaches the optimal state. IFN-γ expression in tumor and lymph node sites is increased and IFN-γ/THF-α double secreting CD8+ T cells in a corresponding increase in the frequency of occurrence, suggesting that PD-1/CTLA-4 dual inhibition of tumor-specific T cells and effect cells to function, the tumor microenvironment by the infiltration of lymphocytes into immune suppression and immune clearance status (26).
Clinical data of combination immunotherapy
Phase I studies in patients with advanced MM showed that NIVO + IPI combined treatment had an ORR of 43% and a 1- and 2-year OS rates of 85% and 79%, respectively. A significant increase incidence in grade 3/4 treatment-related adverse events (TRAE) versus monotherapy (>60%) was observed, but AE types are similar with IPI monotherapy, and AE can be controlled effectively through early corticosteroids or immune modulator medication (27).
CheckMate-069 II of the study (14) compared the combination therapy (IPI 3 mg/kg Q3W + NIVO 3 mg/kg Q2W) with single-agent therapy in the BRAF wild-type patients, results showed that combination therapy ORR, complete remission (CR) rate, median PFS were significantly improved. The ORR was 61% in the combined treatment group and 11% in the IPI + placebo group. 16 patients (22%) achieved CR in the combination group and no CR in the IPI monotherapy group when the study was published, the combined treatment group had not reached the median PFS, while the IPI monotherapy group was 4.4 months. The results of this Phase II study motivated researchers to carry out an expanded Phase III study.
CheckMate-067 Phase III study (13) is still encouraging, and FDA approved the use of combination therapy in patients with advanced MM in. The study showed that IPP combined with NIVO was used in newly diagnosed III/IV MM patients with mPFS of 11.5 months, significantly longer than IPI monotherapy (2.9 months, P<0.001) or NIVO monotherapy (6.9 months, P<0.001) The Patients with PD-L1 expression were more likely to benefit from combination therapy. The incidence of TRAE was grade 55% in combination therapy group and 27.3% and 16.3% in IPI or NIVO group, respectively.
Other studies of PD-1 inhibitors and CTLA-4 inhibitors in MM have been ongoing, as IPI is the first and only publicly licensed CTLA-4 inhibition in most studies So the vast majority of combined regimens include IPI. The Keynote-022 preliminary data was announced on American Society of Clinical Oncology (ASCO) conference in 2016 (28), IPI 1 mg/kg + PEMBRO 2 mg/kg Q3W treatment ORR reach 57%, of which 10% (15/153 cases) of patients reached CR. 6 months PFS rate of 70%, OS rate of 93%. To the data reported, the median patient PFS has not yet reached, 98% of the relief continues.
Another widely discussed case of combination therapy is Wolchok doctors, etc. (29) published in 2014, a case report. One case of postoperative recurrence of MM female patients with only IPI + NIVO combination therapy, the lower part of the breast to the size of grapefruit tumors in 3 weeks completely disappeared, leaving only a necrotic tumor tissue formation of the residual cavity. This extremely intuitive, significant anti-tumor effect is remarkable.
Treatment thinking of combination immunotherapy in melanoma
Crowd selection
A suitable population for immunotherapy has been continually exploring changes based on clinical research data. Early studies have shown that BRAF mutations benefit from immunosuppressive therapy in patients with less pronounced, and the indications for CTLA-4 and PD-1 inhibitors are confined to BRAF wild-type patients, while NCCN guidelines recommended BRAF/MEK inhibitor combination therapy for mutant patients. However, CheckMate-067 studies have shown that (13), regardless of BRAF mutation status, advanced MM patients can benefit from IPI plus therapy or NOVO monotherapy significantly. Therefore, the FDA extended the NIVO indications to BRAF-positive MM patients in 2016. The combination of immunotherapy in this patient group is also very promising. In addition, in 2015, the European Society for Medical Oncology (ESMO) MM treatment guidelines (30) for patients with BRAF mutations recommendation has been controversial. ESMO guidelines that although PD-1 inhibitors ORR in these patients is less than the targeted combination therapy, but the anti-tumor effect is more durable, and therefore PD-1 inhibitor based combination therapy is a considerable option (30).
PD-L1 expression status earlier is also an important indicator of the prognosis of combined therapy. Previous studies have shown that PFS with NIVO monotherapy may be similar to combination therapy (median about 14 months) in patients with positive PD-L1 expression. Of course, the combined tumor volume may be significantly reduced compared with monotherapy, but accordingly, the incidence of drug-related adverse events in combination therapy is also higher than monotherapy. For PD-L1-negative patients, the effectiveness of combination therapy was more pronounced. Therefore, it is generally believed that PD-L1 expression in patients with positive, should be based on individual patient status analysis, weighing the benefits and risks of medication, and for PD-L1 negative, and adverse reactions controllable patients, the author think combined immunotherapy may be more beneficial. However, CheckMate-067 study (13) in PD-L1-positive patients receiving combination therapy ORR and short-term prognosis was significantly better than monotherapy NIVO, this result gives researchers more thinking and enlightenment. In view of this, I believe that for patients with PD-L1-tolerant treatment, whether to consider the combination of treatment is worth, perhaps CheckMate-067 study of long-term follow-up data can give us more answers.
