Ultrasound image-guided core biopsy of the breast
Ultrasound (US) image-guided core biopsy of the breast
Recent trends in breast cancer treatment have favored breast conservation surgery with an emphasis on improved cosmesis. In the last twenty years of breast surgery, one of the most important advances has been the ability to diagnose breast cancer outside of the operating room utilizing the techniques of percutaneous core needle biopsy (CNB), stereotactic biopsy, and vacuum-assisted biopsy techniques. As nearly 80 percent of mammographic abnormalities are benign, the need for open biopsy has dramatically decreased, in turn supporting the use of minimally invasive percutaneous techniques. Utilizing excisional biopsy for diagnostic purposes often requires repeat surgery to establish clear margins in cases of a cancer diagnosis, propelling a shift towards percutaneous biopsy (1-5). The ability to obtain a diagnosis of cancer prior to surgery can allow for proper pre-operative planning, decrease the subsequent positive margin rate, and thus decrease the re-excision rate.
Percutaneous CNB is the preferred minimally invasive technique for diagnosing both palpable and non-palpable lesions. An international interdisciplinary consensus conference held in 2001, 2005, and again in 2009 agreed that percutaneous biopsy of breast lesions should be the gold standard biopsy method with use of US guidance if the lesion is amenable and stereotactic biopsy for calcifications not visualized on US (6). The Agency for Healthcare Research and Quality published an evidence report in 2014, that included 160 studies, and concluded that women were 15 times more likely to have their cancer treated with a single surgical procedure if they underwent image guided biopsy rather than open excisional biopsy (7). Breast surgeons are increasingly gaining expertise in breast US and US-guided core needle biopsies (CNB) (2,4). Surgeon-performed breast biopsies have shown to be cost-effective and associated with high patient satisfaction rates (3). Surgical societies are increasingly providing training programs and are certifying surgeon competency in these areas (2,4). In the United States, percutaneous CNB has nearly replaced fine needle aspiration (FNA) as the pre-operative diagnostic method of choice for breast lesions, as it provides a more definitive histological diagnosis and adequate tissue for prognostic markers (5-9). Performance of percutaneous biopsies for tissue diagnosis allows for optimization of surgical planning with concomitant staging of the axilla, therefore, decreasing the need for re-operation.
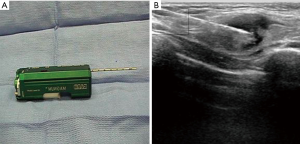
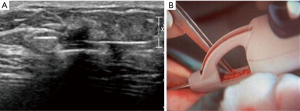
US-directed biopsy is performed for lesions that are palpable, but also for non-palpable, mammogram-detected lesions with a corresponding abnormality visualized on US. US-guided core biopsy is technically easier than stereotactic-guided biopsy as real-time imaging allows the surgeon to visualize the biopsy as it occurs. Using sterile technique and local anesthesia, a small puncture is made with an 11-knife blade and the needle is placed near the edge of the lesion and fired, which inserts it into the lesion for a sample to be retrieved. When placed parallel to the needle the position of the needle can be visualized on US (Figure 1).
A 12- or 14-gauge needle spring-loaded large core biopsy gun is used to remove several cores of tissue and in some instances completely remove the lesion by manually moving in and out of the insertion site, although automated core biopsy guns are also available. It is recommended that 5–10 cores be taken from a single lesion for adequate sampling.
If the core biopsy result is benign and is concordant with imaging findings, continued surveillance is acceptable. If the result is indeterminate or image-discordant, surgical excision is indicated to rule out malignancy. In order to determine the appropriate management and surveillance of a lesion, the histologic, imaging, and clinical findings must be taken into account for an assessment of concordance to be performed. When benign histology is obtained via CNB from a lesion that is concerning for malignancy either on imaging or on clinical exam then the biopsy is considered discordant. Discordance necessitates additional evaluation of such lesions by methods including repeating percutaneous biopsy or proceeding with surgical excisional biopsy (10). In addition, surgical excision is indicated for a core biopsy that demonstrates atypical hyperplasia (lobular or ductal) or lobular carcinoma in situ or neoplasia as the incidence of coexisting ductal carcinoma in situ or invasive carcinoma may be as high as 30% due to potential sampling error (11-14).
