Surgical management of intrahepatic cholangiocarcinoma in the modern era: advances and challenges
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer and it has been increasing in incidence and mortality (1,2). This increase can be at least partially attributed to our improved ability to accurately diagnose ICC and differentiate it from other adenocarcinomas (3).
Unlike hepatocellular carcinoma (HCC) which arises in the setting of chronic liver disease [i.e., from viral hepatitis, alcohol abuse, and/or nonalcoholic steatohepatitis (NASH) (4)], ICC often occurs in patients with no existing risk factors. Therefore, the application of prevention or screening strategies is not feasible. Consequently, unlike HCC where the status of the underlying liver parenchyma condition is crucial in the selection between resection versus transplantation, ICC usually does not appear in a cirrhotic environment making surgical resection the mainstay of treatment.
Hepatic resections can now be performed safely in tertiary centers with minimal mortality and acceptable morbidity (5,6). The 5-year survival rate for patients who undergo resection with curative intent however is only 21% to 35% in the largest series (7-10). Most patients suffer from disease recurrence, and the majority of these recurrences occur within the liver itself (11). Due to the lack of phase 3 trials there is no established adjuvant treatment. Extrapolating treatment strategies from advanced biliary cancer, gemcitabine based regimens are usually utilized in the adjuvant setting, most often as a doublet with cisplatin or oxaliplatin (12,13).
The technique of hepatic resection itself is changing. Minimally invasive hepatic resections are increasingly being utilized for the treatment of primary and metastatic liver cancer. Their advantages of faster recovery, decreased pain and better cosmetic outcome coupled with comparable morbidity, mortality and oncologic outcomes has made minimally invasive hepatic resection a desirable option.
For advanced ICC, locoregional treatments are increasingly being utilized in an effort to control the liver disease. However, their application has never been studied in phase 3 clinical trials making their use limited. Small studies have described varying degrees of effectiveness (14-16). New treatment modalities such as proton beam therapy (PBT) have started to emerge in an effort to control liver disease effectively.
Even though the changes in systemic chemotherapy are not a primary aim of this article, highlighting some recent changes is important. After the introduction of the gemcitabine/cisplatin doublet as a gold standard for advanced biliary cancer (12) our increasing knowledge of the genetics of biliary cancer helps us adopt a targeted approach and introduce new agents to the existing gemcitabine based regimens for improved outcomes (17).
Methods
A literature search was conducted using PubMed and the most recent literature regarding treatment of resectable and advanced unresectable ICC was reviewed. Selected studies were utilized for this review based on their significance and innovation.
Early intrahepatic cholangiocarcinoma (ICC): surgical resection
Surgical resection represents the mainstay of treatment for patients with early ICC. However, only 20–40% of patients with potentially operable disease are offered surgical resection (14). Furthermore, patients who undergo resection with curative intent experience recurrences and a poor 5-year survival of 21–35% (7-10,18).
Most of the large surgical studies are multi-institutional given the rarity of the disease. Common characteristics of the largest modern series are: major hepatic resections in 70–86%, large tumors with median sizes of 5.5–7.7 cm and lymph node metastases in 22–37% (7-10). Recurrence has been reported to occur in up to 50–60% of patients with a median-disease free survival of 26 months (15). Factors associated with tumor recurrence and survival in the largest series include multiple tumors, vascular invasion, and lymph node metastases (Table 1) (7-10).
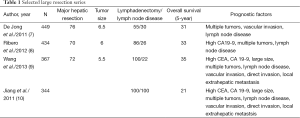
Full table
Lymphadenectomy
The role of routine lymphadenectomy during hepatic resection for ICC represents still an issue of debate. Lymph node disease seems to occur in 22–37% and is associated with a worse median survival (7,15). Opponents to routine lymphadenectomy outline the lack of any proven therapeutic benefit associated with this procedure along with a risk of complications (19).
Besides its prognostic role it seems that lymphadenectomy can potentially serve to select patients for adjuvant treatment. Patients with nodal positive disease seem to benefit the most from adjuvant treatment (20).
Adjuvant treatment
Given the lack of phase 3 clinical trials regarding adjuvant treatment after resection with curative intent, the current practice extrapolates data from advanced biliary cancers (12).