Treatment programs
CTLA-4 inhibitors are commonly used in combination with PD-1 inhibitors in patients with melanoma, although the efficacy is significant, but also increases the risk of severe AE. Therefore, the researchers have been thinking and exploring the optimal treatment model, cannot affect the effectiveness of the patient at the same time try to reduce the side effects.
CheckMate-064 study (31) evaluated the efficacy and safety of NIVO followed by IPI or IPI followed by NIVO therapy in patients with advanced MM. The study included 140 patients with newly diagnosed or previously treated systemic anti-tumor therapy, ECOG PS score of 0 to 1, unresectable MM patients. Patients were randomized to receive the following induction therapy at 1:1 ratio: NIVO 3 mg/kg Q2W ×6, followed by IPI 3 mg/kg Q4W ×4 (n=68); IPI followed by NIVO treatment n=70). At the end of induction therapy, patients still in use may continue to receive NIVO Q2W maintenance until disease progression (PD) or persistent adverse drug reactions are not tolerated. According to the results, it was presently believed that NIVO followed by IPI was used in the induction phase, with ORR of 41.2% (95% CI: 29.4% to 53.8%) in NIVO followed by IPI group and 20% in IPI followed by NIVO group at week 25% (95% CI: 11.4% to 31.3%). The AE of the two scenarios is similar. Therefore, studies suggest that NIVO induces followed by IPI or is the best treatment regimen for this stage.
However, with the combination therapy had a large Phase II/III study compared (13,14), AE incidence for sequential treatment seem similar with simultaneous administration, but less effectiveness than simultaneous administration. Because it is not a head-to-head comparison, this inference requires more prospective research to confirm. However, in clinical decision-making, for patients who can tolerate, clinicians may be appropriate to consider whether to consider the use of simultaneous dosing regimen.
The prospect of immunotherapy in other tumors
Research status quo
Unlike the mechanisms of many molecular-targeted drugs, the cytotoxicity of the immunological checkpoint inhibitor to the tumor is usually independent of a particular mutation and specific histological type, so the investigator has found that for other refractory tumors other than MM, Immunotherapy combined therapy may also play its unique role. The corresponding clinical studies were followed and echoed with the great success of MM in immunotherapy, and a number of studies in other tumors were also encouraging and continued to expand in the late stages of the combined treatment.
The interim results of phase I study(CheckMate-012) showed that ORR reached up to 22% with NIVO plus IPI in refractory NSCLC patients (32). Investigator reported the latest cohort results of CheckMate-012 on 2016 ASCO conference (33), IIIB/IV NSCLC, previously untreated patients were randomly divided into three groups: NIVO 3 mg/kg monotherapy (n=52); NIVO 3 mg/kg Q2W + IPI 1 mg/kg Q6W (n=39); NIVO 3 mg/kg Q2W + IPI 1 mg/kg Q12W (n=38). Three groups of patients had ORR of 23%, 39% and 47%, respectively. The median PFS was 3.6 (2.3–6.6), 3.9 (2.6–13.2) and 8.1 (5.6–13.6) months, respectively. The results suggest that regardless of PD-L1 expression status, combined therapy can benefit patients.
A Phase Ib study (Study-006) (34) also showed that tremelimumab (CTLA-4 antibody) and durvalumab (PD-1 antibody) combination therapy can make locally advanced or metastatic NSCLC patients benefit which is independent of PD-L1 expression. With combination treatment, ORR reached up to 23% in patients, of whom PD-L1 positive patients were 22% (6/26) and negative patients were 29% (4/14), respectively.
Keynote-021 study (35) included IIIB by ≤2 kinds of programs relapse after treatment of 11 cases/IV NSCLC were given PEMBRO + IPI Q3W ×4 combination therapy followed by maintenance therapy using PEMBRO. Treatment was observed in 11 patients in each dose group at 6 weeks of treatment, including 1 case of CR (9%) and 5 patients with partial remission (PR, 45%); all patients received disease control. The results suggest that IPI + PEMBRO combined therapy for patients with recurrent NSCLC has acceptable toxicity and potent antitumor activity; the use of lower doses of IPI and PEMBRO does not affect the effect and the risk of adverse events is reduced.