Image-guided vacuum-assisted core biopsy
Vacuum-assisted core biopsy is based on the same general principle as the CNB but represents a significant advance in technology (15-17). Vacuum is used to pull tissue into a sampling chamber, where it is removed with high-speed internal rotating knives. The specimen is then suctioned to a chamber outside the breast, where it can be retrieved. Multiple samples can be removed through this single-insertion technology, which has been approved for complete removal of benign imaged abnormalities under US or stereotactic guidance (Figure 2).
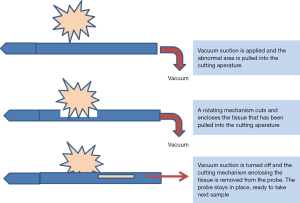
Image-guided vacuum-assisted biopsy (8- or 11-gauge) uses single-insertion technology with vacuum assistance for removal of imaged abnormalities via a suture-less incision (Figure 3).
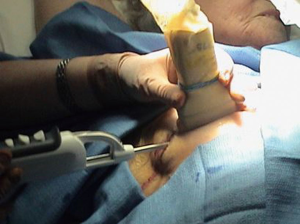
Standard core needle (12- & 14-gauge) biopsy limits the amount of tissue available to pathologists for establishing the histologic diagnosis and biochemical markers. For example, a standard core biopsy specimen may show ductal carcinoma in situ, but the final pathology of the lumpectomy specimen may demonstrate focal areas of infiltrating carcinoma. The vacuum-assisted core biopsy systems that are available provide for a more accurate diagnosis because larger specimens are removed than are possible with core biopsy and, in the case of image-guided vacuum-assisted excisional breast biopsy (IVEB), benign lesions are fully excised via removal of multiple cores. There are a plethora of vacuum-assisted devices available (Table 1).

Full table
Vacuum-assisted core biopsy offers the ability of obtaining larger (3–5 mm × 19 mm) contiguous samples from the same area by rotating the device rather than by withdrawal and reinsertion, as is necessary with the core biopsy needle. Theoretically, this minimizes seeding of the core tract and affords more accurate diagnosis (18,19). Patients diagnosed by vacuum-assisted or standard CNB have shown no difference in recurrence rates compared with patients diagnosed by excisional breast biopsy, suggesting that with radiation limited seeding of the needle tract does not affect outcome (20).
Image-guided vacuum-assisted excisional breast biopsy (IVEB)
The utility of the vacuum-assisted breast biopsy device under stereotactic guidance is well established (21,22). We hypothesized that the hand-held device could be used for diagnostic purposes as well as for therapeutic management (23). We facilitated the complete removal of benign-appearing lesions under US guidance with minimal complications and better cosmesis. Patients enrolled in this study underwent an US-guided minimally invasive excisional breast biopsy through a 3-mm incision. Complete removal of the imaged abnormality was accomplished with a hand-held 8- or 11-gauge image-guided vacuum device (Mammotome®, Ethicon Endo-Surgery, Cincinnati, OH, USA), which uses a high-speed rotating cutter to remove intact cores of breast tissue (Figure 4). Cores measuring 3–5 mm × 19 mm were removed until the visualized abnormality was completely excised. The gauge of the device was determined by lesion size. Lesions 1.5 cm2 and larger were excised with the 8-gauge device. If smaller lesions were removed, the 11-gauge mammotome device was used.
A total of 101 lesions were excised. The average size of the lesions was 1.9±1.1 cm (range, 0.5–2.5 cm). Complete removal of the US-visualized abnormality was achieved in 100% of patients. Ninety-five lesions (95%) had benign pathology. There were five lesions (5.0%) that were malignant and one atypia (1.6%), which was negative on routine pathologic review after re-excision open biopsy.
Hemostasis is achieved through the same tract with pressure most of the time (10 minutes), but sometimes the hematoma can be from injury to an artery resulting in surgical bleeding. Biopsy can result in a large expanding hematoma that causes high levels of anxiety for the patient and the surgeon. In the past, the only way to stop this surgical bleeding was by taking the patient to the operating room for exploration. We described a novel technique to control the bleeding utilizing a pediatric foley catheter to tamponade the vessel from inside and out (24).
