Reirradiation of locally recurrent nasopharyngeal cancer: history, advances, and promises for the future
Local recurrence in nasopharyngeal cancer (NPC)
NPC, while rare in Western countries, is one of the most commonly diagnosed head and neck malignancies in Asian countries and is endemic in Southern China. The histologic subtype and etiology are between these two global regions are different as well. In Western countries, most cases (40%) are subtypes as keratinizing squamous cell carcinoma (formerly classified as WHO Type I) and are associated with exposure to alcohol and tobacco. However, the majority of Asian cases are of the undifferentiated non-keratinizing subtype (formerly WHO Type III) and are strongly associated with EBV co-infection (70% of these cases have positive EBV titers) (1). Keratinizing cases represent less than 5% within the endemic regions.
Radiation therapy is the only curative treatment modality for non-metastatic NPC. For more locally advanced cases, the addition of concurrently administered chemotherapy has significantly improved outcomes, including and especially overall survival (OS). Inspection of Table 1 clearly speaks to this benefit.

Full table
The prevailing use of intensity-modulated photon based radiation therapy (IMRT) has also significantly improved the treatment outcomes including local, regional control, and the reduction of acute and late side effects (5-8). The utilization of advanced diagnostic technology such as MRI and PET/CT together with IMRT have also been expected to minimize the instances of marginal misses of the primary disease and neck adenopathy. However, despite these improvements, approximately 10% of patients who have completed IMRT of 70 Gy still fail locally and represent a substantial challenge to clinicians.
Because of this paradigm shift in treatment towards near-universal use of IMRT, the nature of recurrences is most likely changing as well. In the older era of 2D or 3D conformal radiotherapy, NPC patients may have failed locally due to marginal misses or under-dosing to the clinical targets. Thus, a portion of this recurrent patient population may have its origins within previous technical limitations. However, in the setting of modern radiation treatment techniques, local recurrence after high-dose photon radiotherapy (with and without concurrent chemotherapy) may have a biologic basis, as it may be due primarily to radio-resistant cancer cells that survived initial course of treatment (9).
It is reasonable to postulate that subgroups of cells within the gross disease may be more resistant to adequate-coverage photon radiotherapy. Whether such features of radio-resistance are caused by hypo-oxygenation, inherently resistant clones, or the presence of cancer stem cells or stem cell-like cells remain to be investigated. Nevertheless, their characteristics of radio-resistance to photon-based IMRT appear to confer a more dismal outcome after re-irradiation using IMRT. Salvage radiation treatment using photon therapy to the same or lower dose, usually in the range between 60~70 Gy at standard fractionation, may not produce long-term disease control. This is highlighted in a recent study prepared by Kong et al in which re-irradiation using IMRT produced worse outcome, in-terms of OS and local control in NPC patients who failed locally after IMRT for their primary NPC, as compared to those failed locally after their initial, definitive 2D or 3D-radiotherapy as has been reported in the literature. Not only 40% of patients experienced mucosal necrosis after salvage IMRT, but also close 70% of those patients developed the severe mucosal reaction within 6 months after the completion of reirradiation. The earlier onset of mucosal necrosis is substantially different from what we have observed in other salvage IMRT studies for patients who locally failed 2D conventional radiation therapy. Clearly, previous treatment using IMRT in NPC poses additional challenges to re-irradiation for local recurrence, as this clinical scenario requires the targeting of biologically-selected and proven radio-resistant disease while in the setting of organs at risk, which have already received significant doses.
Treatment options of local recurrence in nasopharyngeal cancer
Just as radiation is the mainstay treatment for definitive NPC treatment, re-irradiation remains the principle modality for patients with locally recurrent NPC (10). Surgery is used only in the very select few cases in which patients are robust enough to tolerate an invasive procedure and have small recurrent tumors (T1and T2) that are technically accessible (not near vital structures) and resectable, and carry with them the potential for increased complication rates due to healing issues in previously irradiated tissues (11-13). Chemotherapy alone is not sufficient to establish local control, as is observed in the primary setting. It may be given adjuvantly, but there must be a component of local therapy for there to be any benefit or chance of salvage (14).
