Percutaneous biliary interventions and complications in malignant bile duct obstruction
Introduction
Biliary procedures are an important part of the management of patients with both benign and malignant hepatobiliary disease. These include a variety of interventions including percutaneous transhepatic cholangiography (PTC), percutaneous transhepatic biliary drainage (PTBD) or stent placement, biliary stone management, and bile duct biopsy. In malignant bile duct obstruction, these interventions are used for palliation of symptoms including anorexia and pruritus, to reduce the serum bilirubin to allow administration of chemotherapy, and to preserve liver function. In some centers, biliary drainage is also performed preoperatively but its use in this context is controversial (1).
The purpose of this article is to review the indications, techniques and complications of percutaneous biliary interventions.
PTBD
Patients with biliary obstruction develop elevation in serum alkaline phosphatase, gamma-glutamyl transpeptidase, and serum bilirubin level. In the absence of prior biliary intervention, patients usually present with jaundice when the serum bilirubin level is more than 3 mg/dL. However, not every patient with jaundice needs to be drained.
PTBD is a minimally invasive procedure that is associated with potential complications and often an exteriorized catheter that can negatively impact quality of life (2). Therefore, careful pre-procedure selection of patients that will likely benefit from PTBD is crucial.
Basic terminology and definitions
Treatment options and prognosis vary depending on the level of bile duct obstruction. Prior to any biliary intervention, cross-sectional imaging should be performed to determine the location of obstruction as clearly as possible.
Biliary obstruction can be defined as “low” or “high”. Low bile duct obstruction occurs when the obstruction is below the insertion of the cystic duct. Low bile duct obstruction is best managed endoscopically because in this situation the entire biliary tree can be drained through the native orifice with a single stent without the need for an external catheter. If endoscopy is not successful, or in cases that endoscopic approach is not technically feasible, a percutaneous approach to low bile duct obstruction is an option.
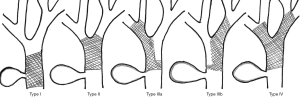
High bile duct obstruction is when the obstruction occurs at or above the common hepatic duct. In patients with high bile duct obstruction, percutaneous approach is preferred because PTBD allows for targeting a specific duct for drainage to maximize drainage of the functional liver. High bile duct obstruction is classified into four types using Bismuth classification (3). Bismuth type I is obstruction of the common hepatic duct, without extension to the bifurcation. Bismuth type II obstruction extends to the bifurcation without extension into the intrahepatic bile ducts. In Bismuth IIIa and IIIb the obstruction extends to the bifurcation of the right or left hepatic ducts, respectively. And finally in Bismuth IV the obstruction involves the bifurcations of both the right and left hepatic ducts (Figure 1).
In the setting of high bile duct obstruction ducts may be isolated from one another. We describe this as complete isolation when isolated ducts are not opacified at cholangiography. Effective isolation refers to the situation in which isolated ducts are opacified with contrast during cholangiography but do not drain effectively. Impending isolation is when bile ducts are opacified and drain slowly due to central narrowing (Figure 2). Effective and impending isolations are likely to progress to complete isolation and both increase the risk of subsequent episodes of cholangitis.
Indications for biliary intervention
Cholangitis
Acute cholangitis is a morbid clinical condition that involves acute inflammation and infection of the bile duct. Acute obstructive cholangitis was initially described by Reynolds and Dargan in 1959 as a syndrome consistent of fever, jaundice, abdominal pain and lethargy or mental confusion, also known as Reynolds’s pentad (4). Acute obstructive cholangitis may rapidly progress to serious and fatal infection. Cholangitis occurs when two factors are present: biliary tract obstruction and bacterial contamination of the biliary tract. Without prior intervention the biliary tree is aseptic, and obstructed patients usually present with jaundice or pruritus. Cholangitis almost always occurs in patients that have been previously instrumented or have had manipulation of the biliary tree from direct or enteric contamination of their biliary system (5).