In patients >40 years, a 6-month follow-up with mammography documented total resolution of the mammographic lesion, demonstrating that vacuum-assisted excisional breast biopsy under US guidance is an effective technique for therapeutic management of benign lesions, with minimal morbidity and optimal cosmesis. At present, we routinely remove benign-appearing lesions in this fashion (23).
Preparation for CNB or IVEB is carried out by the clinic staff. Local anesthesia will be accomplished with at least 5 cc of lidocaine HCL 1% with epinephrine 1:100,000 and 5 cc bupivacaine 0.5%. Patients who desire can be premedicated with oral valium. The breast lesion is removed with the Mammotome Breast Biopsy System® (Ethicon, Cincinnati, OH, USA) using US or mammographic guidance for placement of the 8- or 11-gauge probe. The vacuum-assisted stereotactic biopsy is commonly used at our institution to obtain diagnosis and has been reported to completely remove cancerous lesions (pathologically) nearly 30–48 percent of the time (25,26). Thus, IVEB by itself may not be considered a therapeutic procedure for cancer, even when the target lesion is completely removed.
We have extensive experience using IVEB, not only under stereotactic guidance, but also under US guidance for same-day excisional biopsy of benign lesions. Our experience and that of others has shown that when stereotactic IVEB is used with intention to only incisionally biopsy a suspicious lesion, the result is complete lesion removal in approximately 30–48% of cases (25,26). This indicates its potential utility for complete lesion removal of mammographically detected lesions and determination of size utilizing IVEB (20). Another disadvantage of using IVEB to both obtain a diagnosis and completely excise a lesion is the inability to reconstruct the margin status of a malignant tumor. Thus, after an IVEB procedure for diagnostic purposes, a malignant diagnosis requires lumpectomy for margins. Since our long-term goal is to develop a same-day diagnosis and first-line treatment regimen for small, unicentric breast cancers, we performed a pilot trial of percutaneously resect (IVEB)/percutaneous margin ablation/open resect protocol (23). In this way, using IVEB in combination with ablative techniques, we achieved oncologic excision of tissue for evaluation and negative margins by ablation with 100 percent success (23).
Alternative image-guided excisional techniques may remove the tumor intact. Presently available options are less well developed and were not chosen for this work secondary to significant concerns of tract seeding, cautery distortion of diagnosis (Neothermia™), and larger incision size [Advanced Breast Biopsy Device (ABBI™)] while still not accomplishing margin negativity or improved cosmesis (17).
US-core biopsy of axillary lymph nodes
The most important prognostic indicator involved in the evaluation of a patient with invasive breast cancer is axillary staging (27). This data is typically obtained in the operating room under general anesthesia with utilization of a nuclear medicine technetium injection in order to perform sentinel lymph node biopsy. This process can be a time consuming and costly undertaking requiring many healthcare resources. A variety of non-invasive imaging modalities in conjunction with biopsy techniques have been studied to evaluate the axilla for metastatic disease in an effort to comply with efficiency and cost savings standards during this era of healthcare reform. PET/CT has a sensitivity and specificity of identifying malignant nodes of 56 and 96 percent, respectively (28-30). Similar results were demonstrated with MRI, finding a sensitivity of 66% and specificity of 93% (31-33). Ultrasonography used for axillary staging has a sensitivity of 87% and specificity of 98%, and its use provides significant cost savings in comparison with both PET-CT and MRI (34). With ultrasonography being at least equal in accuracy and more cost efficient than the alternatives, this modality has been increasingly employed as a method to determine nodal positivity (34-36).
Suspicious nodes on US imaging can be specifically targeted for tissue confirmation through use of FNA or core biopsy or vacuum-assisted biopsy methods under local anesthesia, in turn saving the patient a trip to the operating room (37,38). Positive nodal tissue on pathology review is an indication for the patient to proceed to neoadjuvant chemotherapy. A negative node biopsy potentiates the decision to proceed with staging axillary sentinel node biopsy or definitive standard management based on breast cancer stage.
