
Systemic therapy for non-serous ovarian carcinoma
Introduction
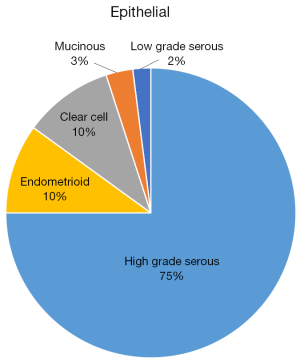
Ovarian cancer is the eight most common cancer in women around the world. In 2018 alone, 295,000 women were diagnosed with ovarian cancer and 184,000 died as a consequence of the disease (1). Ovarian cancer is a heterogeneous disease which can be classified in epithelial and non-epithelial origin. Among epithelial ovarian cancer (EOC), high-grade serous carcinoma is the most common, comprising around 70% of all cases. Other less common epithelial tumors include endometroid, mucinous, clear cell carcinomas (CCC), and carcinosarcomas. Among those with non-epithelial origin, germ cell tumors and sex cord-stromal tumors are most frequent (2) (Figure 1) These subtypes differ in their clinical presentation, genomics, biomarkers, response to chemotherapy, and prognosis (Table 1).

Full table
Management of these types of tumors might be challenging due to their rarity and the scarce information on the efficacy of treatment. The main purpose of this review is to summarize the published literature on the systemic management of patients with non-serous EOC.
Mucinous epithelial ovarian carcinoma
Mucinous epithelial ovarian carcinoma (mEOC) accounts for 3% of EOC (3). Median age at diagnosis is 53 years, and tobacco smoking is considered an associated clinical risk factor (12). Between 65–80% of patients present at localized stages, since these tumors are usually very large and cause symptoms early. The 5-year overall survival (OS) for stage I tumors is 92%, while the median OS in advanced stage ranges from 12–33 months (8). These tumors evolve in a stepwise fashion from benign epithelium to a pre-invasive lesion, to carcinoma, similar to the development of colorectal cancer. In addition, they commonly have mutations in various genes, including KRAS, HER2 and TP53, among others (19,20). Mucinous neoplasms generally display complex glandular arrangements with areas of stromal invasion, expressing gastrointestinal markers such as CK7, CK20, CDX2, which makes it difficult to differentiate them from metastatic tumors from the gastrointestinal tract (21). Clinical suspicion of a mucinous metastasis, rather than a primary mEOC, includes tumor size <10 cm, bilateral tumors, peritoneal spread, evidence of metastatic lesions, or a combination of these findings (12). In these cases, a comprehensive workup must be performed to rule out an occult gastrointestinal cancer, including colonoscopy, upper gastrointestinal endoscopy, and endoscopic ultrasonography (22).
The management of mEOC is primarily surgical. In stage I, fertility-sparing surgery is appropriate for young patients, including unilateral salpingo-oophorectomy with peritoneal staging procedures (cytology, peritoneal biopsies, and omentectomy) (23). The risk of recurrence is lower than that reported for women with stage I serous cancers (6% vs. 20%) (24). In older patients, bilateral salpingo-oophorectomy is preferred. In advanced stages, debulking surgery with the objective of a macroscopically complete resection should be attempted, followed by the administration of systemic treatment.
In contrast with that seen at early stages, the prognosis of women with advanced mEOC is worse than that of patients with other common subtypes [hazard ratio (HR) for death 2.81; 95% CI, 2.47–3.21] (25), due to a worse response to chemotherapy. A case-control study comparing the effectiveness of platinum-based regimens between patients with mEOC and those with other EOC subtypes found that response rates (RR) were significantly lower in mEOC (26.3% vs. 64.9%, P=0.01) with a significantly shorter OS of 12 vs. 37 months (P<0.001) (26). Alexandre et al. (27) retrospectively collected data from five randomized clinical trials (RCT) including 1,118 patients with advanced EOC [International Federation of Obstetrics and Gynecology (FIGO) stages IIB–IV] mostly treated with paclitaxel-carboplatin based chemotherapy, of which 54 (5%) had mEOC. Compared with non-mucinous tumors, RR were found to be lower (60% vs. 80%, P<0.001), and progression free survival (PFS) and OS were shorter (11 vs. 17.5 months, P=0.002; and 21.6 vs. 47.2 months, P<0.001 respectively). A worse prognosis was also observed among patients with FIGO stage IV and optimally debulked stage IIB–IIIC disease (27). In contrast, an exploratory subgroup analysis of the ICON3 RCT (28) that enrolled 2,074 patients with advanced ovarian cancer (7% mEOC), reported no difference in OS/PFS for paclitaxel-carboplatin versus either carboplatin or cisplatin-cyclophosphamide-doxorubicin between mEOC and other more common types of EOC.