As the incidence of malignant disease, sclerosing cholangitis, and non-surgical instrumentation of the biliary tract have been increasing so too has the incidence of cholangitis (6-8). Cholangitis is now commonly seen in the setting of malignancy. When cholangitis occurs in the setting of bile duct isolation, it is critical to identify and drain (if possible) the ducts that were previously colonized to effectively treat cholangitis.
Pruritus
Pruritus is a well-known symptom of biliary obstruction and can be debilitating for patients. Pruritus is usually seen in the setting of jaundice, but can occur as the only manifestation of cholestasis and the degree of pruritus can be disproportion to the serum bilirubin level (9). Clinically, most patients with cholestatic pruritus present with a diurnal variation of itch intensity, with the worst itch in the late evening and early night-time. Another typical feature of cholestatic pruritus is the tendency to affects the limbs, soles of the feet and palms although generalized pruritus may occur. Itching is worst with heat, contact with certain fabrics like wool and psychological stress. Recent studies suggest that increase in serum levels of two mediators: lysophosphatidic acid (LPA-a potent neuronal activator) and autotaxin (an enzyme forming LPA) cause pruritus. Patients with cholestatic pruritus demonstrate significantly higher levels of LPA in their blood compared to patients without pruritus (9). When biliary obstruction is the cause of pruritus, drainage of even a small portion of the biliary tree results in decrease or complete resolution of pruritis (10,11).
Anorexia
Obstructive jaundice may result in alteration in taste of food and decrease in appetite. Padillo et al. investigated the role of biochemical and hormonal factors in the pathogenesis of reduced food intake in 62 patients with biliary obstruction (12). They observed that decreased food intake in these patients was associated with the serum bilirubin, alkaline phosphatase and cholecystokinin levels. After biliary drainage, the resulting decreases in serum levels of bilirubin, alkaline phosphatase and cholecystokinin were associated increase in appetite and food intake.
Bile leakage
Bile leakage can occur after liver or pancreatic surgery with or without bilioenteric bypass. Other causes for biliary leakage are trauma to the biliary tract and iatrogenic injuries including post-endoscopic retrograde cholangiopancreatography (ERCP). In this situation, biliary drainage is used to divert the bile flow and allow the injured duct to heal.
Post-operative bile duct stricture
One of the most common cause of benign bile duct stricture is iatrogenic, (e.g., post-surgical anastomotic stricture), although they may be post-traumatic, congenital, inflammatory, ischemic and idiopathic. In patients who have undergone biliary reconstruction for malignancy, it can be very challenging to differentiate between an anastomotic stricture and tumor recurrence.
There are several techniques that can be used to establish the etiology of bile duct strictures. Typically brush or forceps biopsy can be performed through an existing tract (13). These techniques are limited by relatively poor sensitivity, however. Percutaneous fluoroscopic guided biopsy (Figure 3) has been used to diagnose strictures with improved sensitivity (personal communication, unpublished data). In this technique, the bile ducts are opacified via existing access (e.g., sheath or catheter) and the stricture is targeted for fine needle biopsy under fluoroscopic guidance.

Pre-operative
Cancers that involve the perihilar region may present with obstructive jaundice. These malignancies included intrahepatic cholangiocarcinoma, hilar cholangiocarcinoma, metastatic disease, and gallbladder carcinoma. Surgical resection is the only curative treatment in these patients. Some patients require an extended hepatectomy to achieve negative margins (14). Some surgeons request preoperative biliary drainage to lower bilirubin prior to surgery and facilitate reconstruction of the bile duct. It is controversial whether this results in an increase or decrease in post-operative mortality (2,14).
Chemotherapy
Chemotherapy agents that are excreted or metabolized in the liver require the patients to have near-normal bilirubin levels (usually less than 2 mg/dL) for safe administration. In this setting, biliary drainage may be performed to decrease the bilirubin to the acceptable level required to start chemotherapy.
Local therapy
Some patients with unresectable hilar cholangiocarcinoma are treated with local treatments like brachytherapy or photodynamic therapy. Biliary drainage may be performed to provide access to the biliary tree for these treatments.
Pre-procedure evaluation
Imaging
Current images of the patients are used for procedure planning. The patient should have a current high quality thin-cut CT, mutli-detector computed tomography (MDCT) or MRI with contrast (15). When available, 3 dimensional-image-reconstructions can be helpful.