In addition, NIVO + IPI as an adjunct to platinum-based chemotherapy for first-line treatment of NSCLC (CheckMate-227) is also ongoing.
Salvage treatment is often limited for SCLC patients who failed platinum chemotherapy, The progress almost stagnant in the systemic treatment of SCLC in two decades. Recently a phase I/II Study (ChakeMate-032) (36) conducted in relapsed SCLC patients, suggest that, NIVO combination therapy with IPI may be a glimmer of hope for the new refractory/relapsed SCLC patients. NIVO 1 mg/kg + IPI 3 mg/kg (N1 + I3, n=61); NIVO 3 mg/kg single dose (N3 group, n=98); NIVO 1 mg/kg + 3 mg/kg + IPI 1 mg/kg (N3 + I1, n=54). The results showed that the ORR of the above three groups were 10%, 23% and 19%, respectively. The 1-year PFS rate was 19% in the N1 + I3 group, and 2 patients achieved CR. The 1-year PFS of the NIVO monotherapy group was 11%. The results show that combination therapy and NIVO monotherapy can achieve sustained antitumor activity in patients with recurrent SCLC, and the combination therapy has a better therapeutic effect and both are safe to manage.
Previous evidence has supported the use of NIVO in patients with high satellite instability (MSI-H) mCRC. Recently, a phase II study (CheckMate-142) explored the efficacy and safety about IPI plus NIVO combination therapy in mCRC patients with MSI-H (37). Fifty-nine patients with MSI-H were randomized to receive NIVO 3 mg/kg Q2W (N3), or NIVO 3 mg/kg + IPI 1 mg/kg Q3W (N3 + I1) ×4 followed by treatment with N3 until PD or for other reasons Stop. The results showed that the ORR of N3 group and N3 + I1 group were 27% and 15% respectively; PFS rates were 55% and 80% in 4 months respectively; 5 months OS rate was 75% and 100% respectively; PFS and OS were 5.3 and 16.3 months, respectively, while the N3 + I1 group did not reach the report. Studies have confirmed that most patients with mCRC can tolerate NIVO monotherapy with NIVO + IPI in patients with MSI-H mCRC, the two programs obtained clinical efficacy and survival data are encouraging. The study is still ongoing.
In addition to the association between check point inhibitors, checkpoint inhibitors and vaccines, targeted drug research is also ongoing, the results of different studies. PD-1 inhibitors combined with MEK inhibitors in advanced CRC patients with ORR of 20%, Phase III clinical studies are being prepared; a Phase I clinical trial about tyrosine kinase inhibitors (TKI) combined with PD-1 antibodies in metastatic renal cells cancer also showed that the overall response rate was 40% to 50%; the PD-1 inhibitor/paclitaxel combination also produced a response rate of 38% in the late triple-negative breast cancer. However, the combination of PD-1 inhibitors and another promising immunostimulant OX40 agonist, MOXR0916, was disappointing, with only 2 of the 28 patients enrolled in PR; a PD-1/TKI (EGF816) Of the combined protocol failed to meet expectations; another BRAF inhibitor combined with anti-CTLA-4 inhibitor treatment of clinical trials terminated due to liver toxicity. Therefore, there are still many possible directions for the future application of the combined use of the combined therapy, and there is still a long way to go.
Treatment thinking
Combination therapy has a good effect in a variety of tumors, however, because the combination therapy is still a new, is still exploring the treatment, clinical applications, there are many problems worth thinking about. Such as whether the dose of immunotherapy combined need to be fixed, whether the need for patients based on the characteristics, including clinical features and biological characteristics to develop targeted dose, how to balance the immune treatment combined with the relationship between the toxicity and efficacy. Whether the combination of immunotherapy and chemotherapy/targeted therapy can also be expanded need to carry out further clinical research.
Immunotherapy combined with chemotherapy/targeted therapy are going to be explored in MM and other solid tumors such as NSCLC, SCLC, mCRC, breast cancer, etc. It is possible to improve the efficacy, prolong survival and give a key direction in the future.
For the combination of immunotherapy and chemotherapy, the appropriate combination of the best combination of the program and the order is not determined. Recent studies have shown that some chemotherapeutic drugs are actually caused by necrosis or apoptosis via enhancing the immune response to the tumor, rather than directly killing. Such “immune tumor cells” can activate local infiltrating T cells and DC through purine receptors or Toll-like receptor-4 (TLR-4), respectively, and thus induce such deadly cytotoxic drugs to activate antitumor immune responses, has become the best choice combination therapy (38). Because different cytotoxic drugs have different immune activation mechanism, and different chemotherapy drugs may also make patients in different time of immunosuppression state, so the optimal strategy is a very sophisticated choice, based on the selected chemotherapy drugs and immunotherapy. At the same time, the study also found that, for effective cross-antigen presentation, TLR ligand and antibody in the same position is very important, immunotherapy and chemotherapy arrangements may affect the body of this dynamic process. To date, there is not enough research to summarize the optimal options and treatment arrangements for checkpoint inhibitors combined with chemotherapy, and more animal models and small clinical studies may be helpful to us.