Given the histologic similarities between primary mEOC and gastrointestinal carcinomas, an attractive option is the use of chemotherapy regimens proven to work in gastrointestinal malignancies. The Gynecology Oncology Group (GOG) RCT 0241 (13) included exclusively patients with mEOC and evaluated four different treatment regimens (paclitaxel plus carboplatin or oxaliplatin plus capecitabine, with or without bevacizumab) in 50 patients with FIGO stage II–IV disease or with recurrence after treatment for a stage I tumor. After a median follow-up of 59 months, median PFS was 16.4 months, with no difference between treatment arms. Median OS was 27.8 months (33.9 for capecitabine/oxaliplatin vs. 27.7 months for paclitaxel/carboplatin, HR 0.77, P=0.48), with no benefit added by bevacizumab (29).
Based on this evidence, the National Comprehensive Cancer Network (NCCN) guidelines recommend surgery alone for stage IA or IB mEOC and adjuvant platinum-based chemotherapy (either paclitaxel/carboplatin or oxaliplatin with fluorouracil or capecitabine) for more advanced disease (14).
Genomic profiling studies have shown that the mutational landscape of mEOC is different from serous carcinoma (18). Mucinous neoplasms are not associated with BRCA mutations or defects in homologous recombination, making them unlikely to benefit from poly ADP ribose polymerase (PARP) inhibitors. However, they frequently display mutations or amplifications that might be targetable. Epidermal growth factor receptor (EGFR) amplification or mutations in BRAF, FGFR, or STK11 have been detected in human epidermal growth factor receptor-2 (HER2) negative tumors with wild-type KRAS, suggesting that these tumors may be responsive to targeted therapies (12,30). Just as the absence of a KRAS mutation identifies a subset of patients with colorectal cancer who are more likely to benefit from EGFR-inhibition, preclinical studies have shown that cetuximab inhibits proliferation in mEOC cell lines with wild type KRAS, whereas it has no antitumor effect in a model of KRAS-mutated mEOC (31).
About 15–20% of mEOC is associated with defects in DNA mismatch-repair system resulting in high microsatellite instability (MSI-h) (32). These tumors have a high mutational burden and dense immune infiltrates, and characteristically respond to immune checkpoint inhibition. Although there are no specific studies of immunotherapy in mEOC, 15 patients with MSI-h ovarian cancer (representing 6.4% of the entire patient population) were included in the phase II KeyNote-158 trial (33) that assessed the efficacy of pembrolizumab in non-colorectal MSI-h tumors. In these patients, the RR to pembrolizumab was 33% (3 complete and 2 partial responses), with a median PFS of 2.3 months (95% CI, 1.9–6.2 months) (34).
Endometrioid ovarian carcinoma
Endometrioid carcinoma accounts for 10% of EOC (1). This tumor type presents most frequently in women aged 40–50 years, with a mean age at diagnosis of 56 years (3). Compared with serous tumors, endometrioid carcinomas are more commonly identified at earlier stages and tend to be relatively sensitive to chemotherapy, translating into a better prognosis (3). In a Canadian cohort of 533 patients with EOC (9) (18% endometrioid and 81% serous), 5-year OS was significantly better for those with endometroid tumors (81% vs. 35%), translating in a significantly lower risk of death (HR 0.41, 95% CI: 0.26–0.66) (9). The most widely known risk factor for this type of tumor is endometriosis, which increases the risk of by approximately 14% [odds ratio (OR) 2.04, 95% CI: 1.67–2.48] (35). Endometrioid carcinoma has also been found in women with Lynch syndrome, accounting for 10–15% of hereditary EOC, with the lifetime risk being higher for women with mutations in MLH1 (20%) or MSH2 (24%) genes (36).
Macroscopically, endometrioid tumors are cystic, solid or hemorrhagic, without papillary formations (10). High-grade endometrioid carcinomas have similar profiles to high grade serous carcinomas, with expression of p53, p16, and WT1 (11). Ovarian endometrioid carcinomas most frequently harbor alterations in ARID1A (33%), PTEN (32%), CTNNB1 (28%), PIK3CA (26%) and KRAS (26%) (11).
Primary treatment includes complete cytoreductive surgery with comprehensive staging followed by adjuvant treatment or observation. Fertility-sparing surgery is an option for stage IA-IC1 tumors, with half of recurrences in these cases occurring in the contralateral ovary and therefore amenable to rescue by subsequent surgery (24). Evidence of the role of adjuvant chemotherapy in stage I is scarce. Data from a retrospective Surveillance, Epidemiology and End Results (SEER) cohort (37) including 3,552 patients with FIGO stage I endometrioid ovarian cancer showed that 5-year OS was 90% in patients who received adjuvant chemotherapy versus 89% for those who did not (P=0.80). Only in the subgroup of patients with FIGO IC, grade 3 tumors chemotherapy was associated with an improvement in 5-year OS (81% vs. 62%; HR, 0.58, P=0.03) (37). Based on this data, it is fair to say that women with stage IA/IB, grade 1/2, tumors who have had complete surgical staging have a 5-year disease-free survival (DFS) of over 90% and do not require adjuvant chemotherapy. Patients with grade 3 (any stage) and stage IC-II tumors have a less favorable prognosis, and adjuvant platinum-based chemotherapy should be considered (38).
