Appropriate margin for lumpectomy excision of invasive breast cancer
Introduction
Breast-conserving therapy (BCT), consisting of lumpectomy and whole-breast irradiation, is a preferred treatment option for women with early-stage breast cancer. Six randomized prospective trials, some with follow-up of 20 years or more (1,2) have demonstrated no difference in survival between early-stage breast cancer patients treated with mastectomy compared to breast-conserving surgery and radiotherapy (RT). Over time, rates of local recurrence after BCT have declined steadily and are now considerably less than 10% at ten years of follow-up (3,4). However, the appropriate extent of surgical resection needed to maintain local control following lumpectomy remains a matter of debate, and the lack of consensus regarding what constitutes an adequate negative margin results in multiple trips to the operating room for margin re-excision in a significant number of patients, and unnecessary mastectomies in others (5).
The demonstration in the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview that differences in local control between treatments of 10% to 20% at 5 years are associated with significant differences in breast cancer-specific survival at 15 years (6) has focused new attention on the importance of local control. For many years, disease burden as defined by margin status was felt to be the primary determinant of local control. Over time, it has become increasingly clear that both the underlying biology of the tumor and the availability of effective systemic therapy are also critical components of local control. In this article, we will review the available data on the relationship between margin status and local control for invasive cancer, and discuss the impact of molecular subtypes of breast cancer and systemic therapy, including targeted therapy, on local control outcomes.
Margin evaluation
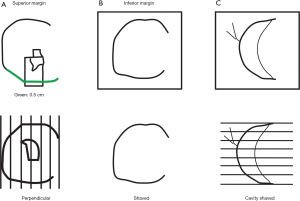
There is no standard method of margin evaluation for lumpectomy specimens, nor are there a standard number of histologic sections which are examined from each margin surface. Margins can be evaluated using a radial (perpendicular) method, a shaved (en face) method, or by shaving the walls of the lumpectomy cavity (separate shaved cavity margins) (Figure 1). Although each method has its own advantages and disadvantages, the separate shaved cavity margin technique avoids disorientation of the specimen by having the surgeon designate the margin and has been shown by several investigators to reduce the rate of re-excision for close margins when compared to traditional margin assessment (Table 1) (7-13). A recent prospective randomized study comparing the cavity shave technique to standard perpendicular margin assessment confirmed a lower rate of positive margins in the cavity-shave group (19% vs. 34%, respectively; P=0.01), although the positive margin rate was high in both groups (13).
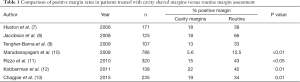
Full table
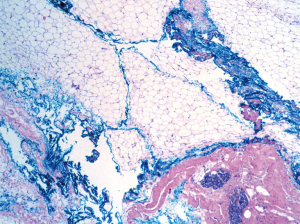
In addition to the method of margin assessment, other factors related to specimen processing may influence the margin width or the rate of margin positivity. Graham et al. compared the measurement of the mean height of the lumpectomy specimen measured by the surgeon in the operating room to the measurement in the pathology lab in 100 consecutive specimens (14) and found that breast specimens lost almost 50% of their height after surgical removal; this “pancake phenomenon” clearly impacts margin assessment. Other factors, such as running ink, imprecise margin orientation, and surface complexity may also compromise margin evaluation. Running of the ink from the irregular fatty specimen surface to the inside of the specimen, and different color inks running into each other, occur frequently, leading to possible over-interpretation and false-positive margins (Figure 2).
The relationship between margin width and local recurrence in invasive cancer
What constitutes a “negative” margin?