The purpose of reviewing these images is to determine the level of obstruction and assess functional hepatic parenchyma. Liver that is replaced by tumor or does not have an intact portal vein are not considered functional. This is due to the fact that the portal vein provides 80% of the blood supply to the normal liver parenchyma and when the portal vein is occluded, atrophy of the affected segments follows, especially when the ipsilateral bile duct is also occluded (16). Drainage of non-functional liver parenchyma or liver without an intact portal venous supply will not result in improvement of liver function. In patients that have not received chemotherapy and are not cirrhotic, drainage of as little as 30% of the functional liver parenchyma may be adequate.
Approach
Cross sectional images help determine which specific duct or ducts are most amenable to drainage and likely to result in the desired outcome. Typically the bile duct that drains the maximum amount of functional hepatic parenchymal should be targeted. Because the volume of the right liver is usually larger than the left side, the right hemi-liver is often the target of drainage. However, the right hepatic duct is shorter than the left and therefore more susceptible to isolation in high bile duct obstruction. If isolation is detected in the right hemi-liver, left sided drainage may be indicated. In patients with ascites, a left-sided approach is recommended because it is anti-dependent and therefore pericatheter ascites leakage is less common.
Antibiotics
All patients undergoing biliary drainage should receive intravenous prophylactic pre-procedure broad-spectrum antibiotics within 1 hour of the start of the procedure because transient bacteremia can occur in these patients during or after the procedure (17).
Technique
Image guidance
Ultrasound can help with the initial puncture, particularly on the left and when the ducts are dilated. When using ultrasound for the right it is important to make sure the puncture remains at or below the 11th rib to decrease the risk of transpleural access. After accessing the targeted duct, the remainder of the procedure is performed under fluoroscopic guidance.
Sedation
PTBD is a procedure that requires sedation. Conscious sedation using a short acting benzodiazepine and narcotic can provide adequate sedation and analgesia for biliary intervention, although general anesthesia may be necessary in some patients (18). Patients should be well hydrated.
Access
It has been written that for right-sided approach, the 11th intercostal space in the midaxillary line and for left sided approach, three fingers below the sub-xyphoid should be used (19). However, after review of the images the best approach should be planned based on the location of the target duct. For example, if the target duct is more posterior, an approach that is more posterior to the midaxillary line should be used. If a patient has had a partial hepatectomy and the regenerated liver is in an in the left upper quadrant, a left-intercostal approach may be most appropriate.
The skin is prepped and draped and 1% lidocaine is injected in the dermatotomy site. A 22- or 21-gauge double-walled needle e.g., Chiba needle is advanced under ultrasound or fluoroscopic guidance. The stylet is removed and contrast is gently injected while retracting the needle until a bile duct is opacified.
Lee et al. suggested a single-wall puncture technique, in which a Y-adaptor was connected to the Chiba needle and diluted contrast medium was injected through the side are of the Y-adaptor while the needle was slowly being advanced into the liver. They report less complication when compared to the double-wall technique (20).
A peripheral duct should be used for drainage to minimize the risk of bleeding and maximize the number of side holes in the catheter above the obstruction. If a central duct is punctured while attempting to access a peripheral duct, contrast injection can be used to opacify a peripheral duct for targeting with a second needle.
Once the target bile duct is accessed, a 0.018 wire is advanced into the duct and through a coaxial system the wire is upsized to a 0.035 guide wire. Through this larger system a directional catheter in combination with a hydrophilic guide wire are used to cross the obstruction and the catheter and wire combination is advanced into the small bowel.
The hydrophilic wire is exchanged for a stiffer wire and a multi-side-hole drainage catheter is advanced into the bowel over this wire. Catheters that pass the ampulla and end in the small bowel are called “internal-external” catheters because they allow for drainage of bile both externally into a bag and internally across the catheter into the small bowel. If the obstruction cannot be crossed, an obligatory external drainage catheter can be placed (Figure 4).

Percutaneous transhepatic biliary stent placement
Patients with malignant biliary obstruction have a short life-expectancy in the range of months rather than years (1,21) and having an exteriorized catheter can limit quality of life.