Various radiotherapeutic strategies, including 2D external beam therapy, 3D conformal, brachytherapy, stereotactic radiosurgery (SRS) (Table 2), and, more recently, IMRT have been utilized in an attempt to control local recurrent NPC. Although brachytherapy and SRS can usually sufficiently spare the organs at risk surrounding the disease foci, their utilization is usually limited by the extent of disease, where, similar to surgery, it is reserved for only small T-stage (T1 and T2) recurrences (16,21,22). 2D and 3D conformal radiotherapy have also resulted in very high complication rates (48–73% 5-year actuarial rate of symptomatic late complications with a treatment mortality rate of 2% to 4%), due to their imprecise treatment delivery in the setting of previous irradiation (14,23).
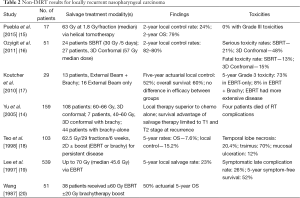
Full table
The use of IMRT with or without chemotherapy has emerged as the standard of care for locally recurrent NPC. Most patient series reports originate mainly from endemic areas. Several groups in China have reported the long-term results in patients re-irradiated with IMRT for their patients diagnosed with locally recurrent NPC (24-26). The documented long-term OS rates range from 45~65%, and patients with more advanced disease at recurrence suffer from far a more dismal outcome, usually with an OS rate of <40%. Table 3 summarizes the results of published series in patients who primarily received IMRT-based re-irradiation for recurrent NPC.
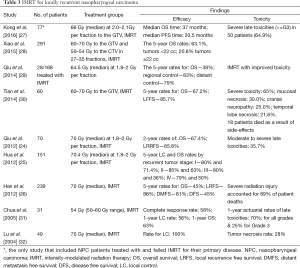
Full table
Predictive factors of the efficacy of salvage radiation therapy
The extent of the recurrent disease appears to be the most important factor in predicting the likelihood of local failure from the second course of radiation. Lee and colleagues reported patients with recurrent T1 tumors had a local control rate of 35%, but with recurrent T3 tumors, it was only 11% (19). Similarly, Han et al. observed 5-year OS rates of 67–85% in recurrent T1-T2 tumors, while only 32–40% rates for T3-T4 tumors (26). The initial, presenting tumor stage also appears to have a long lasting effect, as it is both associated with the extent of recurrence and long-term survival, though was itself an independent factor on multivariate analysis. For patients receiving external beam radiation alone for their salvage course, the likelihood of local failure decreases by 1.7% for every Gy of Biologic Effective Dose delivered (assuming the tumor α/β ratio of 10) (19). Further, investigators have concluded that doses under 60 Gy are inadequate to control recurrent (20,25,26). Doses that exceed this amount may only portend serious long-term side-effects, with minimal therapeutic gain.
Thus, the treatment of locally recurrent NPC after high-dose radiation is clinically challenging for several reasons. The high-dose areas of the initial radiation therapy for primary disease usually encompass not only the gross tumor volume but also the surrounding organs-at-risk such as nasopharyngeal mucosa, temporal lobes of the brain, brain stem, and optic nerve/chiasm for subclinical disease. A substantial portion of the temporal lobes of the brain and most parts if not the entire nasopharynx may be covered by a high dose of >66 Gy especially in T3 or T4 NPC. The incidence of severe adverse effects increases significantly when the combined radiation dose from initial and re-irradiation exceeds 100 Gy (33). Thus, re-irradiation to a dose of 60 Gy or more may cause severe long-term radiation-induced toxicities. Given the location of the nasopharynx and the adjacent critical structures, the potential for catastrophic/life-threatening long-term side effects is not surprising. Chen and colleagues reported a 25% mortality rate from severe late adverse effects in their series of 54 NPC patients who received 70 Gy in their second course of radiotherapy (34).