Given the lack of standardization in pathology methods, it is not surprising that there has historically been little consensus regarding what constitutes an adequate negative margin. Azu et al. (15) surveyed a population-based sample of 318 surgeons identified from breast cancer patients in the Surveillance, Epidemiology, and End Results (SEER) registry. Surgeons were asked ”What negative margin width precludes the need for re-excision in a 60-year-old with a 0.8 cm invasive cancer which is estrogen receptor (ER), progesterone receptor (PR), and HER2 negative?” and offered the options of tumor not touching ink, >1–2, >5, or >10 mm. No single answer was selected by more than 50% of respondents. Only 11% endorsed margins of tumor not touching ink, 42% of >1–2 mm, 28% of >5 mm and 19% of >10 mm. Similar variation exists among radiation oncologists. In a survey of 1,133 North American and European radiation oncologists, 45% of those from North America endorsed a margin of tumor not touching ink, while those in Europe favored more widely clear margins, with greater than 5 mm being the most common answer, selected by 29% (16). The net result of the lack of consensus on what constitutes an adequate negative margin is the frequent performance of re-excision to obtain more widely clear margins. Morrow et al. (5), reporting on a population-based sample from the SEER registry of 800 women attempting BCT, observed that although the procedure was successful in 88%, 22% underwent a re-excision to obtain wider margins. Other studies report a wide variation in re-excision rates ranging from 6% (10) to 49% (17), with the majority noting re-excision in 15% to 30% of patients (7,18,19).
The prospective randomized trials that established the safety and efficacy of BCT (1,2,20-23) do not provide much guidance on the margin question since only the NSABP B06 trial used a microscopic definition of a negative margin, which was tumor not touching ink (1). Although the other trials are often perceived as requiring more widely clear margins, they relied upon gross margin definitions, making the actual margin width impossible to assess. Similarly, although a trial by Veronesi et al., which randomized patients to quadrantectomy or a more limited tumorectomy, demonstrated a lower rate of local recurrence in the quadrantectomy group (2.2% vs. 7.0%), this study also relied on gross margin assessment. The tumorectomy was performed with a gross margin of 1 cm, but in a subset of patients who had microscopic margin evaluation, 16% of those in the tumorectomy group had positive margins (24). The uncertainty over margin status in this trial makes it impossible to conclude that a larger quadrantectomy type procedure is associated with a lower rate of local recurrence than a more limited resection with negative inked margins.
A systematic review of margin width and local recurrence
Houssami et al. reported the results of a methodologically rigorous meta-analysis of the relationship between margin width and local recurrence in women with invasive breast cancer. The meta-analysis included 33 studies with 28,162 patients and 1,506 local recurrences with a median follow-up of 79.2 months. The relationship between positive margin status and local recurrences was verified, with an odds ratio (OR) for local recurrence of 2.44 for positive or unknown vs. negative margins. No relationship between negative margin width, defined as 1 vs. 2 vs. 5 mm, and local recurrence was identified (25). Although a non-statistically significant numeric trend for a benefit of more widely clear margins was seen in some models, this did not persist after adjustment for other factors such as age, the use of a radiation boost, or receipt of endocrine therapy. This analysis included information on a large number of factors relevant to local recurrences, such as date of study enrollment, patient age, use of radiation including a boost, and pathologic tumor features such as lymphovascular invasion (LVI), extensive intraductal component (EIC), and tumor grade, and provides the most convincing evidence to date that margins more widely clear than tumor not touching ink do not have a major impact upon local control in the era of modern multidisciplinary therapy.
Although this may seem counterintuitive, it becomes much more logical if one considers that even mastectomy, which provides the widest possible margin, does not entirely eliminate the risk of local recurrence. In the initial randomized trials comparing BCT to mastectomy in which at least grossly negative margins were required, only the Milan study (2), which included T1 cancers treated with radical mastectomy, showed a statistically significant reduction in local recurrence for mastectomy compared to BCT (Table 2) (1,2,20,21). This, coupled with the observation from the EBCTCG overview (6) that, even with the addition of postmastectomy RT, the incidence of local recurrence is higher in node-positive women than it is in node-negative women, indicates that local recurrence may be due to either excessive tumor burden or aggressive biology. The failure to observe a decrease in local recurrence with surgical margins more widely clear than tumor on ink suggests that once disease burden is reduced to this level (i.e., no clinically detectable cancer), tumor biology is the main determinant of local control.