Because of the different mechanisms of immunotherapy and targeted therapy, the combination of the two strategies cut both ways. The combination of the two is theoretically reasonable and may have stronger anti-tumor effect compared with the single drug. Targeted therapy, with genomics guidance and driven mutations identification, may have a significant effect in most patients with target mutations/target lesions, but due to the instability of the tumor’s internal genome resulting in multiple acquired resistance and the complexity of the target spectrum, the effective time of a single gene targeting strategy is often very short; on the contrary, the check point inhibitor can enhance the anti-tumor response of T cells, the mechanism of action affect multiple pathways and targets, and working in many parts of the body (such as tumor tissue and lymphocytes). Patients usually can get long-term response, but only for some patients effective. According to the above synergies, it is believed that the combination of immunotherapy and targeted therapy in the near future will continue to be the direction of the researchers’ efforts. In-depth understanding of how different targeted drugs affect the function of the immune system and the effect of immunotherapy can help the drug choice, followed by the focus on strategies for drug toxicity, eligible dose and (or) time of administration (39).
Conclusions
The combination of immunotherapy is currently beneficial in MM applications, providing new options for the treatment of MM patients, and also posing a number of questions to the treatment, such as: in order to benefit the patients most, how to optimize the treatment model, how to optimize the treatment model, how to choose the appropriate treatment of the crowd, how to balance the risk of patients, etc., these issues need more research to give us the answer.
At the same time, the combination of immunotherapy in other types of tumors also cut a striking figure for cancer treatment provides an encouraging new direction. However, due to the limited clinical data available, more studies are needed to prove efficacy and to answer more clinical questions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science 2014;346:945-9. [Crossref] [PubMed]
- Khayat D, Bernard-Marty C, Meric JB, et al. Biochemotherapy for advanced melanoma: maybe it is real. J Clin Oncol 2002;20:2411-4. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [Crossref] [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomized controlled trial. Lancet 2015;386:444-51. [Crossref] [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [Crossref] [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [Crossref] [PubMed]
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol 2006;90:51-81. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol 2016;28:383-91. [Crossref] [PubMed]
- Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012;61:1019-31. [Crossref] [PubMed]
- Taube JM, Young GD, McMiller TL, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res 2015;21:3969-76. [Crossref] [PubMed]
- Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PloS One 2014;9:e87705. [Crossref] [PubMed]
- Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD-1 and other interest for clinical application. Nat Immunol 2013;14:1212-8. [Crossref] [PubMed]
- Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg 1956;144:366-83. [Crossref] [PubMed]
- Greene MH, Young TI, Clark WH Jr. Malignant melanoma in renal-transplant recipients. Lancet 1981;1:1196-9. [Crossref] [PubMed]
- Clemente CG, Mihm MC Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996;77:1303-10. [Crossref] [PubMed]
- Disis ML. Mechanism of action of immunotherapy. Semin Oncol 2014;41 Suppl 5:S3-13. [Crossref] [PubMed]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561-84. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reducing regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275-80. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Long GV, Hamid O, Hodi FS, et al. Phase 2 study of the safety and efficacy of pembrolizumab (pembro) in combination with dabrafenib (D) and trametinib (T) for advanced melanoma (KEYNOTE-022). J Clin Oncol 2016;34:abstr TPS9596.
- Chapman PB, D'Angelo SP, Wolchok JD. Rapid eradication of a bulky melanoma mass with one dose of immunotherapy. N Engl J Med 2015;372:2073-4. [Crossref] [PubMed]
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v116-25. [Crossref] [PubMed]
- Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomized, phase 2 trial. Lancet Oncol 2016;17:943-55. [Crossref] [PubMed]
- Antonia SJ, Gettinger SN, Chow LQ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 2014;32:abstr 8023.
- Hellmann MD, Gettinger SN, Goldman JW, et al. CheckMate 012: Safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. J Clin Oncol 2016;34:abstr 3001.
- Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumur activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. [Crossref] [PubMed]
- Patnaik A, Socinski MA, Gubens MA, et al. Phase 1 study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort D. J Clin Oncol 2015;33:abstr 8011.
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Overman MJ, Kopetz S, McDermott RS, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol 2016;34:abstr 3501.
- Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin conscious dictates the immunogenicity of cancer cell death. Nat Med 2007;13:54-61. [Crossref] [PubMed]
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205-14. [Crossref] [PubMed]


