
Narrative review of sentinel lymph node biopsy in breast cancer: a technique in constant evolution with still numerous unresolved questions
Introduction
The sentinel lymph node (SLN) is the first lymph node (LN) that collects the lymphatic flow from the tumor (1). It has been established that if the LN is not invaded by cancerous cells, the other LN in the axillary area are in most cases free of metastases. Axillary LN dissection (ALND) has therefore no benefit. Conversely, if the SLN contains cancerous cells, the other LN removed may be healthy or metastatic, leading to an ALND in some selected cases.
The SLN biopsy (SLNB) has been evaluated in numerous international trials (2-5), showing that the SNLB allows to avoid about 70% of ALND and thus to significantly reduce the morbidity associated with ALND (lymphedema, shoulder mobility problems, reduced sensitivity, pain, etc.). Moreover, due to the progress of pathological analysis, the SLNB leads to more accurate staging and thus an optimization of the therapeutic strategy. Currently, after a learning curve, the SLNB is routinely performed by most breast surgeons.
The aim of this narrative review was to describe the history of SLNB, the different SLN identification techniques, the indications and contraindications of SLNB for invasive and in situ breast carcinoma (BC), the indication of ALND after SLNB, its place in case of neoadjuvant systemic treatment (NST), and finally its expected evolution.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/cco-20-207).
Methods
This narrative review followed the recommendations in the PRISMA statement (6,7). The two authors independently searched and reviewed the relevant studies assessing the accuracy and the utility of SLNB in staging the axilla in case of BC. The MEDLINE database was used for all human studies. The discrepancies were resolved by consensus.
Inclusion and exclusion criteria
Studies with the following inclusion criteria were reviewed: (I) published in French or English; (II) published between 01/01/1994 and 15/08/2020; (III) SLNB was done to detect ALN involvement in patients with BC; (IV) histopathological analysis of ALN obtained by SLNB or ALND procedure were used as the reference standard test.
We excluded studies with the following criteria: (I) patients with metastatic ALN ipsilateral to the BC; (II) no histopathological reference standard was required; (III) patients without BC; (IV) experimental subject was an ex vivo procedure; (V) the type of study was a case report, or a letter to the editor and (VI) we were unable to get the full text.
Data extraction and quality assessment
Data were extracted by one author, checked by the second, and discrepancies resolved by discussion. Study quality was assessed using the QUality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist (8).
Results
Concept and history of SLNB
As LN metastasis is one of the most important prognostic factors for survival, the assessment of regional LN is essential in the staging of many epithelial cancers, ascertaining a prognosis, and determining optimal adjuvant treatments. The SLNB consists in recognizing and removing the first LN(s) that filters lymphatic fluid from the tumor. The complete LND is no longer needed if the SLN is normal.
The SLNB was first used by Cabanas (9) in 1977 for penile cancers. But it took almost 15 years for it to enter in clinical practice with Morton et al. (10) in 1992 for melanoma and with Giuliano et al. (11) in 1994 for BC.
This technique has undergone considerable development in many epithelial cancers due to numerous medical benefits such as the maintenance of normal LN, which act as an anti-tumor immunological barrier, and the more accurate staging thanks to the detection of occult metastases in the SLN leading to a better choice for adjuvant therapies. In addition, the SLNB is generally performed in outpatient surgery and allows a reduction in economic costs compared to an ALND.
Since the early 2000s, SLNB has become a widely accepted method of LN staging in selected invasive or in situ BC, due notably to the significant reduction of the ALND-associated morbidity, in particular lymphedema, shoulder adduction deficit, arm numbness and tingling.
SLN identification and pathological evaluation
The different SLN non-specific marking
The initial identification of SLN was based on two methods of non-specific marking of LN macrophages using a radioisotope (rhenium sulphide or albumin associated with the 99mTc monophotonic emitter) and a lymphophilic dye (Figure 1).
Blue dyes
The main pitfall of SLNB is the failure to visualize SLN, resulting in incorrect tumor staging, leading to suboptimal treatment or axillary recurrence (12). To reduce the false negative rate of the SLN procedure, the use of combined methods (technetium and dye) was initially recommended. The different blue dyes initially used were triarylmethane dyes [patent blue and its isomer: isosulfan blue dye (IBD)] and methylene blue dye (MBD).
Patent blue was used in Europe while its isomer IBD (also called lymphazulin) was used in the United States. The protein-dye complex has a vivid affinity for lymphatic system, with particle size small enough to travel through the lymph vessels but large enough to be trapped in the SLN. Clinical studies reported that IBD, and patent blue (5) had high SLN detection rates.
MBD is a smaller molecule which does not bind to plasma proteins (13). Using a feline model, Wong et al. (14) demonstrated that, when MBD was injected intradermally, it proved to be less satisfactory in defining the lymphatic drainage because of poor uptake in the lymphatics as well as staining of the tissue. As a result, MBD was not adopted in a first time for use in SLNB.
However, it rapidly appeared that IBD and patent blue were associated with a significant number of allergic reactions (0.1–3%), some of which being life-threatening (15-19). For example, multicenter, randomized studies (ALMANAC and NEW START) conducted in the United Kingdom, revealed that 72 patients out of 7917 (0.9%) had allergic reactions to patent blue (20,21). Patent blue is also a food colorant that has been banned in the United States and in several parts of the world due to its side effects. However, is still allowed in the European Union, although upon re-evaluation in 2013 of the European Food Safety Authority, its acceptable daily intake was lowered. Moreover, in 2000s, an international shortage of IBD triggered a rush for alternative dyes for SLN mapping in BC.
