
Advances in percutaneous lung tumor therapy: 2019 update
Introduction
Malignant lung tumors are a leading cause of death worldwide (1). Surgical resection is the gold standard treatment, but surgical candidates are fewer than 30% of all patients because of poor respiratory function, advanced age, and multiple comorbidities (2). For these patients, alternative treatments include systemic therapy including cytotoxic chemotherapy, molecular target therapy and immune check inhibitors, radiation therapy, and percutaneous thermal ablation therapy such as radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation. We herein describe the latest evidence and future prospects for percutaneous ablation therapy of lung cancer.
Post-ablation imaging findings
Post-ablation imaging findings from RFA and MWA are similar. On CT images after immediately after the ablation, ground-glass opacity with surrounding dense rim appears around the tumor (Figure 1). According to earlier reports, the surrounding dense rim and ground-glass opacity within the rim appear respectively as granulation tissue on repair processes and ongoing necrosis (3). Therefore, the endpoint of RFA or MWA must be ground-glass opacity covering the entire tumor as judged from CT results immediately after ablation.
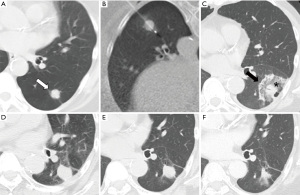
A salient benefit of cryoablation is that the ice ball of the treated area can be readily viewed on images throughout the periprocedural period. However, ice ball formation is difficult to see within the lung because of inability to view low-density ice against a background of air, especially during the initial freezing cycle (4). After the initial thaw cycle, an ablation zone is identifiable as a ground-glass opacity because of hemorrhage development. The ice ball corresponds to the 0 °C isotherm, but 0 °C is not cytotoxic. Given that the cytotoxic isotherm of −20 °C is typically several millimeters within the edge of the ice ball, the ice ball edge is expected to be 5 mm outside the tumor edge (5).
The treated mass on follow-up CT images taken within 6 months after the ablation can be larger than that on pre-ablated CT images. Then its size decreases gradually (Figure 1). The mass diameter can be increasing; alternatively, a nodule along the margin might appear if the tumor is not ablated completely (6). Reportedly, 18F-FDG PET/CT is more useful than CT for detecting local tumor progression. At 3 months after ablation, local tumor progression can appear as a focal FDG uptake area (7).
RFA outcomes
Among percutaneous ablation therapies, RFA is the most common and widely performed all over the world: related published data are abundant. Definitely, RFA is a feasible, safe, and effective treatment option for lung tumors. According to a recent meta-analysis, its complete ablation rate was 86.1% (95% CI: 78.5–92.4%), with estimated median local tumor progression-free survival of 22.0 months (95% CI: 11.8–32.2) (8). Major risk factors of local tumor progression are the tumor diameter and large vessel/bronchus adjacent to the tumor. Local tumor progression has been reported as 7–33% when the tumor is smaller than 2 cm. It was 27–50% when the tumor was larger than 2 cm (9). Tumor diameter clearly increases the possibility of tumor control failure. Using a switching controller is a promising means of treating a large tumor. Local tumor progression of tumors measuring 2–5 cm decreased to 12.7% using a switching controller (9). Another risk factor of local tumor progression is the large vessel/bronchus adjacent to the tumor. Local tumor progression occurred frequently because of the heat-sink effect, especially for a contacting vessel/bronchus larger than 3 mm (10).
Overall survival (OS) rate and estimated pooled median survival after lung RFA were found to be 83–92% at 1-year, 70–75% at 2-year, 40–58% at 3-year, 25% at 5-year, and 30.9 months (95% CI: 20.9–35.8 months) in patients with stage I primary lung cancer. A large tumor (>2 cm) was reported as a factor of poor prognosis (8,11-13). In patients with metastatic lung cancer, OS was 89–92% at 1-year, 64–66% at 2-year, 57–60% at 3-year, 45% at 5-year, and 34.8 months (95% CI: 27.6–42.1 months) (8,12). Several poor prognostic factors have been reported, including extrapulmonary metastasis, local tumor control failure, high tumor marker, large tumor (>2 cm), and a short disease-free interval (14,15).
MWA outcomes
An emerging treatment option is MWA ablation. No extensive prospective data and only few retrospective data have been reported to date. Second-generation MWA with high power (100–180 watts) has become available recently. Usage of MWA for lung tumor treatment is spreading widely. High-power MWA provides a large ablation zone in a short time and a more complete and uniform ablative margin by reducing the heat sink effects of large blood vessels compared to RFA (16). Therefore, more favorable outcomes have been anticipated using MWA.