Severe long-term toxicity after reirradiation
Mucosal ulceration of the nasopharynx represents a complex, morbid and potentially life-threatening complication of patients who receive very high cumulative doses of radiation to this site, as seen in patients who receive salvage radiotherapy for recent NPC. A study of this condition by Hua and colleagues (35) certainly illustrate this point. In 28 patients, pathologically proven necrosis was found within the soft-tissues (mucosa, muscle and tendons) of 14, and skull base osteoradionecrosis was seen in the other 14. Thirteen of the 28 patients had erosion of tissue sufficient enough to expose the carotid artery. Patients most frequently present with severe headaches. The loss of tissue architecture can foster opportunistic infections. Indeed, numerous gram-negative, anaerobic, and fungal pathogens were detected within the necrotic tissue of this patient series. The foul odor created by necrosis and infection has a detrimental impact on the patients’ quality of life, as well. Surgical debridement and antibiotic therapy are the mainstays of management; however, if the necrosis is extensive, only supportive care can be given. Those with osteonecrosis and carotid artery exposure tend to have the worst and often fatal outcome. Uncontrolled mucosal necrosis and ulceration is a serious problem that requires prompt attention. In the large series reported by Han and colleagues (26), 69% of the patient deaths (120 out of 239 recurrent NPC patients treated with IMRT) were attributed to radiation injuries, the majority of which were severe cases of radiation necrosis of the nasopharyngeal mucosa.
Temporal lobes are clearly organs at risk for the development of radiation-induced necrosis due to its close proximity to the nasopharynx. Dose to the temporal lobes is unavoidable, regardless of the treatment planning strategy employed. Temporal lobe necrosis (TLN) can be asymptomatic, being detected only by radiographic changes noted on follow-up MRI, or can be overtly symptomatic, causing focal neurological deficits, memory loss, severe headache and other neurocognitive dysfunction. Su and colleagues analyzed 40 cases of TLN out of 870 NPC patients (4.6%) treated definitively with radiotherapy for predictive dose-volumetric parameters (36). Their major conclusion was that the 5-year incidence of TLN was <5% if less than 10% of the temporal lobes received ≥40 Gy or if less than 5 cc of this structure received greater than ≥40 Gy. However, the incidence of TLN exceeded 20% if greater than 15% of the temporal lobes received ≥40 Gy or if the absolute volume receiving ≥40 Gy was 10 cc or greater. Given the cumulative doses experienced in the setting of nasopharyngeal re-irradiation, though there is some biologic recovery against the life-time dose limitations to CNS structures (37), it is not surprising the rates of TLN are high for these patients. Tian and colleagues observed a TLN rate of (21.6%) in their series of 60 patients re-treated with IMRT (30). Asymptomatic or mild cases of TLN can be treated conservatively with glucocorticoids and observation. For more severe cases, craniotomy with decompression and resection of the necrotic tissue is used. Necrosis accompanied by edema and inflammation can become life threatening if the increased intracranial pressure is not mitigated.
One of the most devastating, but fortunately rare complication of head and neck irradiation is carotid blowout. For those who receive only a definitive course of radiotherapy (60–70 Gy at 1.8–2.0 Gy/fraction), this is rarely observed. However, in the setting of re-irradiation, the discussion of carotid blow-out should certainly be part of the informed consent process. In an exhaustive review of the literature, McDonald and colleagues abstracted the results of 1,554 patients who received a second course of radiotherapy to the head and neck and found that 41 patients (2.6%) developed this serious complication (76% of these cases were fatal) (38). While nearly all of the patients analyzed in this study received over 110 Gy in their cumulative dose, the rates were higher in patients who received accelerated hyperfractionation schemes within their re-irradiation courses (where the daily dose exceeded 2.5 Gy per day). Acute carotid rupture occurs as a result of the compromised arterial wall losing its ability to maintain its structural integrity against the patient’s arterial blood pressure. This is caused by several phenomena: the heavily irradiated supporting soft tissues can fibrotic, leading to perfusion-loss and desiccation of the artery, nasopharyngeal necrosis (as described above) can erode into the carotid artery, infection can occur, recurrent/persistent tumor can invade and weaken the wall or combination of these factors. Open surgical intervention (if addressed in time) has been the standard of care where the artery is repaired primarily, grafted, oversewn, or ligated. In recent years, the approach has shifted to less-invasive management with endovascular/interventional approaches that include coil, balloon, or stent, technology (39).