In 2001, Simmons et al. published the first study of MBD injection for SLNB in BC describing localization rates of 90%, i.e. unexpectedly comparable to IBD and patent blue. MBD is widely used in different diagnostic and therapeutic procedures (surgery of nipple discharge, Fallopian tube patency evaluation, chromoendoscopy…) and it has been rarely associated with potentially life-threatening adverse events. Since it has been shown in numerous other studies to be equally effective in SLN identification, it was proposed as an alternative technique to IBD or patent blue for SLN procedure. Furthermore, compared to IBD and patent blue, MBD offered a substantial cost reduction. However, localized reactions, due to MBD intradermal injections for SLN procedure, including necrosis of skin and subcutaneous tissues and necrotic abscesses, were described, leading to the recommendation of MBD intraparenchymal injections. For patients with glucose-6-phosphate dehydrogenase deficiency, thalassaemia or drepanocytosis, MBD may aggravate methemoglobinaemia or precipitate haemolytic anaemia and its administration is therefore contraindicated (22) (Figure 1).
Radioisotopes
The combination of different techniques (for example radioisotope and dye) is recommended to avoid non-identification of SLN leading to a complete ALND (1). The use of a radioisotope also allows pre-operative imaging of SLN by lymphoscintigraphy.
SLN isotope detection is generally performed by pre-operative injection of a radioisotope solution (99mTc colloidal) in the subareolar area of the breast or in the peritumoral area. Lymphoscintigraphy is performed a few hours after injection with a gamma camera. The surgery, generally performed the day after lymphoscintigraphy, begins with the injection of a blue dye in the subareolar or peritumoral area. Radioactive SLN are detected peri-operatively by an intraoperative probe. Individual removal of all labelled or dyed nodes is followed by pathological examination of the SLN.
Even though SLNB is widely developed worldwide since the 2000s, the amount of radioactivity administered by injection site remains highly variable as it may range, depending on the medical team, between 1.8 and 370 MBq. The Society of Nuclear Medicine and Molecular Imaging and the European Association of Nuclear Medicine recommend the administration of 50 MBq if the injection takes place the day before the surgery (23). Only van der Ent et al. (24) claimed to improve detection sensitivity by administering high doses at the injection site. This was not confirmed in any other studies.
Concerning the injected radioactivity, Bailly et al. (25) reported their experience on the irradiation of nursing and medical staff during the surgery for breast pathology. Their monocentric and prospective study included dosimetric measurements on medical and nursing staff. The mean activity of 99mTc-colloids of injected albumin was 50.1±2.4 and 90.4±3.2 MBq respectively on the day of surgery and the day before. The average doses received for each procedure by the surgeon, surgical assistant and nurse were 5, 3.75 and 0 µSv for whole body exposure and 17.5, 15.6 and 16.2 µSv for extremity exposure, respectively. The authors concluded that, for the surgeons, performing less than 30 SLNB per year, the whole body and extremity irradiation of each was below the regulatory annual thresholds. These conclusions rise to a few comments. As a result of the recommendations of the International Commission on Radiological Protection, the French and European regulations set the annual limit of effective dose (whole body) for the public at 1 mSv over 12 consecutive months. Based on the results obtained by the authors, this corresponds to the exposure for a surgeon performing about 200 SLNB. However, the demand for SLNB has increased, as these are routinely performed for BC, the most common cancer for women. The figure of 200 a year can therefore be easily reached by a surgeon specialized in senology.
By contrast, some teams use low dose injections (about 16 MBq on average) the day before surgery. With such low doses, in a prospective clinical trial (22), the authors showed that SLN identification was possible in 99% of the cases (94% of the SLN were “hot” and 65% were stained). In addition, a dosimetric measurement campaign using thermoluminescent dosimeters showed mean exposure levels per procedure of 4.7±2.6 µSv at the surgeon’s index finger and a whole-body exposure of less than 1 µSv. Thus, extrapolating doses to 200 procedures a year gives for a single surgeon an extremity dose of 940 µSv, i.e. more than 50 times lower than the regulatory limit for the public for extremities (50 mSv/12 months). These data confirmed that it is possible to reduce the medical staff’s exposure without losing intraoperative detection sensitivity (Figure 1).
Indocyanine green (ICG)
Researchers have developed new tracers to overcome side effects of radioisotopes (exposure to radiations, need to have a nuclear medicine center) and blue dyes (notably allergic reactions). Innovative tracers such as ICG, superparamagnetic iron oxide (SPIO), and microbubbles have been explored (26).
ICG is a fluorescent dye. After injection in breast tissue and migration through lymphatic vessels, ICG is tracked using an excitation illumination system combined with a camera that detects, in the near-infrared spectrum, the emitted fluorescence (27). ICG has been used since the 1950’s in the medical field and specifically in BC since the last decade (28). ICG has certain advantages such as the transcutaneous real-time detection (27) and the absence of radiation exposition (29). Compared to radioisotopes, ICG is cheaper, and the involvement of a nuclear medicine department is useless. However, it is necessary to have an intraoperative fluorescence imaging navigation system and to operate under dimmed light conditions (30) which can interfere with the surgical procedure. In addition, after resection of the first SLN leading to lymphatic vessels section, ICG may leak in the surgical field making it difficult to detect the following SLN (31). Low molecular weight is another reported disadvantage. It is supposed that ICG can travel faster than blue dye, resulting in unnecessary extensive dissection and removal of SLN (31). For instance, a recent meta-analysis found higher mean number of SLN removed with ICG (1.31–3.8) compared to radioisotope (1.35–2.3) (26). Nonetheless, identification rates of ICG, with or without blue dye, seems to be equivalent to the gold standard “blue dye and radioisotopes” or “radioisotopes alone” (27,32,33). A recent meta-analysis of ICG compared to radioisotopes found similar detection rates between these 2 techniques alone, and better results when performed together (26). In conclusion, the body of information from the current data suggest that ICG is a good alternative to blue dye and radioisotopes and is suitable for surgeons in resource-constrained setups, i.e. without nuclear medicine department (Figure 1).