However, although it is based on qualitatively and quantitatively insufficient evidence, clinical outcomes of MWA seem not to be markedly superior to those of RFA. The complete ablation rate was 81.1% (95% CI: 75.8–86.0%), with estimated median local tumor progression-free survival of 31.5 months (95% CI: 19.0–44.0 months) (8). The reported OS rate ranges widely: it was 65–100% at 1-year, 43–93% at 3-year, and 16–50% at 5-year in patients with stage I primary lung cancer, and 80–94% at 1-year, 37–82% at 2-year, and 11–17% at 4-year in patients with metastatic lung cancer (17-21). A meta-analysis by Yuan et al. revealed that survival was markedly poorer after lung MWA than after RFA (8). Additional large and prospective studies must be conducted to prove the theoretical benefits of MWA.
Cryoablation outcomes
Compared to RFA and MWA, cryoablation presents the great benefit of less procedural pain by virtue of the analgesic effects of freezing, with shortcomings of more frequent bleeding and the necessity of longer procedural times. Few published data are available for the outcomes of cryoablation. Local tumor progression was found in 14.9–23.8% (22-24). Large tumor (>3 cm) and large vessels measuring 3 mm or more have been reported as risk factors (22,23). Moore et al. reported excellent results from their retrospective study of 45 primary lung cancer patients: 1-year OS was 89%; 3-year was 78%; and 5-year was 68% (24). Cryoablation is apparently a useful treatment option for lung malignancies. Nevertheless, existing evidence is too weak to assess the usefulness of cryoablation.
Complications
Characteristics and rates of complications are similar among RFA, MWA, and cryoablation. Irrespective of the ablation method which is used, pneumothorax is the most common complication, with incidence of around 30% (8,23-25). Several risk factors have been reported including emphysema, tumor number, electrode position, and electrode trajectory through the aerated lung (8,23). Compared to RFA, cavitation and hemoptysis seem to occur often, respectively, after MWA and cryoablation (17,24,25). Among other complications, pleural effusion, pneumonitis, lung abscess, hemorrhagic complication, tumor seeding, air embolism, or nerve injury can occur (17,25).
Lung thermal ablation is a safe procedure, but several lethal complications have also been reported (8,25). Causes of death include acute exacerbation of idiopathic pulmonary fibrosis, hemorrhagic complication, and cardio-pulmonary failure. Especially when a patient has a history of radiation therapy for lung tumors or idiopathic pulmonary fibrosis in their lung background, indication of thermal ablation should be determined prudently in multidisciplinary discussion.
Future prospects
Treatment of malignant lung tumors has long been challenging. Outcomes of treatment other than surgery have not been satisfactory. However, recent development of molecular target therapy or immune checkpoint inhibitor might alter the treatment strategy.
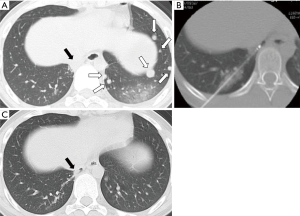
A new era is dawning in the field of thermal ablation. Thermal ablation leads not only to local tumor destruction but also to systemic effects. Various changes were visible in the blood circulation after thermal ablation. Tumor antigen (carcinoembryonic antigen or prostate specific antigen) and danger signals (heat shock proteins or high morbidity group box-1) of both pro-inflammatory and anti-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-10, TNF-α, and TGF-β) were elevated. Some immune cells (neutrophils, NK cells and tumor-antigen-specific T cells) were increased; some immune cells such as regulatory T cell were decreased (26,27). Especially, abscopal effects as systemic effects after thermal ablation are gathering great attention. Abscopal effects are a phenomenon of remission of cancer cells distant from a locally ablated tumor. This phenomenon has been described in reports of several cases (28). We also experienced a case with spontaneous lung metastases disappearing after RFA for one lung metastasis (Figure 2). However, no abscopal effect after thermal ablation has been regarded under a strong research spotlight, perhaps because of its extremely low incidence rate of 0.001% (29). The mechanism of abscopal effects after thermal ablation remains somewhat unclear. Thermal ablation might be increasingly important as a cancer treatment strategy if it is possible to elucidate details of its mechanism and increase the frequency of its use. Recently, Soule et al. reported a case in which spontaneous shrinkage of bone metastasis was achieved after debulking of primary renal cell carcinoma and cryoablation of a contralateral metastatic renal tumor combined with local administration of immune checkpoint inhibitor (30). Several clinical trials of thermal ablation combined with an immune checkpoint inhibitor such as anti-programmed death 1 antibody or anti-cytotoxic T lymphocyte antigen 4 antibody are ongoing. The study results are eagerly anticipated (26). In addition, further basic investigations must be made of local and systemic effects of ablative therapy.