The optimal duration of chemotherapy is not well defined. The GOG-157 trial (39) evaluated three versus six cycles of adjuvant carboplatin (AUC 7.5) and paclitaxel (175 mg/m2) every 3 weeks in high risk early stage EOC, including 25% patients with endometrioid histology. The risk of recurrence was found to be lower for patients receiving six cycles (HR 0.76; P=0.18), although longer treatment duration was associated with more toxicity (39). Therefore, six cycles of standard carboplatin plus paclitaxel +/− bevacizumab is the recommended therapy for stage II/IV endometrioid carcinomas, as well as for high grade serous carcinoma.
Similar to breast cancer, the majority of EOC express estrogen receptors (ER) (40). Several preclinical models have demonstrated the role of estrogen in terms of tumor proliferation and progression in EOC through direct [tumor vascular endothelial growth factor (VEGF) production via ER signaling] and indirect (increased cell migration via protein kinase signaling) pathways (41). Sieh and colleagues (42) reported a tissue-microarray-based analysis of ER and progesterone receptor (PR) expression in 2,933 women with invasive EOC and found significant receptor expression in endometrioid tumors (77% ER and 67% PR). The expression of one or both independently was associated with improved survival (HR 0.33, P=0.001). Furthermore, receptor expression was also associated with lower grade tumors (P=0.007) and absence of macroscopic residual disease after surgery (P=0.041) (42).
Since 1982, more than 50 trials have evaluated the use of endocrine therapy in EOC, but no RCT have been reported to date. Almost all trials included patients with prior treatments. Overall, studies have large variabilities in the type of endocrine agent used (tamoxifen, letrozole, anastrozole, exemestane, etc.), and poorly defined histological subtype/ER expression thresholds, which may explain the inconsistent results. However, positive hormone receptor status and histologic subtype seem to be relevant when it comes to response to endocrine therapy (40). In a meta-analysis involving 2,490 patients, Paleari and collegues (43) found a clinical benefit rate of 41% in patients with EOC treated with endocrine therapy, suggesting that there is subgroup of patients with tumor biology that responds well to treatment. Tamoxifen showed the highest clinical benefit rate (43%), compared with 39% for aromatase inhibitors. Based on this evidence, NCCN guidelines recommend hormonal therapy in the adjuvant and recurrent setting in patients with Grade 1 endometrioid tumors (2B recommendation) (29).
CCC
CCC accounts for almost 10% of EOC (44). There are differences in its prevalence by geographic area and/or ethnicity; for example, in East Asia the prevalence of ovarian CCC is higher (45) (23%) than in the United States (5%) (15), and this is also true when comparing Asian vs. Non-Hispanic White populations in the US (11.1% vs. 4.8%) (15). That being said, it is not clear whether this is due to genetic or environmental factors. CCC often presents at an early stage (stage I or II) compared to serous cancers (15,16) but both DFS and OS adjusted for stage is worse for patients with CCC (15,46), which can be explained by the reduced sensitivity of CCC to platinum-based chemotherapy (16,47).
The treatment strategy for CCC is very similar to that for other histologic types (17,48). Despite the fact that these tumors may be more resistant to cytotoxic treatments, adjuvant chemotherapy should be strongly considered for all patients with early stage (I or II) CCC due to its poor prognosis, as clear-cell histology is considered to be a high-risk factor for recurrence (16,47). Although there is limited evidence regarding the benefits of adjuvant chemotherapy in women with stage IA CCC, guidelines also recommend adjuvant treatment with a platinum-based doublet in these cases (48-50). Other regimens, such as irinotecan plus cisplatin, have failed to demonstrate survival benefits (51).
Advanced CCC should initially be treated with surgical cytoreduction (maximal debulking) followed by first-line chemotherapy (17,48). While responses are much lower than for other subtypes, combination chemotherapy with paclitaxel plus platinum leads to improved survival in patients with advanced CCC, especially those with optimal cytoreduction (18). Unfortunately, most women with advanced-stage CCC will relapse and require additional treatment. The prognosis of relapsing CCC is very poor when compared with serous carcinoma (52). A retrospective Japanese study showed that, in both platinum-sensitive and platinum-resistant tumors, responses were observed in less than 10% of patients with CCC (53). In general, no correlation between the efficacy of second line chemotherapy and histological subtypes has been shown due the small numbers of CCC included in trials of recurrent disease (54,55).
The presence of specific molecular characteristics in CCC, such as the activation of the PI3K/AKT/mTOR, VEGF, IL-6/STAT3, MET, and HNF-1β pathways has been proposed as the reason for resistance to cytotoxic chemotherapy (56). Therefore, pathway inhibitors are currently being evaluated as treatment strategies for CCC, either as single-agent therapies or in combination with cytotoxic agents. For example, the combination of temsirolimus with carboplatin/paclitaxel was investigated in patients with advanced CCC of the ovary. However, compared to conventional treatments, this regimen did not significantly increase PFS (57).
