Comparison of short-term outcomes between laparoscopic-assisted and open complete mesocolic excision (CME) for the treatment of transverse colon cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide (1), which is a leading cause of cancer death (2). Complete mesocolic excision (CME) is considered to be the standard CRC surgeries due to it can reduce local recurrence and improve long-term survival (3). Since the first application of laparoscopic surgery in 1991 (4), laparoscopic technique has been introduced to many surgical fields such as the treatment of CRC with the advanced laparoscopic instruments and technique (5). Previous studies have reported that LCME is a superior approach for CRC benefit from less pain and blood loss, shorter recovery and hospital stays, better cosmetics and short-term outcomes, and reduced morbidity (6,7). However, there was no significant difference in disease-free survival of patients between LCME and OCME in a larger trials (8). Presumably because of the low incidence and technical difficulty of laparoscopic CME in transverse colon cancer (9) including safely take down and fully mobilization of the splenic flexure and hepatic flexure, large separating surface and high-precision technique (10), there are few studies available on it (11).
In this study, we aim to compare the short-term outcomes of the CME for transverse colon cancer between laparoscopic and open approaches and to identify the safety and feasibility about LCME.
Methods
Patient selection
We retrospectively collected the clinical data of patients with transverse colon cancer between October 2014 and February 2016. All participants gave informed written consent. The research protocols were approved by the ethical committee of our hospital (2014-SRFA-188). Transverse colon cancer was defined as the tumor at 1/3 in the middle of transverse colon. Transverse colon cancer was confirmed by a routine biopsy, colonoscopy, computed tomography (CT) scans and magnetic resonance imaging (MRI). MRI was used to identify the suspicious liver metastatic. A total of 78 patients were included in this study. Both LCME and OCME were treatments for transverse colon cancer and the choice of surgical approach was based on patient’s wishes after the surgical procedures, possible risks and discomforts of these two approaches were informed to patients. Among the 78 patients, 39 patients underwent LCME and the remaining 39 patients underwent OCME. Exclusion criteria for CME were as follows: (I) Patients with severe cardiac, pulmonary insufficiency and can not tolerate surgery after active treatment; (II) recurrent cancer patients after operation; (III) patients with tumor involving other organs; (IV) patients with tumor perforation or acute obstruction; (V) patients with secondary CRC; (VI) patients undergoing LCME converted to OCME; (VII) patients with tumor involving adjacent tissues and organs and can not be cured by resection; (VIII) patients with non-adenocarcinoma.
Surgical techniques
Preoperative evaluations
Before surgery, routine examination including blood, coagulation, blood type, biochemical tests, electrocardiography (ECG), five hepatitis B, HIV, HCV, RPR, urine, stool, colonoscopy, chest and abdomen enhanced CT, and MRI were performed to make definite diagnosis and exclude surgical contraindications. Before surgery, all patients were fasted for 12 h, forbidden to drink for 4 h. On the evening before surgery, all patients took polyethylene glycol electrolyte powder solution for bowel preparation. All the surgeries in both groups were performed with the same surgeon. Endotracheal intubation was performed under general anesthesia and routine catheterization. All patients were placed in supine position with legs separated and fixed. The surgeon stood to the left side of the patient, the first assistant stood to the right side of the patient, while the second assistant holding a mirror stood between the two legs of patients.
LCME
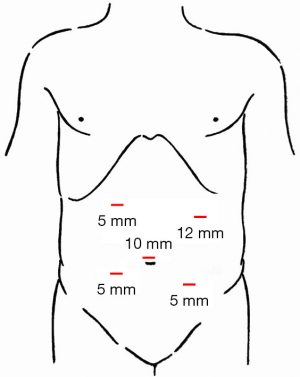
Five-trocar method (Figure 1) was applied in CME. After routine abdominal exploration, procedures were performed with a median-to-lateral approach. Below the horizontal part of duodenum, we dissected the serous membrane, and dissected the sheath of superior mesenteric vein (SMV) (Figure 2A). Dissection was proceeded along the SMV. After entering the sub-fascial space of the pancreatoduodenal fascia, we dissect the superior mesenteric artery (SMA), removed connective tissues and lymph nodes in front of SMA, then enter the right Toldt’s gap, followed by a dissection extending upward to the hepatic flexure ligament of the colon and outward until the lateral fusion fascia of ascending colon. After dissecting the middle colic artery along with SMV, we removed the lymph nodes following by the division of the middle colic artery from the root after the (Figure 2B). Thereafter, we dissected the Henle’s trunk, and divided the right colic vein (Figure 2C) with the preservation of right gastric-omentum vein. After dividing the middle colic vein, we divided the lower edge of pancreas and anterior pancreatic space (Figure 2D), and separated the space between pancreatic and gastric. The surgeon exchanged the position with the first assistant. Then we pulled out the inferior mesenteric vein and dissected the serous membrane and extended upward to the lower edge of pancreas, and downward to the root region of the inferior mesenteric artery. Entered the left side of Toldt’s gap with a dissection extended upward the lower edge of the pancreas tail and outward until the lateral fusion fascia of descending colon. Then the upper region of colon was steered.