We reported our experience on adjuvant treatment in a modern series of resections with curative intent (21). One third of patients received adjuvant treatment most frequently with gemcitabine based doublet regimens. Factors associated with adjuvant treatment administration were younger age and advanced tumor (positive lymph nodes or surgical margins). Patients with nodal disease appeared to have a survival benefit.
Miura et al. reported on 2,751 patients from the National Cancer Database who received adjuvant treatment between 1998–2011 (20). In this study younger age, advanced tumor stage, R1/R2 margins and lymph node metastasis were associated with chemotherapy administration similar to our study. This study did not have details on the chemotherapy regimens, the sequence of treatments, pathology and operative characteristics and recurrences of the tumors. However, a survival benefit of adjuvant chemotherapy was found for ICC patients with nodal metastasis, advanced tumor stage, and positive resection margins. In a similar study utilizing data from the National Cancer Database on 638 patients who underwent resection the presence of positive surgical margins and positive nodes appeared to predict those patients who experience a survival benefit from the administration of adjuvant therapy (22).
Minimally invasive surgery for intrahepatic cholangiocarcinoma (ICC)
Laparoscopic and robotic approaches are increasingly being utilized in liver surgery. Although the amount of existing data is limited, there is growing evidence that minimally invasive operations are associated with lower perioperative morbidity with the same oncologic outcomes (23). These comparable results are seen when a surgeon has overcome his/her learning curve, similar to other laparoscopic procedures (24). Minimally invasive major hepatic resections are becoming more and more common as experience is being accumulated (23,25). Advantages which are frequently associated with laparoscopic surgery in general, such as less analgesia, smaller incisions, better cosmetic result, and faster recovery are applicable to liver resections as well.
Although the first international consensus on laparoscopic liver surgery suggested that the primary indication for laparoscopic approaches are single lesions 5 cm or less in peripheral segments (26), in experienced hands more extended resections can be performed with acceptable perioperative morbidity and mortality (27,28). The CO2 pneumoperitoneum provides some control of back-bleeding during liver resection (29). Major bleeding might be difficult to control laparoscopically and also is associated with specific risks related to laparoscopy such as gas embolism (23). Hand-assisted laparoscopic surgery (HALS) and the hybrid method can be used when intraoperative difficulties are being encountered and they may decrease the rate of conversion to an open procedure (25). HALS is also considered safer when training surgeons to perform laparoscopic liver resections (LRR).
With regards to the long term oncologic outcome a review of the international experience with LRR found 5-year survival rates comparable to open hepatic resections (23). A meta-analysis of studies focusing on long term outcome also reported similar findings (30). With regards to ICC specifically, Uy et al. in a comparison between laparoscopic and open surgery for ICC found similar 3- and 5-year disease free and overall survival rates (31).
The experience with robotic liver resections, albeit smaller, seems to be associated with comparable perioperative outcomes to laparoscopic and open approaches, but more studies on long term oncological outcomes are needed (32,33). Robotic surgery is associated with some intrinsic benefits. Visual advantages include 3-dimensional view, improved depth perception, and a magnification capability. Other technical advantages include the use of articulating instruments, increased degrees of freedom, and filtration of the tremor (34). In the few existing comparative studies, robotic operations appear to be equally effective with open and laparoscopic operations with some authors supporting that it allows for better suturing in confined spaces, facilitating for example biliary reconstruction (35). In a recent review of robotics for oncologic operations, it was shown that robotic surgery is widely used for a variety of operations and they may offer short-term benefits with comparable safety and oncologic outcomes (36). Prospective, randomized, comparative studies are needed before any definite statements can be made, especially in light of a significant cost difference for the robot in a climate of cost cutting measures.
The recent international consensus conference on laparoscopic liver surgery attempted to review the current status of LRR and provide recommendations for its use and development (37). Utilizing the existing reported worldwide experience and categorizing the existing data based on GRADE (38) and the stage of development of the various laparoscopic procedures using the Balliol classification of IDEAL (39). This consensus concluded that minor (two Couinaud segments or less) LRRs have become standard of practice and major LRRs are still innovative procedures and their cautious introduction is recommended. Laparoscopic outcomes were not inferior in terms of short-term outcomes and overall survival and were found to be superior in terms of postoperative complications and shorter length of stay. Based on the existing data the oncologic outcomes appear to be comparable to open hepatic resections. A summary of the most important recommendations based on short and long term outcomes from the second international consensus conference is demonstrated in Table 2.