Carcinosarcoma
Ovarian carcinosarcoma is a rare tumor composed of malignant epithelial and mesenchymal components. It is estimated that carcinosarcoma represents 1–4% of all EOC (58-60). Patients diagnosed with carcinosarcoma are significantly older at the time of presentation (61,62), more often present with advanced stage disease (62), and have a shorter OS when compared to other EOC subtypes (63) (Table 2).

Full table
Although prospective evidence is limited since patients with carcinosarcoma are usually excluded from RCT, the management of carcinosarcoma is similar to other ovarian tumors and includes cytoreductive surgery followed by adjuvant chemotherapy for women with advanced disease (29). Therapeutic strategies for carcinosarcoma have been extrapolated from the management experience of both EOC and of uterine carcinosarcoma, anecdotal experience, and/or small retrospective and prospective series. Treatment regimens utilized for carcinosarcoma have included platinum, paclitaxel, ifosfamide, doxorubicin and dacarbazine as single-agents or in combination. A prospective cohort study led by GOG showed that among 44 patients with carcinosarcoma treated with cisplatin (50 mg/m2), the RR was 20%, with a median PFS and OS of 5.2 and 11.7 months, respectively (64). Other first-line treatments, such as doxorubicin, have shown a disappointingly low RR of only 10% (65), while second-line therapy with ifosfamide plus mesna showed a RR of 17.9% (66).
In the absence of RCT and under the premise that the combination of carboplatin and paclitaxel has proven to be effective in the treatment of EOC, guidelines suggest utilizing this regimen for the treatment of carcinosarcoma (29). Data from a retrospective study conducted at the Massachusetts General Hospital found that first-line carboplatin achieved a RR of 72% in women with primary carcinosarcoma of the ovary (n=26), with an OS of 27.1 months (67). Another case-control study including 50 patients with carcinosarcoma reported a 62% RR and a median OS of 24 months (95% CI, 18–29) when treated with carboplatin and paclitaxel, although this was lower than seen in other EOC subtypes (68). The combination of cisplatin and ifosfamide, which has been proven effective in the treatment of uterine carcinosarcoma, has also been used for ovarian tumors, even achieving better results than paclitaxel/carboplatin in a small study (69).
Unfortunately, patients with advanced stage carcinosarcoma rapidly develop platinum resistant tumors (61) and there is no evidence regarding the use of second-line chemotherapy in these cases. Future areas for research into the treatment of this malignancy include the use of targeted therapies, including those targeting HER2 (70).
Malignant ovarian germ cell tumors (MOGCT)
MOGCT originate from primordial germ cells and sex cord-stromal derivatives, and include dysgerminomas, immature teratomas, embryonal tumors, non-gestational choriocarcinomas, and endodermal sinus (yolk sac) tumors (2). MOGCT account for 3–5% of all ovarian malignant neoplasms, and are more often seen in young women and adolescent girls (71).
Unlike EOC, MOGCTs usually present with abdominal symptoms including pain, a palpable pelvic mass, and/or ascites due to capsular distension, hemorrhage, and necrosis (72). Although symptoms contribute to an earlier diagnosis, the majority of them are unilateral and diagnosed in stage I or II. The recommended initial workup includes serum tumor markers [alpha-fetoprotein (AFP), beta-human chorionic gonadotrophin (B-HGC)], chest X-ray, abdominal computed tomography and pelvic ultrasound (72). B-HGC is elevated in patients with choriocarcinoma; AFP is found with tumors containing yolk sac tumor elements; embryonal carcinoma may produce both serum markers; and dysgerminomas produce low levels of B-HGC and lactic dehydrogenase (LDH). Serum markers play an important role in the diagnosis of MOGCTs and are considered of prognostic value and useful in the management and follow-up of patients, indicating remission or relapse. Other poor prognostic factors include advanced stage, histologic subtype (non-dysgerminoma/immature teratoma), and residual disease (73). The most frequent histology is dysgerminoma, while non-gestational choriocarcinoma and embryonal carcinoma are less common.
MOGCTs have an excellent prognosis after multimodal therapy with surgery and adjuvant systemic treatment which is considered standard of care for these tumors (Table 3). The type of surgery depends on the tumor extension and traditionally fertility sparing surgery is considered acceptable (74,75). It includes unilateral salpingo-oophorectomy, peritoneal washing, careful and systematic abdominal exploration with multiple biopsies of the pelvic and abdominal peritoneum, omentectomy, and retroperitoneal lymphadenectomy, including the bilateral pelvic and para-aortic lymph node areas. Total hysterectomy and bilateral salpingo-oophorectomy is reserved for those with more advanced disease (76).