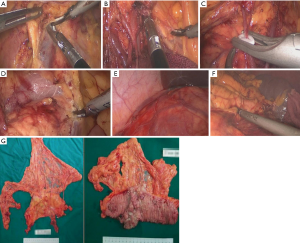
After opening the gastrocolic ligament, we resected the greater omentum. Then we divided the pancreas attachment of the root region of right side of the transverse mesocolon, cut off the hepatic flexure ligament of colon (Figure 2E), divided the outside fusion fascia of ascending colon and converged into the inferior separation of gap. Thereafter we divided the pancreas attachment of the root region of the left side of the transverse mesocolon root, resected the ligament of splenic flexure (Figure 2F), divided the outside fusion fascia of descending colon and converged into the inferior separation of gap. Through above procedures, we completely separated the transverse colon, hepatic flexure, ascending colon, descending colon and splenic flexure. Finally, the resected specimen was obtained (Figure 2G).
OCME
A 5-cm incision protected with the protective sleeve made on the upper abdominal midline. After pulling out the transverse colon in vitro, the classic transverse CME techniques were conducted.
Postoperative treatment
Conventional ECG monitoring was conducted in all patients for 12 hours. Three days after surgery, all patients were routinely given treatment including supplement of body fluid, anti-infection, acid suppression, maintain the balance of acid-base and water electrolyte. On the first postoperative day, the motion of patients was encouraged. Approximately 3 days after surgery, a liquid diet was begun after first flatus which was allowed to transition to a normal diet. On the third postoperative day, blood and biochemical tests were performed to all patients, patients who were anemia or hypoproteinemia were given a blood transfusion, albumin transfusion therapy respectively. On the first or second postoperative day, the catheter was removed. A measurement of drainage fluid and observation characters was performed and the drainage tube was removed on the fifth or sixth postoperative day.
Clinical characteristics, including operation time, intra-operative blood loss, length of incision, number of harvested lymph nodes and length of resected specimen were compared between the LCME and OCME groups.
Short-term surgical outcomes including first flatus, first postoperative motion/movement, postoperative hospitalization time and complications were compared between the LCME and OCME groups.
Statistical analysis
Data were analyzed by using SPSS for Windows (version 18.0). We used chi-square test to compare categorical values between LCME and OCME group. An independent t-test was applied to compare measurement data between each two groups. Quantitative data are expressed as means ± standard deviations (SD), and measurement data are expressed as n or percentage. P values of less than 0.05 were considered statistically significant.
Results
Baseline characteristics
There were 39 cases in LCME group and 39 cases in OCME group, respectively. In LCME group, 21 were men and 18 were women, with a mean age of 58.3±5.8 years (range, 26–84 years). The distribution of tumors according to TNM stage was as follows: Stage I in 4 patients, Stage II in 16, Stage III in 19. In OCME group, 20 were men and 19 were women, with a mean age of 57.5±6.9 years (range, 28–85 years). TNM stage was as follows: Stage I in 3 patients, Stage II in 15, Stage III in 21 (Table 1). There was no statistical significant difference regarding age, gender and TNM stage between the two groups.

Full table
Perioperative outcomes
LCME group were significantly better than OCME group (P<0.05) in intra-operative blood loss (61.6±18.7 vs. 115.4±35.4), length of incision (7.3±1.8 vs. 18.7±4.7), time to first flatus (1.5±0.6 vs. 3.2±0.9), and first postoperative ambulation (1.3±0.4 vs. 2.9±0.8). There were no significant differences in operation time (119.7±27.5 vs. 128.6±30.1), postoperative hospitalization time (8.1±2.9 vs. 10.1±2.2), number of harvested lymph nodes (16.2±3.1 vs. 15.1±3.5), and length of resected specimen (26.5±5.4 vs. 24.8±4.9) between LCME group and OCME group (Table 2).