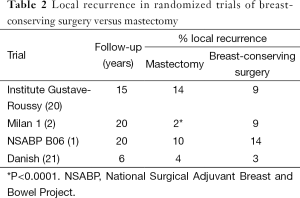
Full table
The influence of histology on margin width
Variations in the growth patterns of different histologic types of cancers raise concerns that the same margin width may not be appropriate for all histologic tumor types. Infiltrating lobular cancers are frequently multifocal and grow as single cells in linear strands separated by normal stroma (26), raising the possibility that margins negative only by tumor not touching ink might be associated with a significant residual tumor burden. However, clinical studies do not document a higher rate of local recurrence after BCT for lobular cancers when compared to ductal cancers (27-29), suggesting that if negative margins are obtained, the growth pattern is irrelevant. Galimberti et al. (30) analyzed 382 patients with pure infiltrating lobular carcinoma treated with BCT to determine if rates of local control differed among those with margins less than 1 cm compared to 1 cm or greater. The local failure rate was 4.6% for the less than 1 cm margin group compared to 3.7% in the 1 cm or greater group, leading the authors to conclude that more widely negative margins were not necessary for patients with infiltrating lobular carcinoma. Sagara et al. evaluated locoregional recurrence in a modern cohort of patients with stage I–III lobular carcinoma. Of 381 patients treated with BCS, in-breast tumor recurrence (IBTR) was significantly increased for patients with positive margins [hazard ratio (HR) =7.5, P=0.01], but it was not increased for patients with margins 1–3 mm (HR =0.57, P=0.60) or margins within 1 mm (HR =0.73, P=0.77) (31). The findings suggest that margins of “no tumor on ink” are adequate in most patients with lobular carcinoma treated with multimodality therapy.
The other group of concern is patients with an EIC in association with their invasive cancer. Early studies performed prior to the routine inking of margins suggested that an EIC was associated with higher rate of local recurrence in patients undergoing BCT (32). Holland et al. documented that approximately 30% of patients with EIC positive cancers had prominent intraductal carcinoma more than 2 cm beyond the primary tumor, compared to only 2% of patients with EIC negative tumors (33), indicating that a substantial number of patients with an EIC treated by excision to grossly negative margins have a heavy residual tumor burden. Despite the concern regarding residual disease in EIC positive cancers, additional studies have demonstrated that when patients with an EIC are excised to negative inked margins, rates of local recurrence are not increased compared to patients without an EIC (34,35). Of note, Schnitt et al. demonstrated a 5-year IBTR rate of 0% in EIC positive patients with a margin of “no tumor on ink” compared to 50% when margins were more than focally positive (36); these results should be interpreted with caution as the number of EIC positive patients in this study was small (n=30). In patients with pure ductal carcinoma in situ (DCIS), Faverly et al. have shown that low- and intermediate-grade tumors often grow with gaps between the DCIS lesions, although these gaps are usually less than 5 mm in size (37), suggesting that margins negative by only tumor not touching ink could be associated with a significant residual tumor burden. The presence of an EIC in association with invasive cancer suggests it may be prudent to consider obtaining a margin of at least 2 mm if large amounts of DCIS are in proximity to the margin. In the case of both infiltrating lobular carcinoma and an EIC, clinical judgment remains important. A single duct of DCIS or microscopic focus of lobular carcinoma in close proximity to the margin is unlikely to be associated with a heavy residual tumor burden, while a large area of tumor immediately adjacent to a margin may be associated with a greater risk of residual disease (38) and should prompt re-excision.