Full table
Combination chemotherapy has dramatically changed the prognosis of patients with MOGCTs. Vincristine, dactinomycin and cyclophosphamide was the standard of care in the early 1970’s. Vinblastine, bleomycin and cisplatin (PVB) was also considered another effective regimen before bleomycin, etoposide and cisplatin (BEP) became the new standard in 1990 (77). BEP was compared with the PVB regimen in patients with germ cell testicular cancer, showing similar efficacy with significantly less toxicity, which led to its adoption for the treatment of women with MOGCTs.
Adjuvant chemotherapy is recommended for all patients with stage II to IV malignant dysgerminomas or immature teratomas; stage I, grade 2 to 3 immature teratomas; and any stage embryonal tumors or endodermal sinus tumors (29). Only selected patients with stage IA or IB are candidates for observation. Four cycles of BEP is considered as the standard regimen, although some patients with low-risk or stage I disease may be treated with three cycles of BEP or three cycles of etoposide/carboplatin, particularly when avoidance of toxicity is critical (77).
Another active regimen is POMB-ACE (cisplatin, oncovin-vincristine, methotrexate, bleomycin, actinomycin-D, cyclophosphamide, etoposide), which was initially used for patients with testicular cancer. It has shown similar RR compared to other regimens and is generally well tolerated (78). Although MOGCTs have a favorable prognosis, recurrences can occur in a small percentage of cases. Due to the rarity of these tumors, treatment for recurrent disease is usually extrapolated from protocols developed for testicular cancer (72).
Malignant sex cord-stromal tumors
Sex cord-stromal tumors are infrequent, representing only 7% of all ovarian tumors. These tumors arise from the primitive sex cords or stromal cells, with the most common being those derived from the granulosa, followed by Sertoli-Leydig cell tumors. Most present as low grade and are of good prognosis, presenting more frequently in younger patients, with the exception being the adult granulosa cell tumor, which presents later in life (up to age 55 years) (79). Sex cord-stromal tumors often have hormone-associated symptoms (including virilization and hypoestrogenism) due to their production of androgens, estrogens and corticoids. Histologic classification includes pure sex cord tumors, pure stromal tumors, and mixed tumors (80). Mutations in DICER1, STK11 and FOXL2 can be found in some of these tumors.
The primary treatment is surgery. Fertility-sparing surgery is recommended for patients desiring to preserve their fertility and with early stage tumors (stage IA or IC). Complete staging (sampling of peritoneal fluid, examination of the contralateral ovary, biopsies of the peritoneum and any suspicious lesions, omental biopsy, and palpation of lymph nodes) is recommended for all other patients (29,79). Adjuvant treatment includes radiotherapy for limited disease and platinum-based chemotherapy for patients with stage II to IV disease. Due to their rarity, few studies have looked at the efficacy of adjuvant therapy (81) (Table 3). Currently, the most commonly utilized chemotherapy regimen is BEP for four to six cycles (82,83), although the combination of paclitaxel plus carboplatin has also shown to be an active regimen (84,85).
Relapse is uncommon, however for those that have a recurrence systemic therapy options include single agent taxanes or taxanes in combination with a platinum compound or ifosfamide. Endocrine therapy, including aromatase inhibitors, tamoxifen, and leuprolide, has also shown efficacy (86). Bevacizumab alone or in combination is another therapeutic option for recurrent disease (87,88).
Conclusions
Non-serous EOC represent a heterogenous and uncommon group of tumors. Most of these are diagnosed in women in their fifties, present at earlier stages, and are less sensitive to conventional platinum-based chemotherapy regimens in comparison to high-grade serous tumors. In general, non-serous EOC have a better prognosis at early stages, with the exception being CCC and carcinosarcoma, which tend to be more aggressive and have less favorable survival rates. On the other hand, MOGCTs and sex-cord stromal tumors mainly affect young patients and have very good prognosis after adjuvant chemotherapy. Although these tumors are rare, understanding the available treatment options, as well as potential strategies to improve their management in the future, is essential for all clinicians providing care for women with ovarian cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Heriberto Medina-Franco) for the series “Ovarian Cancer” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-36). The series “Ovarian Cancer” was commissioned by the editorial office without any funding or sponsorship. YCG reports other from Roche, personal fees from Pfizer, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2018 Available online: https://gco.iarc.fr/today, accessed 20 January 2020.
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. IARC. Lyon, France: International Agency for Research on Cancer, 2014:10-40.