Full table
It cannot be emphasized enough that the reports of LRR and robotic liver resections (RLR) come from high-volume, specialized centers, where surgeons with an extensive experience in both open and minimally invasive surgery are operating on highly selected patients.
Advanced intrahepatic cholangiocarcinoma (ICC) intra-arterial therapies (IATs)
ICC unlike HCC is typically hypovascular, which makes the delivery of therapies more challenging. Hepatic artery-based therapies are based on the dual blood supply of the liver. Normal liver parenchyma derives approximately 75% of its blood from the portal vein whereas liver tumors (both metastatic and primary) derive 80% to 100% of their blood supply from the hepatic artery (40). Thus by utilizing the hepatic artery, treatments can be delivered effectively into the tumor bed while minimizing toxicity to the healthy liver parenchyma. The different types of hepatic artery based treatment modalities that have been utilized for ICC include the following: hepatic arterial infusion (HAI), bland embolization, transarterial chemoembolization (TACE) and radioembolization.
One important topic in advanced ICC is the need of standardization of tumor response to therapy. Current accepted criteria for tumor response include Response Evaluation Criteria in Solid Tumors (RECIST), modified RECIST (mRECIST), and the European Association for the Study of the Liver (EASL) criteria (Table 3). RECIST is based on tumor size reduction, mRECIST on the longest viable tumor dimension, and EASL on the viable tumor reduction in an area of enhancement.
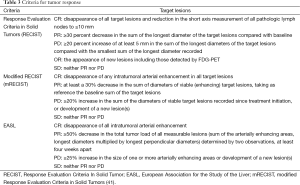
Full table
The European Association for the Study of the Liver recommended a modification of the RECIST criteria in 2000 to take into account tumor necrosis induced by treatment (42). The concept of viable tumor (defined as uptake of contrast agent in the arterial phase of dynamic CT or MRI) was introduced and the modified RECIST (mRECIST) criteria were based on its change in size (43). Bi-dimensional reductions in viable tumor can be assessed using dynamic imaging (44).
Hepatic arterial infusion chemotherapy (HAI) (regional chemotherapy, transcatheter arterial chemoinfusion)
HAI delivers high concentrations of chemotherapeutic agents directly to the liver utilizing a catheter in the hepatic artery. A variety of agents have been utilized including floxuridine (FUDR) (45), mitomycin C (46), epirubicin with cisplatin (47) and 5-fluorouracil (5-FU) (48).
In the US most of the existing experience involves the use of FUDR, which has approximately 95% first-pass clearance. This allows for high concentrations of chemotherapy to be delivered to the liver, minimizing systemic toxicity (49).
The Memorial Sloan Kettering group published two phase II randomized controlled trials with the use of floxuridine with or without bevazizumab (45,50). In the first trial (45) reporting on 26 patients with locally advanced ICC the use of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was found to correlate with survival. In the second trial (50) reporting on 18 ICC patients the addition of bevacizumab did not improve response or survival but was associated with significant biliary toxicity. Therefore FUDR with bevacizumab was abandoned. In a recent update on the 44 ICC patients from these two trials (51), 98% of patients either responded or had stable disease and experienced a median survival of almost 30 months with 23% of patients surviving more than 3 years.
Chemoembolization (TACE)
Bland particle embolization is based on the injection of small particles (40–120 mm) into the arterial supply of the tumor causing terminal vessel blockade and subsequent ischemic necrosis. TACE involves the injection of microspheres loaded with a chemotherapeutic agent (most commonly doxorubicin) into the tumor via the hepatic artery. Chemoembolization results in a cytotoxic and ischemic effect on the cancer cells, and in many institutions has largely replaced plain embolization (52). The advantages of this technique include the ability to treat multiple tumors, match the particle sizes to the vascular characteristics of the tumor, limited toxicity and ability to repeat the procedure (53).
In 2006, the Society of Interventional Radiology published a consensus statement (54) on chemoembolization of hepatic malignancies. Specifically, for ICC chemoembolization was considered a viable option for well compensated patients with unresectable cholangiocarcinoma.