Self-expanding metallic stents have larger inner lumen (usually 8–10 mm) than plastic stents (2–4 mm) and have improved patency resulting in less frequently occlusion (22-25). Stents can occlude for a variety of reasons including reflux of food, sludge, tumor growth at the proximal or distal end of the stent and tumor ingrowth through the interstices of uncovered stents). A mean patency of 6–10 months is generally expected for metallic stents. Covered and partially covered stents have been used in attempt to improve patency; however this has not proven to be the case (24). Metallic stent can be placed primarily at the time of drainage or secondarily after a period of internal/external drainage.
Dahlstrand et al. reported that patients with cancer lived an average of 2–6 weeks with a percutaneous drainage catheter before internalization (23). As mentioned earlier, percutaneous drainage catheters are associated with lifestyle limitations and potential complications including dislodgement, dysfunction, and pericatheter leakage. Therefore, in select patients primary percutaneous stent placement is preferred (21).
Technique
Prior to stent placement, good distal outflow should be confirmed. Cholangiography is performed to identify extent of bile duct occlusion. Through a sheath and over a stiff wire, an appropriate length stent, usually 8 or 10 mm in diameter, is advanced across the obstruction and deployed. Cholangiography through the sheath after stent deployment is used to assess the stent patency. If contrast material does not flow through the stent or demonstrates sluggish flow, additional coaxial stents may be placed or the stent may be balloon dilated. If the flow of contrast is satisfactory the vascular sheath is removed and the tract may be plugged using gelfoam pledges. If there is any question about the patency of the stent or there is poor antegrade drainage, a catheter may be left to retain access for a physiologic trial of stent patency. If there is no pericatheter leakage, pain or fever for 24 hours, this catheter may be removed bedside. Thornton et al. report that by placing primary stents, 90% of patients with malignant bile duct obstruction can live the rest of their lives free of exteriorized catheters (21).
There is data to suggest that placing primary stents above the papilla in order to preserve the function of the sphincter can prevent re-presentation with cholangitis at the time of stent occlusion because the sphincter prevents reflux of enteric flora into the biliary tree (personal communication, unpublished data).
Complications
The interventional radiologist needs to be aware of the potential for complications following percutaneous biliary intervention and be familiar with their diagnosis and management. This section will review the most common complications after biliary interventions, preventive measures and treatment strategies.
Bleeding
Bleeding complications after percutaneous bleeding drainage occurs in 2–5% of patients (1). Because of the close proximity of the hepatic vasculature to the bile ducts, bleeding complications may occur after biliary drainage placement. There is some data to suggest that bleeding following left-sided drainage is more common than right-sided drainage. To minimize the risk of bleeding, a peripheral bile duct should be accessed.
Hemorrhage after biliary drainage may be venous or arterial. Typically venous bleeds occur within a few days of drainage and result from the drainage catheter crossing a hepatic or portal vein branch and present with bleeding into the drainage bag. This often happens when the drainage catheter pulls back and the course of the catheter has traversed a portal or hepatic vein. Patients typically present with intermittent dark blood in the drainage bag or melena if the catheter is not connected to a drainage bag. Diagnosis can be confirmed by a tract study and is usually treated by upsizing of the drainage catheter to tamponade the vessel.
Arterial bleeding usually presents weeks and not days after the procedure and presents with red blood draining through and/or around the drainage catheter. This bleeding is rarely life-threatening and if arterial bleeding is suspected, diagnostic angiography should be performed to assess for structural lesion. It is preferable to perform the angiogram prior to injecting contrast into the catheter because contrast in the biliary tree makes visualization of a subtle arterial abnormality more difficult. Most commonly arterial bleeding is due to a pseudoaneurysm in the arterial branch overlying the course of the drainage catheter (Figure 5). Occasionally all that is seen is arterial spasm. When the site of bleeding is not definitively identified, the drainage catheter can be removed over a wire to remove any tamponade effect and provide better visualization of crossing vessels. Once the bleeding site is found the artery is embolized using coils extending across the lesion. Finally, if the suspicion of an arterial injury is high and no abnormality is seen, in the presence of an intact portal vein empiric embolization of the artery crossing the catheter near the puncture site may be performed.