- Seidman JD, Horkayne-Szakaly I, Haiba M, et al. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 2004;23:41-4. [Crossref] [PubMed]
- National Cancer Institute; Surveillance, Epidemiology, and End Result Program (SEER). Available online: https://seer.cancer.gov/statfacts/html/ovary.html
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609-15. [Crossref] [PubMed]
- McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology 2011;43:420-32. [Crossref] [PubMed]
- Ozols RF, Bundy BN, Greer BE, et al. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group. J Clin Oncol 2003;21:3194-200. [Crossref] [PubMed]
- Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst 2019;111:60-8. [Crossref] [PubMed]
- Bouchard-Fortier G, Panzarella T, Rosen B. Endometrioid carcinoma of the ovary: outcomes compared to serous carcinoma after 10 years of follow-up. J Obstet Gynaecol Can 2017;39:34-41. [Crossref] [PubMed]
- Fadare O, Parkash V. Pathology of endometrioid and clear cell carcinoma of the ovary. Surg Pathol Clin 2019;12:529-64. [Crossref] [PubMed]
- The AACR Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017;7:818-31. [Crossref] [PubMed]
- Morice P, Gouy S, Leary A. Mucinous ovarian carcinoma. N Engl J Med 2019;380:1256-66. [Crossref] [PubMed]
- Gore M, Hackshaw A, Brady WE, et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol Oncol 2019;153:541-8. [Crossref] [PubMed]
- Ryland GL, Hunter SM, Doyle MA, et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med 2015;7:87. [Crossref] [PubMed]
- Chan JK, Teoh D, Hu JM, et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 2008;109:370-6. [Crossref] [PubMed]
- Sugiyama T, Kamura T, Kigawa J, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000;88:2584-9. [Crossref] [PubMed]
- Okamoto A, Glasspool RM, Mabuchi S, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int J Gynecol Cancer 2014;24:S20-5. [Crossref] [PubMed]
- Utsunomiya H, Akahira J, Tanno S, et al. Paclitaxel-platinum combination chemotherapy for advanced or recurrent ovarian clear cell adenocarcinoma: a multicenter trial. Int J Gynecol Cancer 2006;16:52-6. [Crossref] [PubMed]
- Mackenzie R, Kommoss S, Winterhoff BJ, et al. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer 2015;15:415. [Crossref] [PubMed]
- Kurman RJ, Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 2008;27:151. [PubMed]
- Baker PM, Oliva E. Immunohistochemistry as a tool in the differential diagnosis of ovarian tumors: an update. Int J Gynecol Pathol 2005;24:39. [PubMed]
- Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 2003;27:985-93. [Crossref] [PubMed]
- Lee JY, Jo YR, Kim TH, et al. Safety of fertility-sparing surgery in primary mucinous carcinoma of the ovary. Cancer Res Treat 2015;47:290-7. [Crossref] [PubMed]
- Bentivegna E, Fruscio R, Roussin S, et al. Long-term follow-up of patients with an isolated ovarian recurrence after conservative treatment of epithelial ovarian cancer: review of the results of an international multicenter study comprising 545 patients. Fertil Steril 2015;104:1319-24. [Crossref] [PubMed]
- Kelemen LE, Köbel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol 2011;12:1071-80. [Crossref] [PubMed]
- Hess V, A’Hern R, Nasiri N, et al. Mucinous Epithelial Ovarian Cancer: A Separate Entity Requiring Specific Treatment. J Clin Oncol 2004;22:1040-4. [Crossref] [PubMed]
- Alexandre J, Ray-Coquard F, Selle A, et al. Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience. Ann Oncol 2010;21:2377-81. [Crossref] [PubMed]
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet 2002;360:505-15. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 3.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf, Accessed December 10, 2019.
- Jain A, Ryan PD, Seiden MV. Metastatic mucinous ovarian cancer and treatment decisions based on histology and molecular markers rather than the primary location. J Natl Compr Canc Netw 2012;10:1076-80. [Crossref] [PubMed]
- Sato N, Saga Y, Mizukami H, et al. Cetuximab inhibits the growth of mucinous ovarian carcinoma tumor cells lacking KRAS gene mutations. Oncol Rep 2012;27:1336-40. [PubMed]
- Murphy MA, Wentzensen N. Frequency of mismatch repair deficiency in ovarian cancer: a systematic review. Int J Cancer 2011;129:1914-22. [Crossref] [PubMed]
- Diaz LA, Marabelle A, Delord JP, et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. Presented at the annual meeting of the American Society of Clinical Oncology, Chicago, June 2–6, 2017. abstract. Available online: https://meetinglibrary.asco.org/record/144822/abstract
- Marabelle A, Le Dung T, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatchrepair-deficient cancer:results from the phase II keynote-158 study. J Clin Oncol 2020;38:1-10. [Crossref] [PubMed]
- Pearce CL, Templeman C, Roosing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol 2012;13:385-94. [Crossref] [PubMed]
- Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch síndrome. JAMA 2011;305:2304-10. [Crossref] [PubMed]
- Oseledchyk A, Leitao M Jr, Konner J, et al. Adjuvant chemotherapy in patients with stage I endometrioid or clear cell ovarian cancer in the platinum era: a Surveillance, Epidemiology, and End Results Cohort Study, 2000–2013. Ann Oncol 2017;28:2985-93. [Crossref] [PubMed]
- Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant Chemotherapy in Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst 2003;95:105-12. [Crossref] [PubMed]
- Bell J, Brady MF, Young RC, et al. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2006;102:432-9. [Crossref] [PubMed]
- Meszaros A, Schwarz V, Vetter M. Endocrine therapy in epithelial ovarian cancer: New insights in an old target. J Cancer Clin Trials 2018;3:2.