Looking ahead to particle therapy
It appears that IMRT represents the limit of what photon-based radiotherapy can offer to patients with recurrent NPC. Clearly, there is room for improvement, from both an efficacy and toxicity perspective. Particle therapy such as proton or carbon-ion radiation therapy (PRT or CIRT), may represent a more favorable option, as it provides distinct physical characteristics that include: a sharp lateral penumbra: very low energy deposition within the entry path prior to the Bragg peak formed by the steep dose deposition; and, a sharp dose fall-off after the Bragg peak, thus possessing a dose delivery with a finite range. The depth of the Bragg peak is determined by the beam energy. Sparing of normal surrounding tissues is crucial in radiation therapy of head and neck area especially patients who have completed a previous course of high-dose radiation. A number of studies have reported superior dose distributions using particle therapy for primary or recurrent NPC with acceptable clinical outcomes and improved dosimetry (40,41).
In addition to its superior physical properties, carbon ion therapy is a high LET modality and the relative biological effectiveness (RBE) of is significantly higher than those of photon and proton radiation. The value of RBE is 3 to 5 for carbon ion depend on the tissue type and end point of study. It has been suggested that more damage from high LET radiation is in the form of direct DNA double strand breaks, which is more difficult to repair (42). As such, improved clinical results could be expected after high-LET radiation such as CIRT especially for photon-resistant cancer cells.
The use of carbon ion radiation therapy (CIRT) in the setting of heavily previously irradiated sites has been reported for adenoid cystic carcinoma (43), chordoma, and chondrosarcoma (44) with favorable dosimetry, encouraging local tumor control and acceptable toxicity. Trials are ongoing in recurrent NPC as well at the Shanghai Proton and Heavy Ion Center (45). NPC patients who failed their initial treatment with IMRT are accrued and treated in two dose-escalating clinical trials to explore the maximum tolerance dose (MTD) and efficacy at the MTD, with or without concurrent chemotherapy. Results of these trials will reveal the potential of CIRT in the management of locally recurrent NPC.
