Anticipatory extended cholecystectomy: the ‘Lucknow’ approach for thick walled gall bladder with low suspicion of cancer
Introduction
Gall stone disease (GSD) causes inflammation of the gall bladder (GB) in the form of acute cholecystitis (AC) and chronic cholecystitis (CC), both causing thickening (>3 mm) of the GB wall. The GB wall thickening in AC is transient and reversible but that in CC persists. Xantho-granulomatous cholecystitis (XGC), a variant of CC, also causes GB wall thickening. GB wall thickening in AC, CC and XGC is usually diffuse, uniform and regular.
Gall bladder cancer (GBC) also results in GB wall thickening but it is usually focal, non uniform and irregular but may sometimes be diffuse, uniform and regular as in AC, CC and XGC. A diffuse, uniform and regular thick walled GB (TWGB), thus, may be AC, CC, XGC or GBC and it may be difficult to differentiate between these on ultrasonography (US) or even computed tomography (CT) (1). As a matter of fact, it may be difficult or even impossible to differentiate between these even at surgery and, at times, XGC and GBC may even coexist (2). In some patients, an episode of AC may get complicated by intrahepatic GB perforation leading on to an abscess which may look like a mass lesion on US/CT.
While simple cholecystectomy (SC) is the treatment for AC, CC and XGC, GBC should be treated by extended cholecystectomy (EC) (cholecystectomy with liver wedge and lymphadenectomy). In presence of a TWGB, if SC is performed and it turns out to be GBC, it will be inadequate treatment; although completion extended cholecystectomy (CEC) including liver wedge and lymphadenectomy can still be performed at reoperation but tumor planes would have already been breached and outcome compromised. This is more likely to happen if GB perforation and bile spill occurs during cholecystectomy. On the other hand, if EC is performed for a TWGB on suspicion of GBC and it turns out to be AC, CC or XGC it will be an over kill. EC is a safe procedure but operation time is longer than SC and lymphadenectomy can add to post-operative complications e.g., ascitic fluid/chylous leak.
Patients with high suspicion of GBC on US/CT should be taken up directly for EC (3). We propose a new surgical approach, anticipatory extended cholecystectomy (AEC), for TWGB where differentiation between benign (complicated AC, CC or XGC) and malignant (GBC) is not possible on imaging (US or CT) or at operation but there is low suspicion of GBC. This new approach, while being complete treatment for benign disease (AC, CC and XGC), does not violate oncological planes in case the disease is malignant (GBC).
Patients and methods
A retrospective analysis of patients with TWGB (>3 mm) which was suspicious for malignancy on US and CT who were treated with AEC as a prospective protocol from January 2011 to June 2014 at a tertiary-level referral hospital in northern India. Patients with obvious findings suggestive of GBC such as GB mass, GB polyp, significant lymphadenopathy, presence of liver metastasis or ascites on US or CT were excluded from the analysis.
Patients with TWGB (GB wall >3 mm) on US were further evaluated by triple phase (arterial phase, portal phase and hepatic venous phase) CT abdomen. FNAC was not performed as a protocol because a negative FNAC does not rule out GBC and a resectable GBC does not need preoperative histological confirmation.
Details of clinical presentation, findings on imaging (US and CT), results of fine-needle aspiration cytology (FNAC), operation details (findings and procedure), frozen section biopsy and histopathology reports were reviewed.
AEC was performed in the form of removal of the GB with a non-anatomical 2 cm wedge of liver in segments IVB and V (in relation to the fundus and body of GB) and frozen section histopathological examination; lymphadenectomy was not done (cf. EC which includes lymphadenectomy also).
Results
A total of 13 AECs were performed over a period of 3 years; during the same period, 1,673 simple and 116 extended cholecystectomies were performed. The mean age of these 13 patients was 45 (range, 22–67) years; there were 7 men and 6 women. All 13 patients were symptomatic; median duration of symptoms was 3 (range, 2–58) months. All 13 patients had pain abdomen, of which 12 had biliary colic and 1 had continuous dull ache—2 patients reported a recent change in the character of pain (the final histopathology in these 2 patients was XGC). Three patients had history of AC. Two patients also had fever, 2 had weakness and 1 had anorexia; none had jaundice. GB was palpable in five patients.
US was done in all 13 patients. GB calculi were detected in all 13 patients. GB wall was thickened (irregular in 10 and diffuse in 2 patients) in 12 patients and was normal in 1 patient. GB wall thickness was mentioned in US reports in four patients and ranged from 5–10 mm; in other patients it was mentioned that GB wall is thick but the exact thickness was not mentioned. GB mass was seen in seven patients. US raised the suspicion of GBC in 11 patients and CC in 2 patients.