SPIO
The SPIO method consists in injecting a magnetic tracer (contrast agents composed of nanoparticles of iron oxide crystals coated in carbohydrates) which migrates through the lymph vessels and into the SLN. The detection is made by a hand-held magnetometer that generates an alternating magnetic field which temporarily magnetizes the SPIO and senses the particles’ magnetic response. This technique was first developed in 2013 by an international European team (34). The magnetic tracer fades slowly and is still detectable after several months (35). Consequently, this provides a comfortable timeframe as the magnetic tracer can be injected from as early as 15 days before surgery to directly before skin incision. During surgery, the brown color of the magnetic tracer can help the surgeon for the dissection. However, it causes dermopigmentation in up to 20% of patients (36). Moreover, the intramammary persistence of the magnetic tracer can create void artefacts and complicate the interpretation of a postoperative breast magnetic resonance imaging (37). In addition, because of the magnetic field generated by the magnetometer, patients with pacemakers are not eligible as it can cause heart rhythm disorders. Similarly, metallic surgical instruments can interfere with the ferromagnetic signalling and thus constitute another technical limitation. Finally, SPIO has the advantage of being non-irradiating and does not require a nuclear medicine department. A meta-analysis of 7 studies published in 2016 showed that this technique seems non-inferior to the standard technique concerning identification rate, but it has a significantly higher LN retrieval rate (37) which can lead to unnecessarily excessive dissection (Figure 1).
Microbubbles
Another alternative technique for SLNB is contrast-enhanced ultrasound imaging using microbubbles. Phospholipid-stabilized microbubbles contain sulphur hexafluoride gas and act as sonographic contrast agents after intradermal injection and lymphatic migration (38). This technique was initially developed in 2004 to trace lymphatic drainage pathway and SLN in a swine model with melanoma (39). It was later studied in BC patients in 2006 (40). Like ICG and SPIO, there is no need of a radioisotope and consequently potential irradiation and no need for a department of nuclear medicine. Moreover, real-time visualization of SLN is possible, and the contrast agent is cheap. There are no iodine and proteins in sulfur hexafluoride microbubbles, which prevents patients from allergy. However, this technique is slower compared to the others, requires the proficiency of axillary ultrasound examination, has a longer learning curve and remains operator dependent. It has a lower detection rate compared to blue dye, and lower sensitivity.
Subareolar injection or peritumoral injection?
The results of different published data comparing two injection modes (sub-areolar versus peri-tumoral) of different markers (blue dye, isotopes) have shown no difference (1). Moreover, the distribution, in terms of number of LN removed, is strictly the same with a significant proportion of cases with one or two SLN. These results support the notion of “single biological entity” of the SLN of the mammary gland (Figure 1).
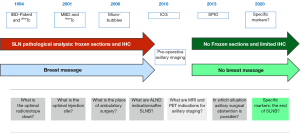
Should breast massage be performed after injection?
In some centers, breast massage (5 to 10 minutes) was performed after injection of radioisotope or blue dye to improve the uptake of these markers in SLN. Different massage techniques have been evaluated, none of them showing to be superior to the others (41). However, concerns have been raised that breast massage, through mechanical transport, may push epithelial cells into a SLN and consequently cause false-positive findings of occult metastases. Diaz et al. found that presence of epithelial cells (without features of established metastases) occurred more frequently in the SLN of patients who underwent breast massage (42).
Nonetheless, even if the presence of isolated tumor cells (ITC) does not alter clinical management, it is associated with a worse prognosis in some published series (43). Breast massage was used at the beginning of the 2000’s (44). As there is no proof of innocuity of breast massage and with no demonstrated benefit with current techniques, it is not performed anymore in routine clinical practice.
Is intraoperative frozen section necessary in SLNB for BC patients?
In the past, many surgeons used intraoperative frozen section for SLN pathological analysis to prevent the need for second surgery to perform an ALND for early stage BC patients (45).
Currently, most teams choose instead to carry out very precise preoperative imaging of the axillary area. In the preoperative settings, ultrasound examination is widely applied followed by fine-needle aspiration or core needle biopsy of abnormal LN. Additional imaging techniques, such as magnetic resonance imaging and fluoro-deoxy-glucose positron emission tomography (PET) have been also proposed to improve axillary staging. The development of preoperative imaging has had an important impact on axillary management. Indeed, preoperatively-proved LN involvement allows to bypass SLNB, these patients undergoing first-line ALND. Similary, a reliable negative preoperative axillary staging had a significant impact on surgical management, leading to a low tumor burden in patients with negative imaging.
Moreover, the false negative rates of frozen section are high (25–30%), and it has been proven that the intraoperative LN examination had only a small impact on the rate of reoperation and increased the time and cost of surgery (46). Frozen section is a less efficient histological technique which can lead to a risk of loss of material. Moreover, frozen section can also increase the risk of overtreatment, leading to an unnecessary ALND in case of micrometastases. So, for all these reasons, SLN intraoperative examinations are not routinely performed anymore. However, in some case of immediate breast reconstruction, SLN frozen sections are still performed to estimate the risk of involved SLN and consequently of radiotherapy, that might alter the cosmetic result of the reconstruction and lead to postpone the reconstruction.