A retrospective review of patients at our institution from 2007–2012, included patients undergoing US of the axilla with CNB (39). A 95 patient cohort was divided into clinically positive (32%) and clinically negative (68%) axillae. The sensitivity and specificity of axillary US guided CNB was 90% (95% CI: 84.8% to 98.8%) and 100% (95% CI: 27% to 59.1%), respectively, and demonstrated cost savings of $485,007 compared to operative sentinel node biopsy. The axillary US guided CNB done under local anesthesia in the office setting can avoid the need for an additional operation, saving significant time and resources in patients with breast cancer who may have nodal involvement (39).
Touch preparation cytology (TPC) on core biopsies
TPC or imprint cytology has proven to be reliable and more efficient than other methods of intraoperative evaluation for tumor diagnosis and margin evaluation (40,41). TPC is accomplished by touching or smearing the core biopsy or lesion to a glass slide. Tumor cells and benign cells will adhere to the slide, but fat cells will not. The slide is then fixed with 95% methanol and stained with hematoxylin and eosin. At our institution, we evaluated 428 patients for intraoperative diagnosis and margin evaluation by touch preparation analysis. There were 83 malignancies, of which 26.5% were in situ. The average tumor size was 2.2±1.9 cm. The diagnostic accuracy was 99.3%; sensitivity was 96.4%; and specificity was 100%. The positive predictive and negative predictive values were 100% and 99.3%, respectively (40). When compared to frozen sections, TPC has also proven to be more efficient and reliable, with lower false positive and false negative rates (41). We published equally accurate results for the intraoperative diagnosis of sentinel lymph nodes evaluated with TPC (42,43). We extended this concept to TPC of CNB specimens as a means of same-day diagnosis in the outpatient setting (44). A total of 55 outpatients presenting with breast lesions underwent 8- or 11-gauge vacuum-assisted core or 14-gauge biopsy gun CNB and TPC for diagnosis. In group I, CNB specimens were sent to pathology for TPC processing. In group II, TPC of the CNB specimen was performed immediately by the surgeon, and slides were then sent to pathology to be read. These results were compared to permanent pathology sections of the CNB. The sensitivity, specificity, positive predictive value, and negative predictive value of TPC in group I was 62.5%, 100%, 100%, and 83.3%, respectively, with a false negative rate of 37.5%. The low sensitivity of Group I was felt to be secondary to drying of the core specimens during transport to the pathologist. Also, in this group lobular cancer was not detected by TPC. When TPC was performed immediately on site (group II), both the sensitivity and specificity of TPC were 100%, with a zero false-negative rate.
Others have reported fair to good results in terms of accuracy in predicting benign versus malignant results (45). This may be due to drying of specimens before they reach pathology. We advocate a coordinated effort between the pathologist and the surgeon such that the surgeon prepares the slides immediately and fixes them in alcohol and then sends these slides to pathology for staining and cytologic interpretation. Alternatively, the cytologist can come to the biopsy suite.
Open excisional breast biopsy
Needle localization breast biopsy (NLBB) is the standard for removal of non-palpable breast lesions after vacuum-assisted core biopsy. Disadvantages include a miss rate of up to 22%, a positive margin rate of up to 75%, and vasovagal reactions (~20%) (46,47). Surgeons cannot rely on needle localization of the clip alone and must be cognizant of potential clip migration. We performed a retrospective review of post-biopsy films in patients who had undergone vacuum-assisted core biopsy with stereotactic clip placement for abnormal mammograms (48). We measured the distance between the clip and the biopsy site in standard two-view mammograms, using the Pythagorean theorem to calculate the distance the clip moved within the breast from the biopsy site. Pathology reports on lesions removed by NLBB versus those removed by intraoperative US-guided breast biopsy, which uses US to localize the core biopsy site created by vacuum excision, were reviewed to assess margin status. A total of 165 post-biopsy mammograms on patients who had vacuum-assisted core biopsy with clip placement were reviewed. In 93 evaluable cases, the mean distance the clip moved was 13.8±1.6 mm (SEM; 95% CI: 10.6–17.1 mm). The range of migration was 0–78.3 mm. The median was 9.7 mm. In 22.5% of patients, the clip was >20 mm from the targeted site. Migration of the clip was not related to the age of the patient, the size of the breast, or the location within the breast. In the subgroup of patients with cancer, margin positivity after NLBB was 42% versus 0% when the clip was ignored and the cavity removed by intraoperative US-guided biopsy (48). These results led us to develop the patented methodology used in the present protocol to locate the vacuum-assisted core biopsy cavity via US.