Bile leakage
Occasionally, bile may leak around the tube into the peritoneum resulting in bile peritonitis or around the catheter causing skin breakdown (1). This usually resolves with upsizing of the catheter, but in some cases may persist. Another source of pericatheter leakage is ascites, particularly with right-sided catheters. In cases of pericatheter bile leak that doesn’t respond to catheter upsizing or in the setting of ascites, placement of an ostomy bag around the catheter to collect the bile and protect the skin is helpful. If a long-standing capped internal/external catheter begins to leak, this is most commonly a result of occlusion of the distal side holes. In this case, simple catheter exchange can remedy the problem. To minimize catheter occlusion, these catheters should be flushed forward only once or twice a day with 10 mL normal saline and routinely exchanged every 3 months.
Pain
Catheter related pain may occur following percutaneous drainage but usually improves over time. In a minority of cases pain may necessitate changing the catheter exit site or an intercostal nerve block.
Cholecystitis/pancreatitis
Placement of drainage catheters or stents across the origin of the cystic and pancreatic ducts can result in cholecystitis and pancreatitis respectively. In the setting of malignant bile duct obstruction, most patients who undergo stent placement do so because they are non-operative candidate. The occurrence of cholecystitis therefore presents a significant problem and many of these patients are treated with cholecystostomy placement. In our experience, approximately 20% of patients who undergo cholecystostomy for cholecystitis that occurs as a direct result of biliary stent placement ultimately are able to have their cholecystostomy catheters removed at a mean of 51 days (unpublished data). Unfortunately, the majority of patients require life-long drainage.
Pancreatitis occurs more commonly after endoscopic biliary intervention than percutaneous. However, when it happens it can be difficult for the patient and physician alike. Management is generally supportive, but if patients have internal/external drains across the origin of the pancreatic duct, conversion to an external drain may be helpful. This complication has been reported more commonly following placement of covered stents compared to uncovered stents (24).
Sepsis
Any biliary intervention is considered at the minimum a clean-contaminated procedure and therefore the recommendation is that all patients scheduled for biliary drainage receive prophylactic antibiotic prior to the procedure (17,25). Transient bacteremia occurs in approximately 2% of patients after biliary intervention (26). If a patient develops fever and/or chills following biliary intervention, antibiotics may be continued, fluid resuscitation should be initiated and the need for blood cultures considered. In some cases, infection does not respond to these measures and additional drainage may be required to address incompletely drained or isolated bile segments. In patients with subsegmental isolation, multiple drains could potentially be required and long-term antibiotic suppression may be favored.
Conclusions
Percutaneous biliary drainage is an important part of the care of many patients with malignant bile duct obstruction, particularly in patients with cholangiocarcinoma or pancreatic carcinoma. Careful pre-procedure assessment is important in determining which patients are likely to benefit from intervention and which are not. Before undergoing biliary intervention, physicians and patients alike must have a realistic expectation of which signs and symptoms are likely to be effectively treated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Robson PC, Heffernan N, Gonen M, et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol 2010;17:2303-11. [Crossref] [PubMed]
- Wang Q, Gurusamy KS, Lin H, et al. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2008.CD005444. [PubMed]
- Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg 1988;12:39-47. [Crossref] [PubMed]
- Reynolds BM, Dargan EL. Acute obstructive cholangitis; a distinct clinical syndrome. Ann Surg 1959;150:299-303. [Crossref] [PubMed]
- Ozden I, Tekant Y, Bilge O, et al. Endoscopic and radiologic interventions as the leading causes of severe cholangitis in a tertiary referral center. Am J Surg 2005;189:702-6. [Crossref] [PubMed]
- Kimura Y, Takada T, Kawarada Y, et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg 2007;14:15-26. [Crossref] [PubMed]
- Gigot JF, Leese T, Dereme T, et al. Acute cholangitis. Multivariate analysis of risk factors. Ann Surg 1989;209:435-8. [Crossref] [PubMed]
- Kremer AE, Beuers U, Oude-Elferink RP, et al. Pathogenesis and treatment of pruritus in cholestasis. Drugs 2008;68:2163-82. [Crossref] [PubMed]
- Kremer AE, Martens JJ, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 2010;139:1008-18, 1018.e1.
- Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc 2002;56:835-41. [Crossref] [PubMed]
- Van Laethem JL, De Broux S, Eisendrath P, et al. Clinical impact of biliary drainage and jaundice resolution in patients with obstructive metastases at the hilum. Am J Gastroenterol 2003;98:1271-7. [Crossref] [PubMed]
- Padillo FJ, Andicoberry B, Naranjo A, et al. Anorexia and the effect of internal biliary drainage on food intake in patients with obstructive jaundice. J Am Coll Surg 2001;192:584-90. [Crossref] [PubMed]
- Jung GS, Huh JD, Lee SU, et al. Bile duct: analysis of percutaneous transluminal forceps biopsy in 130 patients suspected of having malignant biliary obstruction. Radiology 2002;224:725-30. [Crossref] [PubMed]
- Liu F, Li Y, Wei Y, et al. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci 2011;56:663-72. [Crossref] [PubMed]
- Xu AM, Cheng HY, Jiang WB, et al. Multi-slice three-dimensional spiral CT cholangiography: a new technique for diagnosis of biliary diseases. Hepatobiliary Pancreat Dis Int 2002;1:595-603. [PubMed]
- Hann LE, Getrajdman GI, Brown KT, et al. Hepatic lobar atrophy: association with ipsilateral portal vein obstruction. AJR Am J Roentgenol 1996;167:1017-21. [Crossref] [PubMed]
- Venkatesan AM, Kundu S, Sacks D, et al. Practice guidelines for adult antibiotic prophylaxis during vascular and interventional radiology procedures. Written by the Standards of Practice Committee for the Society of Interventional Radiology and Endorsed by the Cardiovascular Interventional Radiological Society of Europe and Canadian Interventional Radiology Association J Vasc Interv Radiol 2010;21:1611-30. [corrected]. [Crossref] [PubMed]
- Hatzidakis AA, Charonitakis E, Athanasiou A, et al. Sedations and analgesia in patients undergoing percutaneous transhepatic biliary drainage. Clin Radiol 2003;58:121-7. [Crossref] [PubMed]
- Venbrux AC, Osterman FA. Percutaneous transhepatic cholangiography and percutaneous biliary drainage:step by step. In: LaBerge JM, Venbrux AC. editors. Biliary Interventions (SCVIR syllabus). The Sociefy of Cardiovascuar and Intervnetional Radiology, 1995:129-50.
- Lee SH, Hahn ST, Hahn HJ, et al. Single-wall puncture: a new technique for percutaneous transhepatic biliary drainage. AJR Am J Roentgenol 2003;181:717-9. [Crossref] [PubMed]
- Thornton RH, Frank BS, Covey AM, et al. Catheter-free survival after primary percutaneous stenting of malignant bile duct obstruction. AJR Am J Roentgenol 2011;197:W514-8. [Crossref] [PubMed]
- Mukai T, Yasuda I, Nakashima M, et al. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci 2013;20:214-22. [Crossref] [PubMed]
- Dahlstrand U, Sandblom G, Eriksson LG, et al. Primary patency of percutaneously inserted self-expanding metallic stents in patients with malignant biliary obstruction. HPB (Oxford) 2009;11:358-63. [Crossref] [PubMed]
- Lee SJ, Kim MD, Lee MS, et al. Comparison of the efficacy of covered versus uncovered metallic stents in treating inoperable malignant common bile duct obstruction: a randomized trial. J Vasc Interv Radiol 2014;25:1912-20. [Crossref] [PubMed]
- Brody LA, Brown KT, Getrajdman GI, et al. Clinical factors associated with positive bile cultures during primary percutaneous biliary drainage. J Vasc Interv Radiol 1998;9:572-8. [Crossref] [PubMed]
- Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol 2001;4:200-6. [Crossref] [PubMed]