Is routine use of immunohistochemistry necessary for SLN analysis?
During the first period of SLN [1994–2010], to accurately determine the pathologic nodal (pN) stage, pathologists examined multiple levels and performed IHC to increase detection of occult LN metastases, defined as tumor cells not identified during the initial histological assessment of a stained section of a SLN. SLN were generally analyzed after staining by haematoxylin-eosin (H&E). Thorough examination of SLN were carried out by sectioning the entire node in 2 mm thick blocks. Duplicate paraffin-embedded sections were cut at different intervals. The SLN that appeared to be free of cancer cells using H&E staining were further submitted to IHC using an anti-keratin antibody that allowed the identification of occult metastases (ITC, micro or macrometastases) (Figure 2).
However, it became quickly clear that patients with limited SLN involvement (ITC or micrometastases) did not benefit from ALND. Retrospective analysis of the NSABP B-32 trial showed a 15.9% increase in detection of occult metastases with the use of IHC (72% of these occult metastases being ITC). The prevalence of occult metastases identified in the ACOSOG Z0010 was 10.5% with the use of IHC. Consistent with the lack of treatment decision information providing by IHC, in 2010, the American Joint Committee on Cancer recommended only histopathologic examination of 2 mm sections of SLNs without routine use of IHC (47). Moreover, this decision lead to cost savings and more effective use of resources.
However, in some cases, notably for invasive lobular carcinoma, IHC is useful to identify occult metastases. Indeed, metastatic lobular carcinoma is difficult to be identified on H&E sections, even in case of micrometastases where the tumor cells might exhibit minimal nuclear atypia (48).
Indications and contraindications of SLNB
Indications and contraindications of SLNB in case of invasive BC
The main risk of the SLNB is related to the presence of metastases in non-SLN when SLN are negative (false negatives). To reduce this risk, there are some contraindications to SLNB. Although these limitations have decreased over time (Figure 3), there is still no consensus. Except the cases with clinically palpable metastasized LN, where the technique is not applicable, all contraindications seek to avoid situations where the risk of false negative would be too high. Thus, the technique is refuted in cases with an history of major breast/axillary surgery or mammary and/or axillary radiotherapy where the alterations in lymphatic drainage are significant. By contrast, an history of limited surgery (e.g., breast biopsy for diagnostic purposes) is no longer a contraindication.
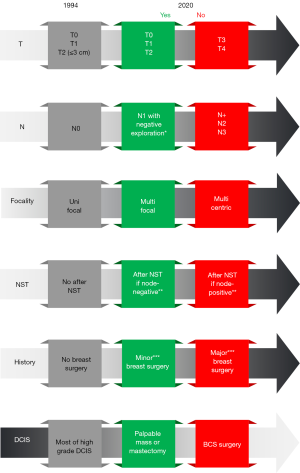
Tumor size is another selection criterion and SLNB is generally reserved for T0-T1-T2 tumors and contraindicated for T3-T4 tumors. In the NSABP B-32 trial, having included 4,439 patients with a T1 BC and 983 patients with a T2 BC, identical rates of identification (96.9% and 98.4%) and false negatives (10.3% and 8.9%) for T1 and T2 respectively were observed.
The exclusion of T3 and T4 BC patients is linked to the fact that the larger the tumor, the higher the risk of LN metastases and therefore the higher the risk of false negatives, to the point of reaching unacceptable levels. However, such a limit (T3) does not in fact seem to be an absolute exclusion criterion.
The surgery during pregnancy also remains a classic exclusion criterion for many teams, because of the high level of LN involvement observed in young women with BC during pregnancy, the lack of large randomized studies, and the teratogenicity of some products used for SLN marking.
Taking these indications and contraindications into account, larger trials including an overall total of 14,700 patients (NSABP B-32, ACOSOG Z0010, AMAROS, ALMANAC…) have achieved SLN identification rates of 94.5% to 98.8% with a mean number of SLN removed of 1.7 to 2 (2-5). However, despite numerous precautions, the rate of false negatives in these trials ranged between 8.8% to 9.7% (2,3).
SLNB indications and contraindications in case of ductal carcinoma in situ (DCIS)
DCIS is a proliferation of malignant epithelial cells within the ductulo-lobular system of the breast showing no evidence of basement membrane disruption and invasion into the surrounding stroma. Twenty years ago, cases of DCIS were rare, representing only 1% to 4% BC cases. Later, BC screening became widely used thus increasing the detection of DCIS. To date, DCIS represent 15% to 20% of newly diagnosed BC.
Using standard histopathological analysis, in case of pure DCIS, the incidence of LN metastases is low (0–4%) (49). Changes in the analysis of LN using serial sections coupled with cytokeratin immunodetection by IHC has increased ITC and micrometastases identification. Depending on the studies, SLN removal associated with exhaustive LN analysis has increased the incidence of LN involvement varying from 0.9% to 8% (49-53). In these studies, most of ALN involvements were limited to isolated or small clusters of epithelial cells (49).
Careful histological examination of the tumor in patients with DCIS is mandatory to exclude microinvasive foci because this lesion is considered as a precursor of invasive carcinoma. However, despite meticulous evaluation of the sample, absolute certainty about the diagnosis of pure DCIS is difficult to reach. For example, foci of invasion may be missed when the tumor size is large or when the stromal reaction around the basal membrane is important. An undetected invasion may explain the occurrence of SLN metastasis in case of DCIS. In such case, the extent of LN involvement is the most important indicator of tumor aggressiveness. However, the presence of epithelial cells in the SLN draining a pure DCIS may not be due to cancer invasion since some authors have reported the passive transport of epithelial cells following a preoperative action such as core or open biopsy and injection of radioisotope or blue dye. Other studies (54,55) have also suggested that the presence of epithelial cells in the LN may be a consequence of preoperative manipulation rather than to cancer cell invasion.