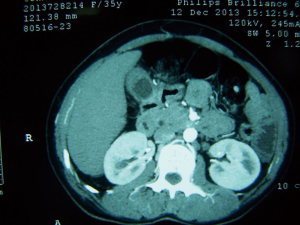
All patients were evaluated with CT because of suspicion of GBC. CT report was available in 12 patients. GB wall was thickened in all 12 patients (irregular in 8 and diffuse in 4 patients) (Figure 1). GB mass was seen in 3 patients; in 2 of these patients, interface between GB mass and liver was lost. Liver infiltration was seen in three other patients. In three patients, pericholecystic stranding was present. CT raised the suspicion of GBC in nine patients; CT diagnosis was XGC, porcelain GB, GB perforation and TWGB in one patient each.
Based on clinical presentation and US/ CT findings, it was not possible to differentiate between CC/XGC and GBC but the suspicion of GBC was low in all the 13 cases.
FNAC had been done in 2 of 13 patients before they were referred to us. In one patient, laparoscopic cholecystectomy was attempted when a GB fundus mass with pericholecystic omental and colonic adhesions was found. Because of suspicion of GBC, intraoperative FNAC was done which was inconclusive. The final histological diagnosis in this patient was XGC. In another patient, FNAC was done because of suspicion of GBC on CT; it was negative for malignancy. The final histological diagnosis in this patient was also XGC.
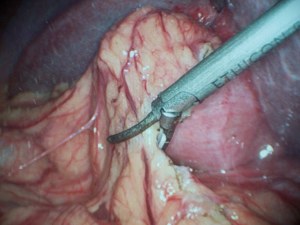
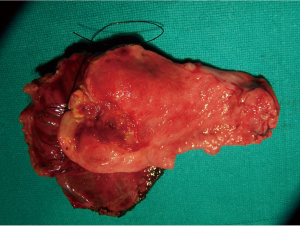
Preoperatively, there was suspicion of GBC in eight patients, TWGB in two patients, XGC, GB perforation and porcelain GB in one patient each. Because of suspicion of GBC, staging laparoscopy (Figure 2) was done to rule out peritoneal dissemination in all 13 patients. After staging laparoscopy, all patients were converted to open procedure for further surgery. GB was removed with 2 cm liver wedge (Figure 3) and the specimen was subjected to frozen section histological examination in all patients. In one patient (in whom frozen section turned out to be positive for malignancy), the GB was adherent to the common hepatic duct which was excised and Roux-en-Y hepatico-jejunostomy was done.
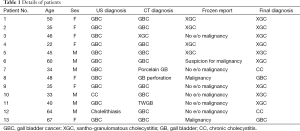
Frozen section histological examination reported malignancy in two patients and suspicious of malignancy in one patient; in ten patients, frozen section was reported as negative for malignancy. Standard lymphadenectomy was done in three patients in whom frozen section report was malignancy or suspicious of malignancy to complete the EC. Two of these were finally confirmed as GBC while one in whom frozen section report was suspicious of malignancy was finally found to have XGC (Table 1).
Full table
One patient developed fever after discharge from the hospital and was found to have an intra-abdominal collection; percutaneous catheter drain (PCD) was placed after readmission which drained bile. Hepato-biliary isotope scan revealed bile leak from the cut liver surface and bilio-enteric ductal continuity was present; endoscopic stenting controlled the bile leak and PCD was removed. One patient had high drain output but it was serous; one patient had a minor wound seroma. Median postoperative hospital stay was 4 (range, 3–14) days. There was no mortality.
Final histopathology was XGC in eight patients, CC in three patients and GBC in two patients. Frozen section histological diagnosis in both patients with GBC was positive and both had T2 N0 disease.
Discussion
We have proposed a new surgical approach, AEC, for TWGB with low suspicion of GBC on imaging (US and/or CT). AEC involves removal of the GB with wedge of liver (but without lymphadenectomy) and frozen section histological examination. If frozen section reveals malignancy (GBC), lymphadenectomy is added to complete EC. AEC is technically easy to perform and does not carry major morbidity. AEC in itself provides complete treatment for benign TWGB (AC, CC and XGC) and at the same time avoids inadequate oncological treatment for malignant TWGB (GBC) also.