Hematoma or cavity-directed US-guided excisional biopsy after stereotactic vacuum-assisted breast biopsy
To decrease the miss rate that can occur with NLBB, we hypothesized that the hematoma-filled cavity resulting from a CNB vacuum-assisted core biopsy could be visualized with US and used to guide excision (Figures 5,6) (49,50). The hematoma-filled cavity visualized by US, and then grossly at surgery, could also be seen microscopically to again confirm lesion removal (49,50). In a study of 455 patients we compared hematoma-directed US-guided procedure for intraoperative localization of non-palpable lesions to needle localization done at the same institution. Needle localization was performed on 126 (28%) patients and 329 (72%) via HUG. The previous core-biopsy site in 100% of patients was successfully excised using HUG: 152 of 329 (46%) were benign and 177 of 329 (54%) were malignant. Margins were positive in 42 of these 177 cases (24%). A total of 88 of 126 (70%) were benign and NLBB 38 of 126 (30%) were malignant; margins were positive in 18 of these 38 (47%) with NLBB. Margin positivity was significantly higher for NLBB than HUG (P=0.045, Fisher exact). This study provides proof of concept as to the effectiveness of US in identifying the cavity of the biopsy site. We have performed the procedure up to 5 weeks after stereotactic biopsy, which allows easier scheduling. In addition, it improves patient comfort over needle localization. We now routinely use hematoma-directed US guidance as a standard localization procedure in lieu of NLBB in this breast program and propose to use it for localization of the vacuum cavity for the ablative procedures.
Summary
In summary, US is an extremely valuable tool in breast surgery, with a variety of uses including screening, diagnostic imaging, image-guided biopsy techniques, image-guided percutaneous excision techniques, as well as image-guided open surgical excision procedures. Technology has advanced to include improved imaging and vacuum-assisted devices making percutaneous biopsies and excisions easier and more efficient. We have found touch prep cytology to be accurate and allows for rapid pathologic results of a tissue specimen, regardless of the source being from breast tissue or from a lymph node. This provides diagnostic capability of a breast lesion with potential staging of the axilla in the clinic setting with cytology results the same day and tissue for marker assessment. We believe this provides optimal care for the patient allowing for thorough pre-operative planning in order to minimize unnecessary additional operations. In the future, we look to improve upon this foundation of tools with implementation of percutaneous excision of lesions followed by ablation of the surrounding tissue for definitive complete treatment of early stage breast cancer in the clinic setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Devaraj S, Iqbal M, Donnelly J, et al. Axillary ultrasound in invasive breast cancer: experience of our surgeons. Breast J 2011;17:191-5. [Crossref] [PubMed]
- Law MT, Bennett IC. Structured ultrasonography workshop for breast surgeons: is it an effective training tool? World J Surg 2010;34:549-54. [Crossref] [PubMed]
- Layeequr Rahman R, Crawford S, Hall T, et al. Surgical-office-based versus radiology-referral-based breast ultrasonography: a comparison of efficiency, cost, and patient satisfaction. J Am Coll Surg 2008;207:763-6. [Crossref] [PubMed]
- Holmes DR, Silverstein MJ. A minimally invasive breast biopsy clinic: an innovative way to teach breast fellows how to perform breast ultrasound and ultrasound-guided breast procedures. Am J Surg 2006;192:439-43. [Crossref] [PubMed]
- Rakha EA, Ellis IO. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol 2007;60:1300-6. [Crossref] [PubMed]
- Silverstein MJ, Recht A, Lagios MD, et al. Special report: Consensus conference III. Image-detected breast cancer: state-of-the-art diagnosis and treatment. J Am Coll Surg 2009;209:504-20. [Crossref] [PubMed]
- Dahabreh IJ, Wieland LS, Adam GP, et al. Core Needle and Open Surgical Biopsy for Diagnosis of Breast Lesions: An Update to the 2009 Report [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Sep.