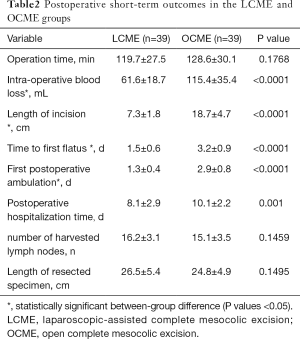
Full table
There was no significant difference in the total incidence of short-term postoperative complications between LCME and OCME groups (12.81% vs. 15.38, P>0.05). However, the incidence of wound infection was significantly lower with laparoscopy (2.56%) than by the open approach (7.69%). Lymphatic leakage, anastomotic leakage, urinary tract infection and wound dehiscence were occurred in 1 group or both 2 groups, there was no significant difference in the incidence (Table 3). None of patients in both groups developed urinary retention, anastomotic bleeding and intestinal obstruction.
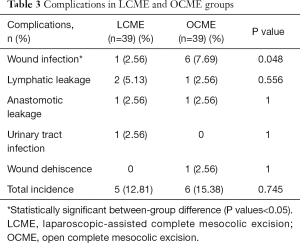
Full table
Discussion
Although previous studies have demonstrated that LCME is a safe and feasible treatment for CRC (6,7,10), the majority of trials excluded cases of transverse colon cancer due to the technical difficulty (10,12,13). Our study compared the feasibility, safety and short-term outcomes between LCME and OCME groups.
In terms of intra-operative outcomes, the length of incision was significantly shorter and the blood loss was less in LCME group than OCME group which suggested that LCME is more precise. Although there was no significant difference in operation time between these two groups, the operation time in LCME is shorter. It suggested that we can overcome the technical difficulty, shorten the operative time and be proficient in LCME. In our study, the numbers of lymph nodes retrieved in these two groups were similar. We can conclude that not only LCME but also OCME have the same oncologic clearance effects.
Previous studies have demonstrated that the postoperative hospitalization time in LCME for CRC is always shorter (7,14). Since the technical difficulty there was no significant difference in postoperative hospitalization time between these two groups, it was still shorter in LCME. In addition, LCME was found to be better in time to the first flatus and first postoperative ambulation after surgery. It suggested that recovery of patients in LCME was faster.
Similar or reduced complications were always reported in LCME for right colon cancer (7,15-18). There was no significant difference in the total incidence of short-term postoperative complications between these two groups in our study. A systematic review and meta-analysis have reported that complications were similar in LCME and OCME for transverse colon cancer, which supported our results (15). However, the incidence of wound infection was significantly less with laparoscopy due to the shorter incision. So LCME is as safe and feasible as OCME.
Due to the high-definition vision, amplification effect of laparoscope, and with the application of ultrasonic knife, laparoscopic surgery can be applied to separation of surgical surfaces, based on the similar operative effects, the length of incision in LCME is shorter and it’s helpful to reduce the blood loss and recover faster.
Moreover, no tumor metastasis and recurrence is crucial for prognosis of CRC. To prevent the metastasis and local recurrence of CRC, intraperitoneal chemotherapy was proposed. Cytoreductive surgery and intraperitoneal chemotherapy may significantly increase the exposure of cancer drugs to cancer present within the peritoneal cavity or liver which may be helpful to repress the metastasis and local recurrence of CRC and are closely associated with prolonged overall survival and even cure of CRC (19-21). In our study, to prevent the formation of the incision planter and further implement the principle of non-contact, we removed the tumor with the incision was protected by protective sleeve. To prevent the formation of planter induced by direct contact between the polluted laparoscopic instruments and the puncture hole, the trocar was fixed to the abdominal wall. To prevent the formation of incision planter induced by intraperitoneal gas which carried tumor cells, we removed the trocar after the discharge with the puncture sheath in the end of surgery. In addition, Kim et al. has reported that central ligation of the main feeding vessels, complete removal of the mesocolon with sharp dissection, and adequate proximal and distal margins were benefit for improved oncologic outcomes. In our study, we resected both ends of the 10cm bowel of tumor, dissected the lymph nodes of mesenteric roots and removed the whole transverse mesocolon to manage for infrapyrolic node or splenic node metastases.
To ensure the security and effectiveness of CME and repress tumor metastase, several key points were concluded from our operative experiences in CME. These principles were as follows: (I) Explore the liver, spleen, stomach, pelvic, mesenteric root, tumor and adjacent organs from far to near; (II) resect adequate bowel (both ends of the 10 cm bowel of tumor) and dissect the lymph nodes of mesenteric roots; (III) separate the Toldt’s gap accurately, maintain the integrity of Toldt’s fascia and resect the lesions bowel and mesentery from roots completely; (IV) apply median- to-lateral approach, ligate the mesenteric vessels on the root firstly and then performed resection and dissociation; (V) precise and gentle operating practice.