GB wall >3 mm on US is defined as TWGB. TWGB on US should be further evaluated with CT (3). Focal, non-uniform and irregular TWGB is highly suspicious of GBC and should be treated as such—by open surgery and EC. Diffuse, uniform and regular TWGB is usually benign (AC, CC or XGC) but can rarely be malignant (GBC) (4). XGC mimics GBC not only on imaging (US and/ or CT) but even intraoperatively and on gross examination of the resected specimen (GB); the two may also coexist (2). Preoperative FNAC may identify most GBC and some XGC (5) but a negative FNAC does not exclude GBC. Moreover, FNAC is not recommended in resectable GBC because of fear of tumor spread along the needle tract. EUS guided FNAC from TWGB has been reported (6) but requires equipment and expertise which is not available easily and everywhere. Again, negative FNAC does not exclude GBC. Diffusion weighted MR imaging (DWI) may help to differentiate between benign and malignant TWGB but is not accurate (7). Tumor markers e.g., CEA, CA 19-9 and CA 125 have not been found to be useful to differentiate between XGC and GBC (8).
Preoperative differentiation between benign and malignant TWGB thus is difficult, especially in a high GBC incidence geographical area such as northern India.
While SC is adequate for AC, CC or XGC, EC is required for GBC. If SC is performed for TWGB harboring GBC, it will result in breach of tumor planes between GB and liver and compromise oncological principles; this will deny the possible chance of cure in an early GBC. Moreover, if SC is done laparoscopically, GB perforation and bile spill are more likely to happen and may result in peritoneal dissemination and port site recurrence in malignant TWGB (GBC). On the other hand, if EC is performed for all TWGBs, it will be an overkill for majority of TWGB which are benign (AC, CC and XGC) resulting in increased morbidity and even occasional mortality. Rammohan et al. (9) reported that 15 out of 77 patients who underwent EC on suspicion of GBC turned out to be XGC on final histology.
Jain et al. (10) reported 22 patients with XGC—radical (extended) cholecystectomy was performed in 11 patients; they used frozen section in 10 patients but after SC. As mentioned earlier, SC when performed in a patient with TWGB harboring GBC violates oncological principles. The authors argued that SC followed by immediate CEC will provide similar oncological outcome as EC but the report did not mention any patients who underwent SC only first and then underwent CEC after frozen section revealed GBC.
Agarwal (1) reported a large experience with 556 GB masses (seen on imaging)—239 were found to be inoperable on preoperative evaluation and 317 were operated. At operation, 129 were found to be unresectable and 198 were resected. Of the resected cases, 167 tuned out to be GBC and 31 were XGC. In these 31 patients with XGC, 25 radical (extended) cholecystectomies (with adjacent organ resection in 10) were performed—an unnecessary procedure for XGC. They also used frozen section in six patients but after performing segment IVB +V resection, which is a major, difficult and more morbid procedure than wedge of liver resection proposed by us. This report also does not mention any patients who underwent SC only first and then underwent CEC after frozen section revealed GBC. In any case, for most patients with malignant TWGB (GBC) liver wedge is adequate (11) and is as good as segment IVB +V resection (12).
Shirai et al. (13) recently reported full thickness cholecystectomy (gall bladder with cystic plate) with limited lymphadenectomy of the first echelon (cystic and pericholedochal) lymph nodes, as an alternative to EC, in 12 elderly patients with GBC. Han (14) reported use of cholecystectomy with wedge of liver but in only 4 out of 39 patients with XGC.
Only one of our patients with benign TWGB (XGC) underwent an unnecessary lymphadenectomy; this was because of a suspicious frozen section report. Ishii et al. (15) also reported a similar patient with TWGB on CT where at operation it was thought to be GBC with infiltration of the transverse colon. Intra-peritoneal wash cytology was positive for malignancy and only palliative resection was done—final histology showed XGC. There was no false positive result of frozen section histological examination in our experience. We, however, do not rely on intra-peritoneal wash cytology as we found it to be of no use in a trial (unpublished data).
Some recent reports describe laparoscopic EC (16) but long term results in terms of recurrence and survival are awaited. We do not practice and do not recommend laparoscopic EC for GBC; we advocate staging laparoscopy (17) followed by open EC. Even AEC can be performed laparoscopically; Cho et al. (18) reported laparoscopic resection of GB together with GB bed in some carefully selected patients with T2 GBC. Han and Cho (19) reported laparoscopic removal of GB with some attached liver tissue in 30 patients with suspected GBC on CT, EUS or lap US—in their experience, however, majority (18 out of 30) of patients turned out to be GBC. We disagree with them in using laparoscopic approach for AEC; also, we recommend AEC in patients with low suspicion of GBC.