Overall, due to the low rate of LN metastases in DCIS, routine removal of SLN is not recommended for conservative therapy. The discovery of invasive cancer during deferred histological analysis of the surgical specimen may lead to SLNB in a second stage. However, this procedure appears to be indicated when there is a risk of occult invasion (palpable mass or suspicious radiological image) or for patients requiring complex oncoplastic surgery, as SLNB is no longer (or hardly) applicable after the operation, in the event of a delayed histological diagnosis of invasion (42) (Figure 3).
Likewise, in patients with large DCIS who will undergo mastectomy, the SLNB should be performed as they have a risk of harboring a micro-invasive component and because it will not be possible to perform SLNB after mastectomy (Figure 3).
In the future, SLNB in cases of DCIS treated by mastectomy could be reserved to particular clinical/radiological presentations (clinical or radiological mass, extensive forms or multiple foci) and omitted in other cases. For some authors, the presence of a micropapillary form should also lead to a SLNB, because of the associated risk of invasive carcinoma (50).
Loco-regional recurrence and axillary morbidity in case of SLNB
The results of the different published series (2,3) are very consistent and show that when SLNB is performed on a selected population of patients, with a rigorous technique by staff with multidisciplinary training and experience, it is extremely reliable with axillary recurrence rates generally below 2% after a follow up of 8–10 years. These rates are comparable to those observed after ALND. Likewise, when the SLN contains ITC or micrometastases, axillary recurrence rates remain low even when ALN is omitted.
In ACOSOG Z0011 trial, patients randomized to ALND had a median of 17 LN removed compared with a median of only 2 SLNs removed with SLNB alone (P<0.001). ALND, as expected, also removed more positive LN (P<0.001). At a median follow-up of 9.25 years, there was no statistically significant difference in local recurrence-free survival (P=0.13). The cumulative incidence of nodal recurrences at 10 years was 0.5% in the ALND group and 1.5% in the SLNB alone group (P=0.28). Ten-year cumulative locoregional recurrence was 6.2% with ALND and 5.3% with SLNB alone (P=0.36) (56). Despite the potential for residual axillary disease, SLNB without ALND offers excellent regional control for selected patients with early metastatic BC treated with breast-conserving therapy and adjuvant systemic therapy.
In the IBCSG 23-01 trial, long-term surgical complications included lymphoedema of any grade in 16 (4%) of 453 patients in the no ALND group and 60 (13%) of 447 in the ALND group, sensory neuropathy of any grade in 57 (13%) in the no ALND group versus 85 (19%) in the ALND dissection group, and motor neuropathy of any grade in 14 (3%) in the no ALND group vs. 40 (9%) in the ALND group (57).
ALND indications and contraindications after SLNB
For all type of surgeries
In case of failure to detect the SLN, or more than 2 metastatic SLN, or capsular effraction, ALND is still indicated.
Concerning women with metastatic BC (stage IV), the optimal management of the axilla remains debated. Among these patients, surgery may increase local control of the disease and avoid discomfort and complication such as skin involvement, infection and axillary lymphatic compression causing lymphedema. However, scientific literature is insufficient in this topic. Only 2 clinical trials were reported in the 2018 Cochrane Review (58): one performed SLNB in node-negative and ALND in node-positive patients (59) while in the other all women underwent ALND and additional supraclavicular LND (60). Nonetheless, authors of the Cochrane Review suggested that surgery (both breast and axillary) may improve local progression‐free and worsen distant progression‐free survival (58). To date, if surgery is performed, we advise that axillary surgery should be chosen considering possible risks and benefits, after multidisciplinary discussion and considering patient opinion. For selected patients with a curative treatment plan, SLNB for node-negative and ALND for node-positive patients is an option.
Breast conservative surgery
Eligible patients of the ACOSOG Z0011 trial were women with clinical T1 or T2 invasive BC, no palpable axillary adenopathy, and 1 or 2 SLN containing metastases. All patients had planned lumpectomy, tangential whole-breast irradiation, and adjuvant systemic therapy. In this trial including 891 patients (446 with SLNB alone and 445 with ALND), the recurrence rates as well as the overall survival and disease-free survival rates were identical whether or not additional ALND were performed after one or two positive SLN (61). The 10-year overall survival was 86.3% in the SLNB alone group and 83.6% in the ALND group (HR, 0.85; 95% CI, 0–1.16; P=0.02). The 10-year disease-free survival was 80.2% in the SLNB alone group and 78.2% in the ALND group (HR, 0.85; 95% CI, 0.62–1.17; P=0.32). Ten-year regional recurrence did not differ significantly between the 2 groups (62). So, for Giuliano et al. (61), complementary ALND can be avoided in the following situations: T0 T1-2 N0 and conservative in sano treatment and ≤2 SLN positive in H&E and systemic adjuvant therapy (chemotherapy and/or hormonotherapy).
Similar results were observed in the IBCSG 23-01 trial which included 931 patients (57). In this multicenter, randomized, controlled, phase 3 trial, patients were recruited from 27 hospitals and cancer centers in nine countries. Eligible women could be of any age with a BC with largest lesion diameter of 5 cm or smaller, and one or more metastatic SLN, all of which were 2 mm or smaller and with no extracapsular extension. Between April 1, 2001, and Feb 8, 2010, 6,681 patients were screened and 934 randomly assigned to no ALND (n=469) or ALND (n=465). Disease-free survival at 10 years was 76.8% (95% CI, 72.5–81.0) in the no ALND group, compared with 74.9% (70.5–79.3) in the ALND group (HR 0.85, 95% CI, 0.65–1.11; log-rank P=0.24; P=0.0024 for non-inferiority). These results were consistent with those of the 10-year follow-up analysis of the Z0011 trial.