- Willems SM, van Deurzen CH, van Diest PJ. Diagnosis of breast lesions: fine-needle aspiration cytology or core needle biopsy? A review. J Clin Pathol 2012;65:287-92. [Crossref] [PubMed]
- Garg S, Mohan H, Bal A, et al. A comparative analysis of core needle biopsy and fine-needle aspiration cytology in the evaluation of palpable and mammographically detected suspicious breast lesions. Diagn Cytopathol 2007;35:681-9. [Crossref] [PubMed]
- Johnson NB, Collins LC. Update on percutaneous needle biopsy of nonmalignant breast lesions. Adv Anat Pathol 2009;16:183-95. [Crossref] [PubMed]
- Landercasper J, Linebarger JH. Contemporary breast imaging and concordance assessment: a surgical perspective. Surg Clin North Am 2011;91:33-58. [Crossref] [PubMed]
- Neal L, Tortorelli CL, Nassar A. Clinician's guide to imaging and pathologic findings in benign breast disease. Mayo Clin Proc 2010;85:274-9. [Crossref] [PubMed]
- Masood S, Rosa M. Borderline breast lesions: diagnostic challenges and clinical implications. Adv Anat Pathol 2011;18:190-8. [Crossref] [PubMed]
- Corben AD, Edelweiss M, Brogi E. Challenges in the interpretation of breast core biopsies. Breast J 2010;16 Suppl 1:S5-9. [Crossref] [PubMed]
- Liberman L, LaTrenta LR, Van Zee KJ, et al. Stereotactic core biopsy of calcifications highly suggestive of malignancy. Radiology 1997;203:673-7. [Crossref] [PubMed]
- Bassett L, Winchester DP, Caplan RB, et al. Stereotactic core-needle biopsy of the breast: a report of the Joint Task Force of the American College of Radiology, American College of Surgeons, and College of American Pathologists. CA Cancer J Clin 1997;47:171-90. [Crossref] [PubMed]
- Velanovich V, Lewis FR Jr, Nathanson SD, et al. Comparison of mammographically guided breast biopsy techniques. Ann Surg 1999;229:625-30; discussion 630-3. [Crossref] [PubMed]
- Youngson BJ, Liberman L, Rosen PP. Displacement of carcinomatous epithelium in surgical breast specimens following stereotaxic core biopsy. Am J Clin Pathol 1995;103:598-602. [Crossref] [PubMed]
- Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR Am J Roentgenol 1999;173:1303-13. [Crossref] [PubMed]
- King TA, Hayes DH, Cederbom GJ, et al. Biopsy technique has no impact on local recurrence after breast-conserving therapy. Breast J 2001;7:19-24. [Crossref] [PubMed]
- The American Society of Breast Surgeons. Performance and Practice Guidelines for Stereotactic Breast Procedures. Revised April 2010. Available online: https://www.breastsurgeons.org/new_layout/about/statements/. Accessed November 5, 2010.
- ACR Practice parameter for the performance of stereotactic-guided breast interventional procedures. Available online: http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/Stereotactically_Guided_Breast.pdf
- Klimberg VS, Boneti C, Adkins LL, et al. Feasibility of percutaneous excision followed by ablation for local control in breast cancer. Ann Surg Oncol 2011;18:3079-87. [Crossref] [PubMed]
- Gadgil PV, Klimberg VS. Management of percutaneous core biopsy tract bleeding. Ann Surg Oncol 2013;20:3348. [Crossref] [PubMed]
- Penco S, Rizzo S, Bozzini AC, et al. Stereotactic vacuum-assisted breast biopsy is not a therapeutic procedure even when all mammographically found calcifications are removed: analysis of 4,086 procedures. AJR Am J Roentgenol 2010;195:1255-60. [Crossref] [PubMed]
- Burak WE Jr, Owens KE, Tighe MB, et al. Vacuum-assisted stereotactic breast biopsy: histologic underestimation of malignant lesions. Arch Surg 2000;135:700-3. [Crossref] [PubMed]
- Trifirò G, Viale G, Gentilini O, et al. Sentinel node detection in pre-operative axillary staging. Eur J Nucl Med Mol Imaging 2004;31 Suppl 1:S46-55. [Crossref] [PubMed]