Last but not least, LCME was reported to reduce the postoperative mortality and to have more clinical significance especially in elderly patients (22). The main drawback in the present study is the deficiency of long-term oncological follow-up and need further research.
In conclusion, despite the technique difficult in surgery, LCME is more safe and feasible for the treatment of transverse colon cancer because it could provide better short-term outcomes including less blood loss and faster recovery compared to that of OCME. Further studies with longer term of follow up are required to confirm the efficacy of LCME/ confirm our findings
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All participants gave informed written consent. The research protocols were approved by the ethical committee of our hospital (2014-SRFA-188).
References
- Zhang GJ, Zhou T, Tian HP, et al. High expression of ZEB1 correlates with liver metastasis and poor prognosis in colorectal cancer. Oncol Lett 2013;5:564-8. [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA-CANCER J CLIN 2015;65:87-108. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 2009;11:354-64; discussion 364-5. [Crossref] [PubMed]
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144-50. [PubMed]
- Kim WR, Baek SJ, Kim CW, et al. Comparative study of oncologic outcomes for laparoscopic vs. open surgery in transverse colon cancer. Ann Surg Treat Res 2014;86:28-34. [Crossref] [PubMed]
- Gao F, Cao YF, Chen LS. Meta-analysis of short-term outcomes after laparoscopic resection for rectal cancer. Int J Colorectal Dis 2006;21:652-6. [Crossref] [PubMed]
- Pecorelli N, Amodeo S, Frasson M, et al. Ten-year outcomes following laparoscopic colorectal resection: results of a randomized controlled trial. Int J Colorectal Dis 2016;31:1283-90. [Crossref] [PubMed]
- Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44-52. [Crossref] [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Okuda J, Yamamoto M, Tanaka K, et al. Laparoscopic resection of transverse colon cancer at splenic flexure: technical aspects and results. Updates Surg 2016;68:71-5. [Crossref] [PubMed]
- Hahn KY, Baek SJ, Joh YG, et al. Laparoscopic resection of transverse colon cancer: long-term oncologic outcomes in 58 patients. J Laparoendosc Adv Surg Tech A 2012;22:561-6. [Crossref] [PubMed]
- Agarwal S, Gincherman M, Birnbaum E, et al. Comparison of long-term follow up of laparoscopic versus open colectomy for transverse colon cancer. Proc (Bayl Univ Med Cent) 2015;28:296-9. [PubMed]
- Nakanishi M, Kokuba Y, Murayama Y, et al. A new approach to laparoscopic lymph node excision in cases of transverse colon cancer. Digestion 2012;85:121-5. [Crossref] [PubMed]
- Athanasiou CD, Markides GA, Kotb A, et al. Open compared with laparoscopic complete mesocolic excision with central lymphadenectomy for colon cancer: a systematic review and meta-analysis. Colorectal Dis 2016;18:O224-35. [Crossref] [PubMed]
- Chand M, Siddiqui MRS, Rasheed S, et al. A systematic review and meta-analysis evaluating the role of laparoscopic surgical resection of transverse colon tumours. Surg Endosc 2014;28:3263-72. [Crossref] [PubMed]
- Storli KE, Søndenaa K, Furnes B, et al. Outcome after introduction of complete mesocolic excision for colon cancer is similar for open and laparoscopic surgical treatments. Dig Surg 2013;30:317-27. [Crossref] [PubMed]
- Bae SU, Saklani AP, Lim DR, et al. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 2014;21:2288-94. [Crossref] [PubMed]
- Huang JL, Wei HB, Fang JF, et al. Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int J Surg 2015;23:12-7. [Crossref] [PubMed]
- Markman M. Intraperitoneal chemotherapy in the management of colon cancer. Semin Oncol 1999;26:536-9. [PubMed]
- Zhuchenko AP, Kalganov ID, Filon AF. Cytoreductive surgery with intraperitoneal chemotherapy in patients with colon cancer and peritoneal carcinomatosis. Antibiot Khimioter 2003;48:31-5. [PubMed]
- Nadler A, McCart JA, Govindarajan A. Peritoneal Carcinomatosis from Colon Cancer: A Systematic Review of the Data for Cytoreduction and Intraperitoneal Chemotherapy. Clin Colon Rectal Surg 2015;28:234-46. [Crossref] [PubMed]
- Law WL, Chu KW, Tung PH. Laparoscopic colorectal resection: a safe option for elderly patients. J Am Coll Surg 2002;195:768-73. [Crossref] [PubMed]