Other factors influencing local control in invasive cancer
It is important to recognize that a “negative” margin does not indicate that there is no residual tumor in the breast. In a landmark study using serial subgross sectioning to evaluate the remaining breast tissue in 264 mastectomy specimens from patients with clinically unifocal cancers 4 cm or less in size, Holland et al. (39) showed that only 39% of cases had no additional tumor beyond the index cancer. In 20% of cases, the additional tumor foci were within 2 cm of the index tumor, and in 41% of cases, the tumor foci were more than 2 cm from the primary tumor. From a practical point of view, a negative margin indicates that the residual tumor burden in the breast is low enough that it is likely to be controlled by RT. The role of RT in maintaining local control is well documented in the EBCTCG overview (6). At five years, the absolute incidence of local recurrence in node-negative women treated with breast-conserving surgery receiving RT was 16% lower than in those not receiving RT, while for node-positive women, a 30% reduction in isolated local recurrence was seen. These reductions in local recurrence at 5 years translate to 15-year survival gains of 5% and 7% in node-negative and node-positive women, respectively. In a prospective randomized trial examining the benefits of boost dose of RT on local control, Bartelink et al. demonstrated statistically significant reductions in local recurrence with the addition of a boost in women of all ages (40). While the role of RT in local control has long been recognized, the effect of systemic therapy on local control is less well recognized.
The majority of women with invasive breast cancer now receive some form of adjuvant systemic therapy in addition to surgery and RT. Both endocrine therapy and chemotherapy significantly reduce the likelihood of local recurrence after BCT. In the NSABP B14 trial, in which node-negative, ER positive women were randomized to tamoxifen citrate or placebo, the 10-year rate of in-breast recurrence was reduced from 14.7% in the placebo group to 4.3% in the tamoxifen group (41). In the NSABP B13 trial, node-negative ER negative women were randomized to chemotherapy or a no-treatment control group (41). At eight years, local recurrence was seen in only 2.6% of those receiving chemotherapy compared to 13.4% of controls (P=0.001). In a report of 3,799 node-negative women treated with BCT participating in five NSABP trials of adjuvant systemic therapy, the cumulative incidence of in-breast recurrence at 12 years for those receiving adjuvant therapy was only 6.6% (3). With the increasing use of systemic therapy, rates of locoregional recurrence are declining over time. Bouganim et al. performed a meta-analysis of 53 randomized clinical trials with a total of 86,598 patients and demonstrated that between 1990 and 2011, the relative frequency of locoregional recurrences was reduced by half, from over 30% to approximately 15% of all recurrences, which was largely related to the use of chemotherapy and/or endocrine therapy (42). Since the time when these trials were conducted, systemic therapy options have improved with the introduction of targeted therapy and better cytotoxic chemotherapy, and this will undoubtedly result in a further decrease in local recurrence rates. For example, in the randomized trials that established the efficacy of adjuvant trastuzumab, the addition of trastuzumab to chemotherapy resulted in a 50% decrease in locoregional recurrence compared to treatment with chemotherapy alone (43). Similar results have been reported in ER positive, node-negative patients when systemic treatment is selected on the basis of the Oncotype DX™ (Genomic Health, Redwood City, CA) recurrence score (RS). Although the RS was developed to predict the risk of systemic recurrence, Mamounas et al. (44) demonstrated that in patients treated with placebo, Oncotype DX score also correlated with risk of locoregional failure. The 10-year estimates for locoregional recurrence (LRR) were 18.4% in patients with a high RS compared to 10.8% in those with a low RS (P=0.022). The addition of tamoxifen, which is considered appropriate treatment for those with low RS, reduced the incidence of LRR by more than 50% to 4.3% in the low-risk group. In contrast, a much more modest reduction in LRR from 18.4% to 15.8% was seen with tamoxifen in patients with a high RS. However, when chemotherapy was added, the 10-year LRR decreased to 7.8% in those with a high RS.