Summary
Recurrent NPC represents a substantial public health issue in the endemic areas of Asia. Despite the technological improvements in the management of this disease entity in the primary setting, the likelihood of local recurrence is significantly high. While IMRT has emerged as the best modality of care, the local control rates and long-term toxicity outcomes from this salvage therapy are dismal as compared to those observed in the definitive setting. This is due to a confluence of technical and biological factors, including the limitations of normal tissue re-irradiation and the selection of radio-resistant clonal cell populations. Clearly, there is a need for improvement and new treatment options should be explored.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol 2007;18:29-35. [Crossref] [PubMed]
- Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2005;97:536-9. [Crossref] [PubMed]
- Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730-8. [Crossref] [PubMed]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [PubMed]
- Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 2006;66:981-91. [Crossref] [PubMed]
- Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002;53:12-22. [Crossref] [PubMed]
- Zhang B, Mo Z, Du W, et al. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol 2015;51:1041-6. [Crossref] [PubMed]
- Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007;25:4873-9. [Crossref] [PubMed]
- Weichselbaum RR, Beckett MA, Schwartz JL, et al. Radioresistant tumor cells are present in head and neck carcinomas that recur after radiotherapy. Int J Radiat Oncol Biol Phys 1988;15:575-9. [Crossref] [PubMed]
- Wei WI, Chan JY, Ng RW, et al. Surgical salvage of persistent or recurrent nasopharyngeal carcinoma with maxillary swing approach - Critical appraisal after 2 decades. Head Neck 2011;33:969-75. [Crossref] [PubMed]
- Wei WI, Kwong DL. Recurrent nasopharyngeal carcinoma: surgical salvage vs. additional chemoradiation. Curr Opin Otolaryngol Head Neck Surg 2011;19:82-6. [Crossref] [PubMed]
- Vlantis AC, Chan HS, Tong MC, et al. Surgical salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma: a multivariate analysis of prognostic factors. Head Neck 2011;33:1126-31. [Crossref] [PubMed]
- Chan JY. Surgical salvage of recurrent nasopharyngeal carcinoma. Curr Oncol Rep 2015;17:433. [Crossref] [PubMed]
- Yu KH, Leung SF, Tung SY, et al. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Head Neck 2005;27:397-405. [Crossref] [PubMed]
- Puebla F, Lopez Guerra JL, Garcia Ramirez JM, et al. Effectiveness and toxicity of helical tomotherapy for patients with locally recurrent nasopharyngeal carcinoma. Clin Transl Oncol 2015;17:925-31. [Crossref] [PubMed]
- Ozyigit G, Cengiz M, Yazici G, et al. A retrospective comparison of robotic stereotactic body radiotherapy and three-dimensional conformal radiotherapy for the reirradiation of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e263-8. [Crossref] [PubMed]
- Koutcher L, Lee N, Zelefsky M, et al. Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys 2010;76:130-7. [Crossref] [PubMed]
- Teo PM, Kwan WH, Chan AT, et al. How successful is high-dose (> or = 60 Gy) reirradiation using mainly external beams in salvaging local failures of nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys 1998;40:897-913. [Crossref] [PubMed]
- Lee AW, Foo W, Law SC, et al. Reirradiation for recurrent nasopharyngeal carcinoma: factors affecting the therapeutic ratio and ways for improvement. Int J Radiat Oncol Biol Phys 1997;38:43-52. [Crossref] [PubMed]
- Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma--treatment techniques and results. Int J Radiat Oncol Biol Phys 1987;13:953-6. [Crossref] [PubMed]
- Shen X, Li Y, Zhang Y, et al. An analysis of brachytherapy with computed tomography-guided permanent implantation of Iodine-125 seeds for recurrent nonkeratin nasopharyngeal carcinoma. Onco Targets Ther 2015;8:991-7. [PubMed]
- Cheah SK, Lau FN, Yusof MM, et al. Treatment outcome with brachytherapy for recurrent nasopharyngeal carcinoma. Asian Pac J Cancer Prev 2014;14:6513-8. [Crossref] [PubMed]
- Hsiung CY, Yorke ED, Chui CS, et al. Intensity-modulated radiotherapy versus conventional three-dimensional conformal radiotherapy for boost or salvage treatment of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2002;53:638-47. [Crossref] [PubMed]
- Qiu S, Lin S, Tham IW, et al. Intensity-modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2012;83:676-83. [Crossref] [PubMed]
- Hua YJ, Han F, Lu LX, et al. Long-term treatment outcome of recurrent nasopharyngeal carcinoma treated with salvage intensity modulated radiotherapy. Eur J Cancer 2012;48:3422-8. [Crossref] [PubMed]
- Han F, Zhao C, Huang SM, et al. Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:569-76. [Crossref] [PubMed]
- Kong L. Salvage intensity-modulated radiation therapy (IMRT) for locally recurrent nasopharyngeal cancer after definitive IMRT: treatment outcomes of a clinically distinct condition in the modern era. (In Press).