Gusani et al. (55) reported on 42 patients treated with TACE using gemcitabine as the intra-arterial drug (alone, 18/42), as well as combining or following with cisplatin and/or oxaliplatin (24/42). The median overall survival was 9.1 months from the date of the procedure. Burger et al. (56) reported on patients treated with one or more cycles of TACE between 1995 and 2004 at Johns Hopkins Hospital. The median survival was 23 months. The investigators suggested that TACE was effective at prolonging the overall survival of patients with unresectable cholangiocarcinoma. Kim et al. (57) treated 49 patients with unresectable ICC with a mixture of techniques: using TACE and/or TACI. Twenty-one patients underwent only TACE (cisplatin, gelform, lipiodol), 13 patients underwent only TACI (cisplatin alone), and 15 patients underwent both TACE and TACI. The median survival periods from the time of diagnosis were 12 months.
Radioembolization
Radioembolization utilizes yttrium-90 (Y90) loaded microspheres administered into the hepatic artery and acts through the emission of beta radiation. The treatment takes place over weeks due to the long half-life (58). The therapy is well tolerated, the beads are of small size and subsequently the embolic effect and occurrence of post-embolization syndromes are low. However, there is risk of shunting of radioactive particles into the lung causing pulmonary fibrosis and radiation related liver toxicity (59). Expertise with the technique is needed as any particles situated more than 3 mm from the tumor, will not have a direct antitumor effect (60).
Small studies with approximately 20 patients demonstrate an overall median survival of 9–22 months for patients with a good performance status and peripheral tumor type (61-64).
Studies comparing different treatments
Most of the studies on IATs are limited to small size and single institution setting (Table 4). A retrospective multi-institutional study that included 198 patients reported on the safety and efficacy of IATs [TACE (65%) and Y90 (23%)]. The median overall survival was 13.2 months and it was similar between the two treatments (65). Kim et al. reporting on 49 patients with unresectable ICC who received either TACE or TACI reported a median survival of 12 months with tumor vascularity being the only independent predictor associated with response (57). Boehm et al. in a recent meta-analysis comparing the existing data on HAI, TACE, DEB-TACE and Y90 for unresectable ICC found the highest median OS for HAI (22.8 months) versus 13.9 months for Y90, 12.4 months for TACE and 12.3 months for DEB-TACE. The authors concluded that for unresectable ICC HAI offered the best outcomes in terms of tumor response and survival, but the therapy is limited due to toxicity (16).
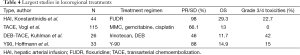
Full table
Other therapies for advanced ICC
Stereotactic radiotherapy
Stereotactic radiotherapy is based on delivery of radiation to the tumor via computer modeling, while sparing the normal liver parenchyma. Consequently, it is a well tolerated, outpatient procedure. Ibarra et al. in a series of 32 patients from 4 institutions, reported a median overall survival of 11 months (66), similar to a recent phase I study in 10 patients with unresectable ICC (67). Zeng et al. reported on 45 patients with unresectable ICC, 22 of whom received external-beam radiation therapy resulting in a 2-year survival of 19% (68).
Photodynamic therapy (PDT)
PDT is a two-stage treatment. A photosensitizer is given systemically and taken up by the cancer cells. Most photosensitizers are modified hematoporphyrins. Normal tissue is unaffected as it does not take up the photosensitizer nor is it subsequently exposed to light. Endoscopic direct illumination of the tumor bed with a specific wavelength of light resulting in the activation of the porfimer, generating oxygen free radicals, which results in ischaemic cancer cell death (69). Tissue penetration which can be achieved is approximately 4 mm and hence it is regarded as a palliative option (69).
Ortner et al. (70) reported on 39 patients who were randomized to receive stenting or stenting with PDT for advanced disease and poor performance status that precluded chemotherapy. The study was terminated early as there was significant survival benefit for PDT (493 vs. 98 days).