The importance of biology and targeted therapy is further supported by the emerging literature on the impact of tumor subtype on local recurrence after BCT or mastectomy. Both Millar et al. (45) and Nguyen et al. (46) have demonstrated that the rate of local recurrence after BCT varies among the intrinsic subtypes of breast cancer as approximated by ER, PR, and HER2 status. In both studies, the lowest rates of local recurrence at 5 years were seen among the ER positive, PR positive, HER2 negative (Luminal A-like) group, and the highest rates were among the triple-negative (basal-like) and ER negative, HER2 positive patients in the absence of adjuvant trastuzumab. However, ER, PR, and HER2 status are not indicators of the need for more widely clear margins, as chest wall recurrences after mastectomy are also more likely in ER negative patients, regardless of HER2 status, as reported in a retrospective analysis of the Danish Breast Cancer Group randomized trials of mastectomy with or without RT (47). A meta-analysis by Lowery et al. (48) evaluated 12,592 patients from 15 studies, of whom 7,174 were treated with BCT and 5,418 with mastectomy. Patients with ER/PR positive tumors had a lower risk of local recurrence than HER2 positive tumors (without trastuzumab) (relative risk 0.34) and triple-negative tumors (relative risk 0.38). Patients with (untreated) HER2 positive tumors had a higher risk of local recurrence than triple-negative tumors (relative risk 1.44). As previously noted, the addition of trastuzumab to chemotherapy has been shown to reduce the risk of local recurrence in HER2 overexpressing patients (43), indicating that targeted therapy is a major contributor to local control. Kiess et al. (49) validated a significant decrease in locoregional recurrence in patients treated with BCT by the addition of adjuvant trastuzumab. Among 197 patients who were treated with BCT immediately before and after adjuvant trastuzumab became available, 3-year rates of locoregional recurrence fell from 10% to 1%. Even in patients considered to have aggressive tumor biology, there is no evidence that wider margins result in a lower risk of local recurrence. Pilewskie et al. (50) evaluated local recurrence rates in 535 triple-negative breast cancers treated with BCT. Seventy-one had negative margins ≤2 mm, and 464 had negative margins >2 mm. The 5-year incidence of local recurrence was 4.7% with margins ≤2 mm and 3.7% with margins >2 mm, a difference which was not significant after controlling for tumor size and the use of chemotherapy. Moreover, in patients with aggressive tumor biology treated with systemic therapy, mastectomy, which provides the widest surgical margin, does not provide improved local control over BCT. In a study of 646 patients with T1-2N0 triple-negative breast cancer, of whom 81% received adjuvant chemotherapy, the 5-year incidence of LRR was 4.2% for patients undergoing BCT compared to 5.4% for patients treated with mastectomy (51). In aggregate, this information validates the importance of systemic therapy in local control, indicates that factors other than disease burden are key determinants of local control, and provides evidence that margins more widely clear than no tumor on ink are not indicated even for high-risk tumor subtypes.
Summary and conclusion on invasive cancer: consensus guidelines
The failure of mastectomy, the most widely clear margin which can be obtained in the breast to achieve rates of local control approaching 100%, is clear evidence that disease burden is not the only factor determining local control. Evidence that margins more widely clear than tumor not touching ink decrease local recurrence in patients receiving whole-breast RT is lacking, and the underlying biology of the tumor and the availability of targeted therapy appear to be major determinants of local control.
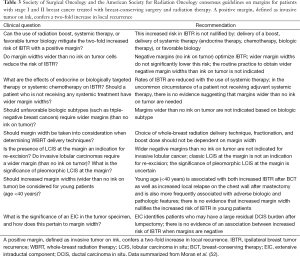
In recognition of the many factors impacting local control, a multidisciplinary panel was convened in 2013 by the Society of Surgical Oncology (SSO) and American Society for Radiation Oncology (ASTRO) to establish consensus guidelines on margin width for patients with invasive cancer undergoing BCT. The meta-analysis of Houssami et al. (25) discussed previously, as well as other published literature, formed the basis for the group’s deliberations. The group concluded that while positive margins, defined as ink on invasive tumor or DCIS, were associated with an increased rate of local recurrence, evidence that margins more widely clear than no ink on tumor reduces the risk of local recurrence is lacking, and the routine use of re-excision to more widely clear margins is not indicated. This conclusion applies independent of age, histology, biologic subtype, the presence of an EIC, or the now-uncommon scenario of no planned adjuvant systemic therapy. The consensus statements are summarized in Table 3 (52). These guidelines have been endorsed by the American Society of Clinical Oncology (ASCO) and the American Society of Breast Surgeons (ASBrS) in addition to the SSO and ASTRO. It is hoped that their adoption will decrease re-excision rates and lower healthcare costs (52). This does not mean that in some circumstances a more widely clear margin is not appropriate; but it does mean that the routine use of unnecessarily large surgical resections or mandatory re-excisions to obtain a more widely clear margin in all patients should be abandoned.