Together, these findings support the current practice of not performing ALND anymore in patients with early BC when the tumor burden in the SLN is minimal or moderate.
Mastectomy
Regional failure rates are low in patients with a positive SLNB who undergo breast-conserving therapy without ALND. The applicability of these findings to total mastectomy patients is not established. Milgrom et al. aimed at evaluating the characteristics and outcomes of SLNB-positive patients who underwent mastectomy and did not receive axillary-specific treatment and to compare them to similar patients who underwent breast-conserving surgery. A total of 535 patients with early-stage BC who underwent definitive breast surgery (210 mastectomies, 325 breast-conserving surgery), had a positive SLNB and did not receive ALND between 1997 and 2009 were identified from an institutional database. Most patients had stage I to IIA, with minimal nodal disease. Compared to the breast-conserving surgery group, mastectomy group were younger, had larger tumors, had higher nomogram scores predicting additional axillary disease and were more likely to receive chemotherapy. Ninety-four percent of the breast-conserving surgery group and 5% of the mastectomy group received adjuvant radiotherapy. At a median follow-up of 57.8 months, the 4-year local, regional and distant recurrence rates were 1.7%, 1.2% and 0.7% in the mastectomy group and 1.4%, 1.0% and 3.7% in the breast-conserving surgery group. The 4-year disease-free and overall survival rates were 94.8% and 97.8% in the mastectomy group and 90.1% and 92.6% in the breast-conserving surgery group. The authors concluded that early-stage BC patients with minimal SLN disease experience excellent outcomes without ALND, whether they undergo BCS or mastectomy (63).
However, in case of mastectomy with metastases affecting one or two SLN, most medical teams recommend either axillary postsurgical radiation therapy or ALND. According to other teams, two situations, depending on whether radiotherapy is indicated, can be identified. If there is an indication for radiotherapy, the abstention of ALND should respect the same rules as conservative treatment. By contrast, if there is no indication for radiotherapy, ALND remains the rule today, but abstention can be considered if ≤2 micrometastatic SLN and no capsular effraction and more than 2 SLN taken and systemic adjuvant treatment (chemotherapy and/or hormone therapy) upon validation by a multidisciplinary team.
The place of the SLNB in NST
NST is currently used not only for locally advanced BC but also for early stages. As a result, there are an increasing number of situations where a patient undergoes both SLNB and NST. This change of practices raised question concerning the optimal management of the axilla. In this situation, SLNB is performed according to the same indications concerning tumor local stage (T0-T1-T2) and contraindications (locally advances tumors, T3-T4), but there are specificities about the nodal status (N).
First, choosing the timing of SLNB is challenging, as it can be performed before or after NST. If performed before NST, SLNB assess the initial nodal status and can lead to a change of chemotherapy protocol. However, this setup implies a second operation after NST for breast tumor removal. Conversely, SLNB can be performed after NST. At the beginning, there was concern about feasibility of SLNB after NST. The GANEA study showed that SLNB performances (detection rate, false-negative rate, and accuracy) were similar in patients with or without NST, thus demonstrating the feasibility of SLNB after NST (64). Still, initial staging may not be accurate if positive nodes become negative after NST, and thus conditioning the radiotherapy protocol. Both setups are possible for all N0 patients and those N1 with negative exploration of the axilla (ultrasound with or without fine needle aspiration or core biopsy).
Then, management of node-positive patients (those with confirmed metastatic LN before surgery) remains an issue still in discussion, and management is not consensual. Results from different clinical trials (65-68) show that in this situation SLNB after NST is accurate when at least 3 SLN are obtained and examined allowing surgical down-staging of the axilla, however long-term outcomes are lacking.
Finally, complementary ALND after SNLB remains indicated for node-positive patients. To date, there is no evidence on clinical outcomes comparing complementary ALND versus no further dissection in NST settings (69). As the ACOSOG Z0011 criteria are not met, patients with 1–2 positive nodes should undergo complementary ALND.
In conclusion, today several major guidelines (ASCO 2017, ESMO 2019, St Gallen 2019, NCCN 2020) recommend that SLNB can be performed in patients receiving NST, for those N0 and N1 with negative exploration of the axilla (ultrasound with or without fine needle aspiration or core biopsy) (70-73).