- Langdon SP, Hirst GL, Miller EP, et al. The regulation of growth and protein expression by estrogen in vitro: a study of 8 human ovarian carcinoma cell lines. J Steroid Biochem Mol Biol 1994;50:131-5. [Crossref] [PubMed]
- Sieh W, Köbel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol 2013;14:853-62. [Crossref] [PubMed]
- Paleari L, Gandini S, Provinciali N, et al. Clinical benefit and risk of death with endocrine therapy in ovarian cancer: A comprehensive review and meta-analysis. Gynecol Oncol 2017;146:504-13. [Crossref] [PubMed]
- Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95 Suppl 1:S161-92. [Crossref] [PubMed]
- Nagase S, Ohta T, Takahashi F, et al. Annual report of the committee on gynecologic oncology, the Japan Society of Obstetrics and Gynecology: Annual patients report for 2015 and annual treatment report for 2010. J Obstet Gynaecol Res 2019;45:289-98. [Crossref] [PubMed]
- Winter WE, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:3621-7. [Crossref] [PubMed]
- Itamochi H, Kigawa J, Sultana H, et al. Sensitivity to anticancer agents and resistance mechanisms in clear cell carcinoma of the ovary. Jpn J Cancer Res 2002;93:723-8. [Crossref] [PubMed]
- Colombo N, Sessa C. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol 2019;30:672-705. [Crossref] [PubMed]
- Takada T, Iwase H, Iitsuka C, et al. Adjuvant chemotherapy for stage I clear cell carcinoma of the ovary: an analysis of fully staged patients. Int J Gynecol Cancer 2012;22:573-8. [Crossref] [PubMed]
- Takano M, Sugiyama T, Yaegashi N, et al. Less impact of adjuvant chemotherapy for stage I clear cell carcinoma of the ovary: a retrospective Japan Clear Cell Carcinoma Study. Int J Gynecol Cancer 2010;20:1506-10. [PubMed]
- Sugiyama T, Okamoto A, Enomoto T, et al. Randomized Phase III Trial of Irinotecan Plus Cisplatin Compared With Paclitaxel Plus Carboplatin As First-Line Chemotherapy for Ovarian Clear Cell Carcinoma: JGOG3017/GCIG Trial. J Clin Oncol 2016;34:2881-7. [Crossref] [PubMed]
- Kajiyama H, Shibata K, Mizuno M, et al. Postrecurrent oncologic outcome of patients with ovarian clear cell carcinoma. Int J Gynecol Cancer 2012;22:801-6. [Crossref] [PubMed]
- Takano M, Sugiyama T, Yaegashi N, et al. Low response rate of second-line chemotherapy for recurrent or refractory clear cell carcinoma of the ovary: a retrospective Japan Clear Cell Carcinoma Study. Int J Gynecol Cancer 2008;18:937-42. [Crossref] [PubMed]
- Pujade-Lauraine E, Wagner U, Aavall-lundqvist E, et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol 2010;28:3323-9. [Crossref] [PubMed]
- Ferrandina G, Ludovisi M, Lorusso D, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 2008;26:890-6. [Crossref] [PubMed]
- Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol 2016;27:e31. [Crossref] [PubMed]
- Farley JH, Brady WE, Fujiwara K, et al. A phase II evaluation of temsirolimus in combination with carboplatin and paclitaxel followed by temsirolimus consolidation as first-line therapy in the treatment of stage III-IV clear cell carcinoma of the ovary. J Clin Oncol 2016;34:5531. [Crossref]
- del Carmen MG, Birrer M, Schorge JO. Carcinosarcoma of the ovary: a review of the literature. Gynecol Oncol 2012;125:271-7. [Crossref] [PubMed]
- Chang J, Sharpe JC, A'hern RP, et al. Carcinosarcoma of the ovary: incidence, prognosis, treatment and survival of patients. Ann Oncol 1995;6:755-8. [Crossref] [PubMed]
- Mano MS, Rosa DD, Azambuja E, et al. Current management of ovarian carcinosarcoma. Int J Gynecol Cancer 2007;17:316-24. [Crossref] [PubMed]
- Rauh-Hain JA, Diver EJ, Clemmer JT, et al. Carcinosarcoma of the ovary compared to papillary serous ovarian carcinoma: a SEER analysis. Gynecol Oncol 2013;131:46-51. [Crossref] [PubMed]
- George EM, Herzog TJ, Neugut AI, et al. Carcinosarcoma of the ovary: natural history, patterns of treatment, and outcome. Gynecol Oncol 2013;131:42-5. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Morris R, Malone JM, Munkarah AR. Survival of women diagnosed with malignant, mixed mullerian tumors of the ovary (OMMMT). Gynecol Oncol 2004;93:506-12. [Crossref] [PubMed]
- Tate thigpen J, Blessing JA, Degeest K, et al. Cisplatin as initial chemotherapy in ovarian carcinosarcomas: a Gynecologic Oncology Group study. Gynecol Oncol 2004;93:336-9.