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol 2009;27:2466-73. [Crossref] [PubMed]
- Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol 2006;24:2028-37. [Crossref] [PubMed]
- Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA 2009;302:1551-6. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Huston TL, Pigalarga R, Osborne MP, et al. The influence of additional surgical margins on the total specimen volume excised and the reoperative rate after breast-conserving surgery. Am J Surg 2006;192:509-12. [Crossref] [PubMed]
- Jacobson AF, Asad J, Boolbol SK, et al. Do additional shaved margins at the time of lumpectomy eliminate the need for re-excision? Am J Surg 2008;196:556-8. [Crossref] [PubMed]
- Tengher-Barna I, Hequet D, Reboul-Marty J, et al. Prevalence and predictive factors for the detection of carcinoma in cavity margin performed at the time of breast lumpectomy. Mod Pathol 2009;22:299-305. [Crossref] [PubMed]
- Marudanayagam R, Singhal R, Tanchel B, et al. Effect of cavity shaving on reoperation rate following breast-conserving surgery. Breast J 2008;14:570-3. [Crossref] [PubMed]
- Rizzo M, Iyengar R, Gabram SG, et al. The effects of additional tumor cavity sampling at the time of breast-conserving surgery on final margin status, volume of resection, and pathologist workload. Ann Surg Oncol 2010;17:228-34. [Crossref] [PubMed]
- Kobbermann A, Unzeitig A, Xie XJ, et al. Impact of routine cavity shave margins on breast cancer re-excision rates. Ann Surg Oncol 2011;18:1349-55. [Crossref] [PubMed]
- Chagpar AB, Killelea BK, Tsangaris TN, et al. A Randomized, Controlled Trial of Cavity Shave Margins in Breast Cancer. N Engl J Med 2015;373:503-10. [Crossref] [PubMed]
- Graham RA, Homer MJ, Katz J, et al. The pancake phenomenon contributes to the inaccuracy of margin assessment in patients with breast cancer. Am J Surg 2002;184:89-93. [Crossref] [PubMed]
- Azu M, Abrahamse P, Katz SJ, et al. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol 2010;17:558-63. [Crossref] [PubMed]
- Taghian A, Mohiuddin M, Jagsi R, et al. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg 2005;241:629-39. [Crossref] [PubMed]
- Waljee JF, Hu ES, Newman LA, et al. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol 2008;15:1297-303. [Crossref] [PubMed]
- Kurniawan ED, Wong MH, Windle I, et al. Predictors of surgical margin status in breast-conserving surgery within a breast screening program. Ann Surg Oncol 2008;15:2542-9. [Crossref] [PubMed]
- Lovrics PJ, Cornacchi SD, Farrokhyar F, et al. Technical factors, surgeon case volume and positive margin rates after breast conservation surgery for early-stage breast cancer. Can J Surg 2010;53:305-12. [PubMed]
- Arriagada R, Lê MG, Rochard F, et al. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol 1996;14:1558-64. [PubMed]
- Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr 1992.19-25. [PubMed]
- Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 2003;98:697-702. [Crossref] [PubMed]
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. [Crossref] [PubMed]
- Veronesi U, Volterrani F, Luini A, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer 1990;26:671-3. [Crossref] [PubMed]
- Houssami N, Macaskill P, Marinovich ML, et al. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol 2014;21:717-30. [Crossref] [PubMed]
- Dillon DA, Guidi AJ, Schnitt SJ. Pathology of invasive breast cancer. In: Harris JR, Lippman ME, Morrow M, et al. editors. Diseases of the Breast. 4th ed. Philadelphia, Pa: Lippincott-Williams & Wilkins, 2010:374-407.