Cholecystectomy with wedge of liver followed by frozen section histological examination has been reported by some surgeons earlier for TWGB but in small number of cases and in retrospective analyses. We have used it in 13 selected patients with TWGB with low suspicion of GBC as a prospective protocol and wish to name it as AEC. AEC triages patients with TWGB into benign (AC, CC and XGC) in whom it avoids an unnecessary lymphadenectomy (in our experience, only 1 out of 11 patients with benign TWGB underwent an unnecessary lymphadenectomy because of a false positive frozen section report) and malignant (GBC) in whom it is an oncologically adequate procedure. This surgical approach is likely to be useful particularly in areas with high incidence of GBC which also report large number of patients with TWGB. We wish to name this surgical approach as the Lucknow approach for TWGB.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Agarwal AK, Kalayarasan R, Javed A, et al. Mass-forming xanthogranulomatous cholecystitis masquerading as gallbladder cancer. J Gastrointest Surg 2013;17:1257-64. [PubMed]
- Rao RV, Kumar A, Sikora SS, et al. Xanthogranulomatous cholecystitis: differentiation from associated gall bladder carcinoma. Trop Gastroenterol 2005;26:31-3. [PubMed]
- Agrawal S, Kapoor VK. Thick-walled gallbladder. Natl Med J India 2006;19:37-8. [PubMed]
- Srikanth G, Kumar A, Khare R, et al. Should laparoscopic cholecystectomy be performed in patients with thick-walled gallbladder? J Hepatobiliary Pancreat Surg 2004;11:40-4. [PubMed]
- Krishnani N, Dhingra S, Kapoor S, et al. Cytopathologic diagnosis of xanthogranulomatous cholecystitis and coexistent lesions. A prospective study of 31 cases. Acta Cytol 2007;51:37-41. [PubMed]
- Ogura T, Kurisu Y, Masuda D, et al. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for thick-walled gallbladders? Dig Dis Sci 2014;59:1917-24. [PubMed]
- Kang TW, Kim SH, Park HJ, et al. Differentiating xanthogranulomatous cholecystitis from wall-thickening type of gallbladder cancer: added value of diffusion-weighted MRI. Clin Radiol 2013;68:992-1001. [PubMed]
- Yu H, Yu TN, Cai XJ. Tumor biomarkers: help or mislead in the diagnosis of xanthogranulomatous cholecystitis?-analysis of serum CA 19-9, carcinoembryonic antigen, and CA 12-5. Chin Med J (Engl) 2013;126:3044-7. [PubMed]
- Rammohan A, Cherukuri SD, Sathyanesan J, et al. Xanthogranulomatous cholecystitis masquerading as gallbladder cancer: can it be diagnosed preoperatively? Gastroenterol Res Pract 2014;2014:253645.
- Jain S, Saluja SS, Sharma AK, et al. Xanthogranulomatous cholecystitis: catching the culprit--clinical and imaging analysis. Dig Surg 2012;29:187-93. [PubMed]
- Goetze TO, Paolucci V. Incidental T1b-T3 gallbladder carcinoma. Extended cholecystectomy as an underestimated prognostic factor-results of the German registry. Chirurg 2014;85:131-8. [PubMed]
- Horiguchi A, Miyakawa S, Ishihara S, et al. Gallbladder bed resection or hepatectomy of segments 4a and 5 for pT2 gallbladder carcinoma: analysis of Japanese registration cases by the study group for biliary surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2013;20:518-24. [PubMed]
- Shirai Y, Sakata J, Wakai T, et al. Full-thickness cholecystectomy with limited lymphadenectomy for gallbladder cancer. Hepatogastroenterology 2012;59:1338-40. [PubMed]
- Han SH, Chen YL. Diagnosis and treatment of xanthogranulomatous cholecystitis: a report of 39 cases. Cell Biochem Biophys 2012;64:131-5. [PubMed]
- Ishii T, Hatano E, Yasuchika K, et al. A case of xanthogranulomatous cholecystitis suspected to be adenocarcinoma based on the intraoperative peritoneal washing cytology. Int J Surg Case Rep 2014;5:138-41. [PubMed]
- Agarwal AK, Javed A, Kalayarasan R, et al. Minimally invasive versus the conventional open surgical approach of a radical cholecystectomy for gallbladder cancer: a retrospective comparative study. HPB (Oxford) 2015;17:536-41. [PubMed]
- Agrawal S, Sonawane RN, Behari A, et al. Laparoscopic staging in gallbladder cancer. Dig Surg 2005;22:440-5. [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Laparoscopic approach for suspected early-stage gallbladder carcinoma. Arch Surg 2010;145:128-33. [PubMed]
- Han HS, Cho JY. Role of laparoscopy in the management of gall bladder cancer. In: Agarwal A, Fong Y, editors. Carcinoma of the gallbladder. New Delhi: Elsevier, 2014:68-77.