- Morrow CP, Bundy BN, Hoffman J, et al. A Gynecologic Oncology Group Study. Adriamycin chemotherapy for malignant mixed mesodermal tumor of the ovary. Am J Clin Oncol 1986;9:24-6. [Crossref] [PubMed]
- Sutton GP, Blessing JA, Homesley HD, et al. A phase II trial of ifosfamide and mesna in patients with advanced or recurrent mixed mesodermal tumors of the ovary previously treated with platinum-based chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol 1994;53:24-6. [Crossref] [PubMed]
- Duska LR, Garrett A, Eltabbakh GH, et al. Paclitaxel and platinum chemotherapy for malignant mixed müllerian tumors of the ovary. Gynecol Oncol 2002;85:459-63. [Crossref] [PubMed]
- Rauh-Hain JA, Growdon WB, Rodriguez N, et al. Carcinosarcoma of the ovary: a case-control study. Gynecol Oncol 2011;121:477-81. [Crossref] [PubMed]
- Sit AS, Price FV, Kelley JL, et al. Chemotherapy for malignant mixed Müllerian tumors of the ovary. Gynecol Oncol 2000;79:196-200. [Crossref] [PubMed]
- Han Ch, Altwerger G, Menderes G, et al. Novel Targeted Therapies in Ovarian and Uterine Carcinosarcomas. Discov Med 2018;25:309-19. [PubMed]
- Smith HO, Berwick M, Verschraegen CF, et al. Incidence and Survival Rates for Female Malignant Germ Cell Tumors. Obstet Gynecol 2006;107:1075-85. [Crossref] [PubMed]
- Gershenson DM. Management of Ovarian Germ Cell Tumors. J Clin Oncol 2007;25:2938-43. [Crossref] [PubMed]
- Lai CH, Chang TC, Hsueh S, et al. Outcome and prognostic factors in ovarian germ cell malignancies. Gynecol Oncol 2005;96:784-91. [Crossref] [PubMed]
- Kurman RJ, Norris HJ. Malignant germ cell tumors of the ovary. Hum Pathol 1977;8:551-64. [Crossref] [PubMed]
- Peccatori F, Bonazzi C, Chiari S, et al. Surgical management of malignant ovarian germ- cell tumors: 10 years experience of 129 patients. Obstet Gynecol 1995;86:367-72. [Crossref] [PubMed]
- Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev 2008;34:427-41. [Crossref] [PubMed]
- Gershenson DM, Morris M, Cangir A, et al. Treatment of Malignant Germ Cell Tumors of the Ovary With BLeomycin, Etoposid, and Cisplatin. J Clin Oncol 1990;8:715-20. [Crossref] [PubMed]
- Bower M, Fife K, Holden L, et al. Chemotherapy for ovarian germ cell tumours. Eur J Cancer 1996;32A:593-7. [Crossref] [PubMed]
- Schultz KA, Harris AK, Schneider DT, et al. Ovarian Sex Cord-Stromal Tumors. J Oncol Pract 2016;12:940-6. [Crossref] [PubMed]
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol 2015;21:277-86. [Crossref] [PubMed]
- Gurumurthy M, Bryant A, Shanbhag S. Effectiveness of different treatment modalities for the management of adult-onset granulosa cell tumours of the ovary (primary and recurrent). Cochrane Database Syst Rev 2014;4:CD006912. [PubMed]
- Homesley HD, Bundy BN, Hurteau JA, et al. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: A Gynecologic Oncology Group study. Gynecol Oncol 1999;72:131-7. [Crossref] [PubMed]
- Pautier P, Gutierrez-Bonnaire M, Rey A, et al. Combination of bleomycin, etoposide, and cisplatin for the treatment of advanced ovarian granulosa cell tumors. Int J Gynecol Cancer 2008;18:446-52. [Crossref] [PubMed]
- Brown J, Shvartsman HS, Deavers MT, et al. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord-stromal ovarian tumors. Gynecol Oncol 2005;97:489-96. [Crossref] [PubMed]
- Brown J, Shvartsman HS, Deavers MT, et al. The activity of taxanes in the treatment of sex cord-stromal ovarian tumors. J Clin Oncol 2004;22:3517-23. [Crossref] [PubMed]
- Fishman A, Kudelka AP, Tresukosol D, et al. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J Reprod Med 1996;41:393-6. [PubMed]
- Tao X, Sood AK, Deavers MT, et al. Anti-angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecol Oncol 2009;114:431-6. [Crossref] [PubMed]
- Brown J, Brady WE, Schink J, et al. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: Results of a phase 2 trial of the Gynecologic Oncology Group. Cancer 2014;120:344-51. [Crossref] [PubMed]