- Xiao W, Liu S, Tian Y, et al. Prognostic significance of tumor volume in locally recurrent nasopharyngeal carcinoma treated with salvage intensity-modulated radiotherapy. PLoS One 2015;10:e0125351. [Crossref] [PubMed]
- Qiu S, Lu J, Zheng W, et al. Advantages of intensity modulated radiotherapy in recurrent T1-2 nasopharyngeal carcinoma: a retrospective study. BMC Cancer 2014;14:797. [Crossref] [PubMed]
- Tian YM, Guan Y, Xiao WW, et al. Long-term survival and late complications in intensity-modulated radiotherapy of locally recurrent T1 to T2 nasopharyngeal carcinoma. Head Neck 2016;38:225-31. [Crossref] [PubMed]
- Chua DT, Sham JS, Leung LH, et al. Re-irradiation of nasopharyngeal carcinoma with intensity-modulated radiotherapy. Radiother Oncol 2005;77:290-4. [Crossref] [PubMed]
- Lu TX, Mai WY, Teh BS, et al. Initial experience using intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2004;58:682-7. [Crossref] [PubMed]
- Pryzant RM, Wendt CD, Delclos L, et al. Re-treatment of nasopharyngeal carcinoma in 53 patients. Int J Radiat Oncol Biol Phys 1992;22:941-7. [Crossref] [PubMed]
- Chen HY, Ma XM, Ye M, et al. Effectiveness and toxicities of intensity-modulated radiotherapy for patients with locally recurrent nasopharyngeal carcinoma. PLoS One 2013;8:e73918. [Crossref] [PubMed]
- Hua YJ, Chen MY, Qian CN, et al. Postradiation nasopharyngeal necrosis in the patients with nasopharyngeal carcinoma. Head Neck 2009;31:807-12. [Crossref] [PubMed]
- Su SF, Huang SM, Han F, et al. Analysis of dosimetric factors associated with temporal lobe necrosis (TLN) in patients with nasopharyngeal carcinoma (NPC) after intensity modulated radiotherapy. Radiat Oncol 2013;8:17. [Crossref] [PubMed]
- Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol 2000;10:200-9. [Crossref] [PubMed]
- McDonald MW, Moore MG, Johnstone PA. Risk of carotid blowout after reirradiation of the head and neck: a systematic review. Int J Radiat Oncol Biol Phys 2012;82:1083-9. [Crossref] [PubMed]
- Mazumdar A, Derdeyn CP, Holloway W, et al. Update on endovascular management of the carotid blowout syndrome. Neuroimaging Clin N Am 2009;19:271-81. Table of Contents. [Crossref] [PubMed]
- Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy--dose-volume histogram analysis. Radiology 1999;213:489-94. [Crossref] [PubMed]
- Brown AP, Urie MM, Chisin R, et al. Proton therapy for carcinoma of the nasopharynx: a study in comparative treatment planning. Int J Radiat Oncol Biol Phys 1989;16:1607-14. [Crossref] [PubMed]
- Huang YW, Pan CY, Hsiao YY, et al. Monte Carlo simulations of the relative biological effectiveness for DNA double strand breaks from 300 MeV u(-1) carbon-ion beams. Phys Med Biol 2015;60:5995-6012. [Crossref] [PubMed]
- Jensen AD, Poulakis M, Nikoghosyan AV, et al. Re-irradiation of adenoid cystic carcinoma: analysis and evaluation of outcome in 52 consecutive patients treated with raster-scanned carbon ion therapy. Radiother Oncol 2015;114:182-8. [Crossref] [PubMed]
- Combs SE, Kalbe A, Nikoghosyan A, et al. Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother Oncol 2011;98:63-7. [Crossref] [PubMed]
- Kong L, Hu J, Guan X, et al. Phase I/II Trial Evaluating Carbon Ion Radiotherapy for Salvaging Treatment of Locally Recurrent Nasopharyngeal Carcinoma. J Cancer 2016;7:774-83.