Emerging therapies-proton beam therapy (PBT)
The application of PBT for gastrointestinal cancers and particularly liver cancer is gaining increasing attention. Protons have a favorable irradiation profile compared to conventional X-rays and consequently they allow for better sparing of organs at risk, as well as reduction in integral dose to the patient giving them dosimetric advantages compared to photons (71,72). This allows the delivery of potentially curative doses to the target tumor, without increased risk of radiation induced liver toxicity. Preservation of non-cancerous liver parenchyma is particularly advantageous for primary liver tumors in the presence of cirrhosis or a heavily pretreated liver.
The biological effectiveness of protons relative to photons [defined as relative biological effectiveness (RBE)] is usually estimated as 1.1 (73). High-dose PBT therapy can be safely combined with locoregional or systemic therapies, and incorporated in combined treatment regimens (74).
Ohkawa et al. reported on 20 patients treated with PBT (12 for cure and 8 for palliation). Median survivals of 27.5 months and 9.6 months respectively were reported (75). The Massachusetts General Hospital experience with PBT in primary and metastatic liver tumors was first reported in a prospective feasibility study in 15 patients demonstrating effectiveness and acceptable toxicity profile of the treatment (76). In a subsequent multi-institutional phase 2 trial reporting on 43 ICCs grade 3 RT-related toxicity was 14%, median progression free survival was 10 months and median overall survival was 23 months (77). These promising results are adding a new weapon in the armamentarium against ICC which can be combined with other treatment modalities.
Systemic chemotherapy
The dublet of gemcitabine/cisplatin was established as a gold standard for advanced biliary cancer after the ABC trial demonstrated a benefit over gemcitabine alone in progression free survival and overall survival (12). Another gemcitabine based dublet, gemcitabine and oxaliplatin (GemOx) leads to similar response and survival rates (13).
Recent genetic studies provide more information about the genetic background of these tumors and help identify potential therapeutic targets (78-80). Exome sequencing of liver fluke-associated tumors revealed TP53 (44%), KRAS (17%), SMAD4 (17%), MLL (15%) as the most frequently mutated genes (81). In a recent multi-institutional genomic profiling study, including our institution, utilizing resected ICC tumor specimens from 200 patients from 7 centers found that most somatic mutations were met in low frequency and only IDH1 and KRAS were mutated in >5% (15.5% and 8.6% respectively) (79). Mutant IDH seems to block liver progenitor cells from undergoing hepatocyte differentiation and promotes biliary cancer as we demonstrated in a novel genetically engineered mouse model of IDH-driven biliary cancer (82).
Other studies have shown that the epidermal growth factor receptor (EGFR) gene is involved in the pathogenesis of biliary tract cancer in 38–100% of patients (83,84). Unfortunately, anti-EGFR antibodies failed to consistently demonstrate a survival benefit (85,86). A phase III study in South Korea, involving 11 hospitals and a total of 268 patients with advanced biliary tract cancer, compared the combination of GemOx with or without erlotinib. Overall survival was equal in both groups at 9.5 months (17). An analysis of the ICC patients revealed a greater increase in the progression free survival with the addition of erlotinib (5.9 versus 3 months).
The vascular endothelial growth factor (VEGF) is the most potent angiogenic factor expressed in ICC (87). The combination of GemOx and bevacizumab was evaluated in a phase II trial of 35 patients with a median PFS of 7.0 months, and OS of 12.7 months (88). Another phase II study reporting on 43 patients with advanced cholangiocarcinoma and 10 patients with gallbladder cancer who received erlotinib plus bevacizumab reported an overall survival of 9.9 months (89). We reported a phase II trial with the use of cabozantinib (XL-184), a dual VEGF receptor and c-MET small molecule inhibitor, for unresectable or metastatic cholangiocarcinoma (18 ICC patients) with progression of disease after 1 or 2 lines of systemic chemotherapy (90). In this unselected population with advanced disease resistant to prior systemic chemotherapy the results were limited (median progression free survival 1.77 months, median overall survival 5.2 months), and the rate of grade 3 and 4 toxicities was high (79%) leading to study early termination.
Conclusions
ICC is increasing in incidence and mortality. Surgical resection is the mainstay of therapy, however the majority of patients present with advanced unresectable cancers. For patients who undergo resection with curative intent lymphadenectomy offers prognostic information and can act as a selection tool for patients who would benefit from chemotherapy. Even patients who undergo resection with curative intent experience frequent recurrences and a dismal prognosis. Thus improvements in the adjuvant treatment are urgently needed.