Conclusions
The results of the literature are quite consistent and show that when SLNB is dedicated to selected BC, is performed with a rigorous technique by staff with multidisciplinary training and experience, it is extremely reliable. The de-escalation of axillary surgery is expected to continue over the next few years, although this change is difficult for many surgeons to accept, as Armando Giuliano recently pointed out: “We have surgically ignored the IM (internal mammary chain) nodes for over 50 years without regret but cannot omit axillary dissection.” (Madrid, 19 October 2019).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Denis Querleu and Cherif Youssef Akladios) for the series “Sentinel Lymph Node Biopsy in Gynecologic Cancer” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/cco-20-207
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-207). The series “Sentinel Lymph Node Biopsy in Gynecologic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mertz L, Mathelin C, Marin C, et al. Subareolar injection of 99m-Tc sulfur colloid for sentinel nodes identification in multifocal invasive breast cancer. Bull Cancer 1999;86:939-45. [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007;8:881-8. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
- Straver ME, Meijnen P, van Tienhoven G, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol 2010;17:1854-61. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Antes G, von Elm E. The PRISMA Statement - what should be reported about systematic reviews? Dtsch Med Wochenschr 2009;134:1619. [Crossref] [PubMed]
- Harms M. The EQUATOR Network and the PRISMA Statement for the reporting of systematic reviews and meta-analyses. Physiotherapy 2009;95:237-40. [Crossref] [PubMed]
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [Crossref] [PubMed]
- Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [Crossref] [PubMed]
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392-9. [Crossref] [PubMed]
- Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994;220:391-8; discussion 398-401. [Crossref] [PubMed]
- Mathelin C, Salvador S, Guyonnet JL. Axillary lymph node recurrence after sentinel lymph node biopsy for breast cancer. J Gynecol Obstet Biol Reprod (Paris) 2007;36:253-9. [Crossref] [PubMed]
- Tsopelas C, Sutton R. Why certain dyes are useful for localizing the sentinel lymph node. J Nucl Med 2002;43:1377-82. [PubMed]
- Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg 1991;214:637-41. [Crossref] [PubMed]
- Cinar H, Koca B, Kesicioglu T, et al. Isosulfan blue-induced anaphylactic reaction during sentinel lymph node biopsy in breast cancer. Breast 2012;21:220-2. [Crossref] [PubMed]
- Bézu C, Coutant C, Salengro A, et al. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol 2011;20:e55-9. [Crossref] [PubMed]
- Sandhu S, Farag E, Argalious M. Anaphylaxis to isosulfan blue dye during sentinel lymph node biopsy. J Clin Anesth 2005;17:633-5. [Crossref] [PubMed]
- Sprung J, Tully MJ, Ziser A. Anaphylactic reactions to isosulfan blue dye during sentinel node lymphadenectomy for breast cancer. Anesth Analg 2003;96:1051-3. table of contents. [Crossref] [PubMed]
- Laurie SA, Khan DA, Gruchalla RS, et al. Anaphylaxis to isosulfan blue. Ann Allergy Asthma Immunol 2002;88:64-6. [Crossref] [PubMed]
- Barthelmes L, Goyal A, Sudheer P, et al. Investigation of anaphylactic reaction after patent blue V dye injection. Breast 2010;19:516-20. [Crossref] [PubMed]
- Barthelmes L, Goyal A, Newcombe RG, et al. Adverse reactions to patent blue V dye - The NEW START and ALMANAC experience. Eur J Surg Oncol 2010;36:399-403. [Crossref] [PubMed]
- Mathelin C, Croce S, Brasse D, et al. Methylene blue dye, an accurate dye for sentinel lymph node identification in early breast cancer. Anticancer Res 2009;29:4119-25. [PubMed]
- Giammarile F, Alazraki N, Aarsvold JN, et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging 2013;40:1932-47. [Crossref] [PubMed]
- van der Ent FW, Kengen RA, van der Pol HA, et al. Sentinel node biopsy in 70 unselected patients with breast cancer: increased feasibility by using 10 mCi radiocolloid in combination with a blue dye tracer. Eur J Surg Oncol 1999;25:24-9. [Crossref] [PubMed]
- Bailly M, Zinsius A, Maia S, et al. Radiation exposure of surgical staff during sentinel node surgery. Is there a risk for the surgeon and his team? Gynecol Obstet Fertil 2014;42:296-300. [Crossref] [PubMed]
- Goonawardena J, Yong C, Law M. Use of indocyanine green fluorescence compared to radioisotope for sentinel lymph node biopsy in early-stage breast cancer: systematic review and meta-analysis. Am J Surg 2020;220:665-76. [Crossref] [PubMed]
- Guo J, Yang H, Wang S, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol 2017;15:196. [Crossref] [PubMed]
- Ersoy YE, Kadioglu H. Review of Novel Sentinel Lymph Node Biopsy Techniques in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Clin Breast Cancer 2018;18:e555-9. [Crossref] [PubMed]
- Hirano A, Kamimura M, Ogura K, et al. A comparison of indocyanine green fluorescence imaging plus blue dye and blue dye alone for sentinel node navigation surgery in breast cancer patients. Ann Surg Oncol 2012;19:4112-6. [Crossref] [PubMed]
- He K, Chi C, Kou D, et al. Comparison between the indocyanine green fluorescence and blue dye methods for sentinel lymph node biopsy using novel fluorescence image-guided resection equipment in different types of hospitals. Transl Res 2016;178:74-80. [Crossref] [PubMed]
- Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014;15:e351-62. [Crossref] [PubMed]
- Agrawal SK, Hashlamoun I, Karki B, et al. Diagnostic Performance of Indocyanine Green Plus Methylene Blue Versus Radioisotope Plus Methylene Blue Dye Method for Sentinel Lymph Node Biopsy in Node-Negative Early Breast Cancer. JCO Glob Oncol 2020;6:1225-31. [Crossref] [PubMed]