- Crippa F, Gerali A, Alessi A, et al. FDG-PET for axillary lymph node staging in primary breast cancer. Eur J Nucl Med Mol Imaging 2004;31 Suppl 1:S97-102. [Crossref] [PubMed]
- Heusner TA, Kuemmel S, Hahn S, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging 2009;36:1543-50. [Crossref] [PubMed]
- Ueda S, Tsuda H, Asakawa H, et al. Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer 2008;8:165. [Crossref] [PubMed]
- Meng Y, Ward S, Cooper K, et al. Cost-effectiveness of MRI and PET imaging for the evaluation of axillary lymph node metastases in early stage breast cancer. Eur J Surg Oncol 2011;37:40-6. [Crossref] [PubMed]
- Michel SC, Keller TM, Fröhlich JM, et al. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology 2002;225:527-36. [Crossref] [PubMed]
- Motomura K, Ishitobi M, Komoike Y, et al. SPIO-enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Ann Surg Oncol 2011;18:3422-9. [Crossref] [PubMed]
- Alvarez S, Añorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol 2006;186:1342-8. [Crossref] [PubMed]
- Britton P, Moyle P, Benson JR, et al. Ultrasound of the axilla: where to look for the sentinel lymph node. Clin Radiol 2010;65:373-6. [Crossref] [PubMed]
- Deurloo EE, Tanis PJ, Gilhuijs KG, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer 2003;39:1068-73. [Crossref] [PubMed]
- Mainiero MB, Cinelli CM, Koelliker SL, et al. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol 2010;195:1261-7. [Crossref] [PubMed]
- van Rijk MC, Deurloo EE, Nieweg OE, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol 2006;13:31-5. [Crossref] [PubMed]
- Henry-Tillman R, Glover-Collins K, Preston M, et al. The SAVE review: sonographic analysis versus excision for axillary staging in breast cancer. J Am Coll Surg 2015;220:560-7. [Crossref] [PubMed]
- Cox CE, Ku NN, Reintgen DS, et al. Touch preparation cytology of breast lumpectomy margins with histologic correlation. Arch Surg 1991;126:490-3. [Crossref] [PubMed]
- Klimberg VS, Westbrook KC, Korourian S. Use of touch preps for diagnosis and evaluation of surgical margins in breast cancer. Ann Surg Oncol 1998;5:220-6. [Crossref] [PubMed]
- Rubio IT, Korourian S, Cowan C, et al. Use of touch preps for intraoperative diagnosis of sentinel lymph node metastases in breast cancer. Ann Surg Oncol 1998;5:689-94. [Crossref] [PubMed]
- Henry-Tillman RS, Korourian S, Rubio IT, et al. Intraoperative touch preparation for sentinel lymph node biopsy: a 4-year experience. Ann Surg Oncol 2002;9:333-9. [Crossref] [PubMed]
- Kass R, Henry-Tillman RS, Nurko J, et al. Touch preparation of breast core needle specimens is a new method for same-day diagnosis. Am J Surg 2003;186:737-41; discussion 742. [Crossref] [PubMed]
- March DE, Walker MT, Bur M, et al. Touch-preparation cytologic examination of breast core biopsy specimens: accuracy in predicting benign or malignant core histologic results. Acad Radiol 1999;6:333-8. [Crossref] [PubMed]
- Acosta JA, Greenlee JA, Gubler KD, et al. Surgical margins after needle-localization breast biopsy. Am J Surg 1995;170:643-5; discussion 645-6. [Crossref] [PubMed]
- Homer MJ, Smith TJ, Safaii H. Prebiopsy needle localization. Methods, problems, and expected results. Radiol Clin North Am 1992;30:139-53. [PubMed]
- Kass R, Kumar G, Klimberg VS, et al. Clip migration in stereotactic biopsy. Am J Surg 2002;184:325-31. [Crossref] [PubMed]
- Smith LF, Henry-Tillman R, Harms S, et al. Hematoma-directed ultrasound-guided breast biopsy. Ann Surg 2001;233:669-75. [Crossref] [PubMed]
- Arentz C, Baxter K, Boneti C, et al. Ten-year experience with hematoma-directed ultrasound-guided (HUG) breast lumpectomy. Ann Surg Oncol 2010;17 Suppl 3:378-83. [Crossref] [PubMed]