Locoregional and systemic therapies are evolving. IATs are used more frequently and can act synergistically with systemic chemotherapy. The current standard of care for systemic chemotherapy remains the doublet of gemcitabine and cisplatin as the addition of biologic agents has failed to lead to significant improvements in survival so far. New therapeutic modalities such as PBT are being introduced and show promising results. A better understanding of the biology of ICC will allow for more targeted treatments to be added to the existing chemotherapy regimens. A multidisciplinary approach will allow for the best combination of the current treatments and is the best hope for the development of new ones.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [PubMed]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [PubMed]
- Ferrone CR, Ting DT, Shahid M, et al. The Ability to Diagnose Intrahepatic Cholangiocarcinoma Definitively Using Novel Branched DNA-Enhanced Albumin RNA In Situ Hybridization Technology. Ann Surg Oncol 2016;23:290-6. [PubMed]
- Okuda K. Hepatocellular carcinoma. J Hepatol 2000;32:225-37. [PubMed]
- Huang ZQ, Xu LN, Yang T, et al. Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl) 2009;122:2268-77. [PubMed]
- Kingham TP, Correa-Gallego C, D'Angelica MI, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg 2015;220:471-9. [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [PubMed]
- Ribero D, Pinna AD, Guglielmi A, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg 2012;147:1107-13. [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [PubMed]
- Jiang W, Zeng ZC, Tang ZY, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol 2011;22:1644-52. [PubMed]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [PubMed]
- Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol 2008;15:600-8. [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [PubMed]
- Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol 2015;111:213-20. [PubMed]
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [PubMed]
- Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011;254:824-9; discussion 830. [PubMed]
- Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery 2009;145:411-6. [PubMed]
- Miura JT, Johnston FM, Tsai S, et al. Chemotherapy for Surgically Resected Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:3716-23. [PubMed]
- Konstantinidis IT, A X, Goyal L, et al. Resected intrahepatic cholangiocarcinoma: patterns of adjuvant therapy and recurrence. AHPBA 2015 Meeting Abstract 2015. Available online: www.ahpba.org/documents/15finalprogram.pdf
- Sur MD, In H, Sharpe SM, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:2209-17. [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [PubMed]
- Vigano L, Laurent A, Tayar C, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 2009;250:772-82. [PubMed]
- Buell JF, Thomas MT, Rudich S, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg 2008;248:475-86. [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [PubMed]
- Dagher I, O'Rourke N, Geller DA, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg 2009;250:856-60. [PubMed]
- Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 2013;257:205-13. [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2014;21:723-31. [PubMed]
- Parks KR, Kuo YH, Davis JM, et al. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB 2014;16:109-18. [PubMed]
- Uy BJ, Han HS, Yoon YS, et al. Laparoscopic liver resection for intrahepatic cholangiocarcinoma. J Laparoendosc Adv Surg Tech A 2015;25:272-7. [PubMed]
- Ho CM, Wakabayashi G, Nitta H, et al. Systematic review of robotic liver resection. Surg Endosc 2013;27:732-9. [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [PubMed]
- Ji WB, Wang HG, Zhao ZM, et al. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg 2011;253:342-8. [PubMed]
- Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: results for 70 resections. Surgery 2011;149:29-39. [PubMed]
- Yu HY, Friedlander DF, Patel S, et al. The current status of robotic oncologic surgery. CA Cancer J Clin 2013;63:45-56. [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [PubMed]
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. [PubMed]
- Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg 1989;76:545-8. [PubMed]
- Yaghmai V, Besa C, Kim E, et al. Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. AJR Am J Roentgenol 2013;201:80-96. [PubMed]
- Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [PubMed]
- Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol 2009;20:1589-95. [PubMed]
- Shitara K, Ikami I, Munakata M, et al. Hepatic arterial infusion of mitomycin C with degradable starch microspheres for unresectable intrahepatic cholangiocarcinoma. Clin Oncol (R Coll Radiol) 2008;20:241-6. [PubMed]