- Valente SA, Al-Hilli Z, Radford DM, et al. Near Infrared Fluorescent Lymph Node Mapping with Indocyanine Green in Breast Cancer Patients: A Prospective Trial. J Am Coll Surg 2019;228:672-8. [Crossref] [PubMed]
- Thill M, Kurylcio A, Welter R, et al. The Central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014;23:175-9. [Crossref] [PubMed]
- Karakatsanis A, Christiansen PM, Fischer L, et al. The Nordic SentiMag trial: a comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res Treat 2016;157:281-94. [Crossref] [PubMed]
- Houpeau JL, Chauvet MP, Guillemin F, et al. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: The French Sentimag feasibility trial. J Surg Oncol 2016;113:501-7. [Crossref] [PubMed]
- Zada A, Peek MC, Ahmed M, et al. Meta-analysis of sentinel lymph node biopsy in breast cancer using the magnetic technique. Br J Surg 2016;103:1409-19. [Crossref] [PubMed]
- Cox K, Sever A, Jones S, et al. Validation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patients with a normal grey-scale axillary ultrasound. Eur J Surg Oncol 2013;39:760-5. [Crossref] [PubMed]
- Goldberg BB, Merton DA, Liu JB, et al. Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 2004;230:727-34. [Crossref] [PubMed]
- Omoto K, Hozumi Y, Omoto Y, et al. Sentinel node detection in breast cancer using contrast-enhanced sonography with 25% albumin--Initial clinical experience. J Clin Ultrasound 2006;34:317-26. [Crossref] [PubMed]
- Haynes G, Garske D, Case D, et al. Effect of massage technique on sentinel lymph node mapping for cancer of the breast. Am Surg 2003;69:520-2. [PubMed]
- Diaz NM, Cox CE, Ebert M, et al. Benign mechanical transport of breast epithelial cells to sentinel lymph nodes. Am J Surg Pathol 2004;28:1641-5. [Crossref] [PubMed]
- de Boer M, van Deurzen CH, van Dijck JA, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med 2009;361:653-63. [Crossref] [PubMed]
- Bass SS, Cox CE, Salud CJ, et al. The effects of postinjection massage on the sensitivity of lymphatic mapping in breast cancer. J Am Coll Surg 2001;192:9-16. [Crossref] [PubMed]
- Marin C, Mathelin C, Neuville A, et al. Sentinel lymph node biopsy with micrometastases in breast cancer: histological data and surgical implications. About a series of 201 axillary dissections after peroperative sentinel node identification. Bull Cancer 2003;90:459-65. [PubMed]
- Godazande G, Moradi S, Naghshvar F, et al. Is Necessary Intraoprative Frozen Section In Sentinel Lymph Node Biopsy For Breast Cancer Patients? Asian Pac J Cancer Prev 2020;21:647-51. [Crossref] [PubMed]
- Matsen CB, Neumayer LA. Breast cancer: a review for the general surgeon. JAMA Surg 2013;148:971-9. [Crossref] [PubMed]
- Harrison BT, Brock JE. Contemporary Evaluation of Breast Lymph Nodes in Anatomic Pathology. Am J Clin Pathol 2018;150:4-17. [Crossref] [PubMed]
- Ruvalcaba-Limón E, de Jesus Garduno-Raya M, Bautista-Pina V, et al. Sentinel lymph node metastasis in patients with ductal breast carcinoma in situ. Cir Cir 2014;82:129-41. [PubMed]
- Ramzi S, Najeeb E, Coulthard J, et al. Does sentinel lymph node biopsy for screening high-grade ductal carcinoma in situ of the breast cause more harm than good? Breast Cancer Res Treat 2020;182:47-54. [Crossref] [PubMed]
- Bertozzi S, Cedolini C, Londero AP, et al. Sentinel lymph node biopsy in patients affected by breast ductal carcinoma in situ with and without microinvasion: Retrospective observational study. Medicine (Baltimore) 2019;98:e13831 [Crossref] [PubMed]
- Holm-Rasmussen EV, Jensen MB, Balslev E, et al. The use of sentinel lymph node biopsy in the treatment of breast ductal carcinoma in situ: A Danish population-based study. Eur J Cancer 2017;87:1-9. [Crossref] [PubMed]
- Sorrentino L, Sartani A, Bossi D, et al. Sentinel node biopsy in ductal carcinoma in situ of the breast: Never justified? Breast J 2018;24:325-33. [Crossref] [PubMed]
- Tamhane R, Dahlstrom JE, McCallum DD, et al. The clinical significance of cytokeratin-positive cells in lymph nodes at the time of mastectomy from patients with ductal carcinoma-in-situ. Ann Surg Oncol 2002;9:999-1003. [Crossref] [PubMed]
- Lara JF, Young SM, Velilla RE, et al. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer 2003;98:2105-13. [Crossref] [PubMed]
- Giuliano AE, Ballman K, McCall L, et al. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 2016;264:413-20. [Crossref] [PubMed]
- Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 2013;14:297-305. [Crossref] [PubMed]
- Tosello G, Torloni MR, Mota BS, et al. Breast surgery for metastatic breast cancer. Cochrane Database Syst Rev 2018;3:CD011276 [PubMed]
- Soran A, Ozbas S, Kelsey SF, et al. Randomized trial comparing locoregional resection of primary tumor with no surgery in stage IV breast cancer at the presentation (Protocol MF07-01): a study of Turkish Federation of the National Societies for Breast Diseases. Breast J 2009;15:399-403. [Crossref] [PubMed]
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380-8. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Milgrom S, Cody H, Tan L, et al. Characteristics and outcomes of sentinel node-positive breast cancer patients after total mastectomy without axillary-specific treatment. Ann Surg Oncol 2012;19:3762-70. [Crossref] [PubMed]
- Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol 2009;27:726-32. [Crossref] [PubMed]
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015;33:258-64. [Crossref] [PubMed]
- Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol 2016;23:3467-74. [Crossref] [PubMed]
- Cavalcante FP, Millen EC, Zerwes FP, et al. Role of Axillary Surgery After Neoadjuvant Chemotherapy. JCO Glob Oncol 2020;6:238-41. [Crossref] [PubMed]
- Lyman GH, Somerfield MR, Bosserman LD, et al. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:561-4. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer, Version 5.20202020.
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 2019;30:1194-220. [Crossref] [PubMed]
- Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 2019;30:1541-57. [Crossref] [PubMed]