- Molland JG, Donnellan M, Janu NC, et al. Infiltrating lobular carcinoma--a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast 2004;13:389-96. [Crossref] [PubMed]
- Peiro G, Bornstein BA, Connolly JL, et al. The influence of infiltrating lobular carcinoma on the outcome of patients treated with breast-conserving surgery and radiation therapy. Breast Cancer Res Treat 2000;59:49-54. [Crossref] [PubMed]
- Santiago RJ, Harris EE, Qin L, et al. Similar long-term results of breast-conservation treatment for Stage I and II invasive lobular carcinoma compared with invasive ductal carcinoma of the breast: The University of Pennsylvania experience. Cancer 2005;103:2447-54. [Crossref] [PubMed]
- Galimberti V, Maisonneuve P, Rotmensz N, et al. Influence of margin status on outcomes in lobular carcinoma: experience of the European Institute of Oncology. Ann Surg 2011;253:580-4. [Crossref] [PubMed]
- Sagara Y, Barry WT, Mallory MA, et al. Surgical Options and Locoregional Recurrence in Patients Diagnosed with Invasive Lobular Carcinoma of the Breast. Ann Surg Oncol 2015;22:4280-6. [Crossref] [PubMed]
- Harris JR. Breast-conserving therapy as a model for creating new knowledge in clinical oncology. Int J Radiat Oncol Biol Phys 1996;35:641-8. [Crossref] [PubMed]
- Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol 1990;8:113-8. [PubMed]
- Bartelink H, Horiot JC, Poortmans P, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med 2001;345:1378-87. [Crossref] [PubMed]
- Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol 2000;18:1668-75. [PubMed]
- Schnitt SJ, Abner A, Gelman R, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer 1994;74:1746-51. [Crossref] [PubMed]
- Faverly DR, Burgers L, Bult P, et al. Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol 1994;11:193-8. [PubMed]
- Darvishian F, Hajdu SI, DeRisi DC. Significance of linear extent of breast carcinoma at surgical margin. Ann Surg Oncol 2003;10:48-51. [Crossref] [PubMed]
- Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 1985;56:979-90. [Crossref] [PubMed]
- Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol 2007;25:3259-65. [Crossref] [PubMed]
- Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 1996;88:1529-42. [Crossref] [PubMed]
- Bouganim N, Tsvetkova E, Clemons M, et al. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat 2013;139:603-6. [Crossref] [PubMed]
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [Crossref] [PubMed]
- Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 2010;28:1677-83. [Crossref] [PubMed]
- Millar EK, Graham PH, O'Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol 2009;27:4701-8. [Crossref] [PubMed]
- Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 2008;26:2373-8. [Crossref] [PubMed]
- Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol 2008;26:1419-26. [Crossref] [PubMed]
- Lowery AJ, Kell MR, Glynn RW, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 2012;133:831-41. [Crossref] [PubMed]
- Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 2012;118:1982-8. [Crossref] [PubMed]
- Pilewskie M, Ho A, Orell E, et al. Effect of margin width on local recurrence in triple-negative breast cancer patients treated with breast-conserving therapy. Ann Surg Oncol 2014;21:1209-14. [Crossref] [PubMed]
- Zumsteg ZS, Morrow M, Arnold B, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol 2013;20:3469-76. [Crossref] [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology–American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer. J Clin Oncol 2014;32:1507-15. [Crossref] [PubMed]