- Cantore M, Mambrini A, Fiorentini G, et al. Phase II study of hepatic intraarterial epirubicin and cisplatin, with systemic 5-fluorouracil in patients with unresectable biliary tract tumors. Cancer 2005;103:1402-7. [PubMed]
- Tanaka N, Yamakado K, Nakatsuka A, et al. Arterial chemoinfusion therapy through an implanted port system for patients with unresectable intrahepatic cholangiocarcinoma--initial experience. Eur J Radiol 2002;41:42-8. [PubMed]
- Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol 1983;10:176-82. [PubMed]
- Kemeny NE, Schwartz L, Gonen M, et al. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology 2011;80:153-9. [PubMed]
- Konstantinidis IT, Do RK, Gultekin DH, et al. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: a potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann Surg Oncol 2014;21:2675-83. [PubMed]
- Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010;52:762-73. [PubMed]
- Erinjeri JP, Salhab HM, Covey AM, et al. Arterial patency after repeated hepatic artery bland particle embolization. J Vasc Interv Radiol 2010;21:522-6. [PubMed]
- Brown DB, Geschwind JF, Soulen MC, et al. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol 2006;17:217-23. [PubMed]
- Gusani NJ, Balaa FK, Steel JL, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg 2008;12:129-37. [PubMed]
- Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 2005;16:353-61. [PubMed]
- Kim JH, Yoon HK, Sung KB, et al. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer 2008;113:1614-22. [PubMed]
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507.e2.
- Burton MA, Gray BN, Klemp PF, et al. Selective internal radiation therapy: distribution of radiation in the liver. Eur J Cancer Clin Oncol 1989;25:1487-91. [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [PubMed]
- Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer 2008;113:2119-28. [PubMed]
- Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. [PubMed]
- Hoffmann RT, Paprottka PM, Schon A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. [PubMed]
- Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. [PubMed]
- Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol 2013;20:3779-86. [PubMed]
- Ibarra RA, Rojas D, Snyder L, et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol 2012;51:575-83. [PubMed]
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-64. [PubMed]
- Zeng ZC, Tang ZY, Fan J, et al. Consideration of the role of radiotherapy for unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 75 patients. Cancer J 2006;12:113-22. [PubMed]
- Berr F. Photodynamic therapy for cholangiocarcinoma. Semin Liver Dis 2004;24:177-87. [PubMed]
- Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology 2003;125:1355-63. [PubMed]
- Engelsman M, Schwarz M, Dong L. Physics controversies in proton therapy. Semin Radiat Oncol 2013;23:88-96. [PubMed]
- Wang X, Krishnan S, Zhang X, et al. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim 2008;33:259-67. [PubMed]
- Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407-21. [PubMed]
- Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 2005;23:8739-47. [PubMed]
- Ohkawa A, Mizumoto M, Ishikawa H, et al. Proton beam therapy for unresectable intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol 2015;30:957-63. [PubMed]
- Hong TS, DeLaney TF, Mamon HJ, et al. A prospective feasibility study of respiratory-gated proton beam therapy for liver tumors. Pract Radiat Oncol 2014;4:316-22. [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high dose, hypofractionated proton beam therapy (HF-PBT) for unresectable primary liver cancers: Long term outcomes in patients (pts) with intrahepatic cholangiocarcinoma (ICC). J Clin Oncol 2015;33:abstr 4020.
- Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012;142:1021-31.e15.
- Zhu AX, Borger DR, Kim Y, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol 2014;21:3827-34. [PubMed]
- Voss JS, Holtegaard LM, Kerr SE, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol 2013;44:1216-22. [PubMed]
- Ong CK, Subimerb C, Pairojkul C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 2012;44:690-3. [PubMed]
- Saha SK, Parachoniak CA, Ghanta KS, et al. Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature 2014;513:110-4. [PubMed]
- Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol 2010;28:3531-40. [PubMed]
- Pignochino Y, Sarotto I, Peraldo-Neia C, et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer 2010;10:631. [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 2006;24:3069-74. [PubMed]
- Park BK, Paik YH, Park JY, et al. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am J Clin Oncol 2006;29:138-42. [PubMed]
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48-54. [PubMed]
- Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491-7. [PubMed]
- Goyal L, Yurgelun MB, Abrams TA, et al. A phase II trial of cabozantinib (XL-184) in patients with advanced cholangiocarcinoma. J Clin Oncol 2015;33:abstr 800.