Conclusions
Thermal ablation for lung malignancies is a safe and effective treatment irrespective of whether it is performed by RFA, MWA or cryoablation. Thermal ablation combined with emerging drugs such as immune check inhibitors might play an important role in systemic treatment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg 2007;83:409-17. [Crossref] [PubMed]
- Tominaga J, Miyachi H, Takase K, et al. Time-related changes in computed tomographic appearance and pathologic findings after radiofrequency ablation of the rabbit lung: preliminary experimental study. J Vasc Interv Radiol 2005;16:1719-26. [Crossref] [PubMed]
- Ito N, Nakatsuka S, Inoue M, et al. Computed tomographic appearance of lung tumors treated with percutaneous cryoablation. J Vasc Interv Radiol 2012;23:1043-52. [Crossref] [PubMed]
- Hinshaw JL, Littrup PJ, Durick N, et al. Optimizing the protocol for pulmonary cryoablation: a comparison of a dual- and triple-freeze protocol. Cardiovasc Intervent Radiol 2010;33:1180-5. [Crossref] [PubMed]
- Chheang S, Abtin F, Guteirrez A, et al. Imaging Features following Thermal Ablation of Lung Malignancies. Semin Intervent Radiol 2013;30:157-68. [Crossref] [PubMed]
- Aarntzen EHJG, Heijmen L, Oyen WJG. 18F-FDG PET/CT in Local Ablative Therapies: A Systematic Review. J Nucl Med 2018;59:551-6. [Crossref] [PubMed]
- Yuan Z, Wang Y, Zhang J, et al. A Meta-Analysis of Clinical Outcomes After Radiofrequency Ablation and Microwave Ablation for Lung Cancer and Pulmonary Metastases. J Am Coll Radiol 2019;16:302-14. [Crossref] [PubMed]
- Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency Ablation Using a Multiple-Electrode Switching System for Lung Tumors with 2.0-5.0-cm Maximum Diameter: Phase II Clinical Study. Radiology 2015;277:895-902. [Crossref] [PubMed]
- Gillams A, Khan Z, Osborn P, et al. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol 2013;36:724-30. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [Crossref] [PubMed]
- Palussière J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg 2018;13:91. [Crossref] [PubMed]
- Yamakado K, Hase S, Matsuoka T, et al. Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: a multicenter study in Japan. J Vasc Interv Radiol 2007;18:393-8. [Crossref] [PubMed]
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. l. [Crossref] [PubMed]
- Kodama H, Ueshima E, Gao S, et al. High power microwave ablation of normal swine lung: impact of duration of energy delivery on adverse event and heat sink effects. Int J Hyperthermia 2018;34:1186-93. [Crossref] [PubMed]
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. [Crossref] [PubMed]
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol 2014;110:758-63. [Crossref] [PubMed]
- Yao W, Lu M, Fan W, et al. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. Int J Hyperthermia 2018;34:1329-36. [Crossref] [PubMed]
- Zheng A, Ye X, Yang X, et al. Local Efficacy and Survival after Microwave Ablation of Lung Tumors: A Retrospective Study in 183 Patients. J Vasc Interv Radiol 2016;27:1806-14. [Crossref] [PubMed]
- Kurilova I, Gonzalez-Aguirre A, Beets-Tan RG, et al. Microwave Ablation in the Management of Colorectal Cancer Pulmonary Metastases. Cardiovasc Intervent Radiol 2018;41:1530-44. [Crossref] [PubMed]
- Yashiro H, Nakatsuka S, Inoue M, et al. Factors affecting local progression after percutaneous cryoablation of lung tumors. J Vasc Interv Radiol 2013;24:813-21. [Crossref] [PubMed]
- McDevitt JL, Mouli SK, Nemcek AA, et al. Percutaneous Cryoablation for the Treatment of Primary and Metastatic Lung Tumors: Identification of Risk Factors for Recurrence and Major Complications. J Vasc Interv Radiol 2016;27:1371-9. [Crossref] [PubMed]
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol 2015;26:312-9. [Crossref] [PubMed]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol 2011;197:W576-80. [Crossref] [PubMed]
- Takaki H, Cornelis F, Kako Y, et al. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn Interv Imaging 2017;98:651-9. [Crossref] [PubMed]
- Haen SP, Pereira PL, Salih HR, et al. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol 2011;2011:160250. [Crossref] [PubMed]
- Sánchez-Ortiz RF, Tannir N, Ahrar K, et al. Spontaneous regression of pulmonary metastases from renal cell carcinoma after radio frequency ablation of the primary tumor: an in situ tumor vaccine? J Urol 2003;170:178-9. [Crossref] [PubMed]
- Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother 2001;50:391-6. [Crossref] [PubMed]
- Soule E, Bandyk M, Matteo J. Percutaneous ablative cryoimmunotherapy for micrometastatic abscopal effect: No complications. Cryobiology 2018;82:22-6. [Crossref] [PubMed]


