
Applications of transcatheter embolotherapy in preparation for liver transplantation and resection
Introduction
Management of primary and secondary hepatic malignancies has long been a cornerstone of interventional oncology. The myriad disease processes that affect the liver combined with the many and varied treatment options available helped to cement the role of the interventional radiologist (IR) as, among other things, an oncologic specialist. However, the full utility of the available tools is often underappreciated and extends far beyond curative treatment. In particular, the supportive role IR can play in establishing and maintaining candidacy for surgical intervention is less commonly recognized and employed. Herein we provide an overview of the available pre-operative interventions with a specific focus on bridging to liver transplantation or resection.
Hepatocellular carcinoma (HCC) and liver transplantation
Overview and staging
Orthotopic liver transplantation (OLT) is the treatment of choice for patients with cirrhosis and early-stage HCC because of its ability to eliminate both the HCC lesion(s) and the underlying stroma which promotes de novo tumor development (1). However, the limited pool of available organs necessitates utilization of a system for prioritization of allotment. The current transplant allocation system is based both on urgency (predicted pre-transplant mortality) and utility (maximization of post-transplant outcome). Because post-transplant HCC recurrence is associated with dismal overall survival (OS), eligibility for transplantation is determined in part by predicted likelihood of disease recurrence (2,3). The most commonly utilized system for this purpose is the Milan criteria, which was first introduced in 1996 (4). It defines candidates for transplantation as those who meet the following conditions: (I) single tumor with diameter less than 5 cm, (II) no more than three HCCs, each not exceeding 3 cm, (III) no macroscopic angioinvasion, and (IV) no extrahepatic disease (5). In those patients who fall within these criteria, the post-transplant 5-year OS is 73.3% (6).
Once eligibility for transplantation has been determined, patients are stratified for deceased donor liver allocation based on disease severity as assessed by the MELD and MELD-Na scores. In the United States, patients with HCC who meet Milan criteria have since 2002 been provided a special allocation benefit, termed MELD exception points, which elevates their status on the United Network for Organ Sharing (UNOS) allocation list. When the policy began in 2002, patients classified by the Organ Procurement and Transplantation Network (OPTN) as very early HCC (T1: 1 lesion <2 cm) and early HCC (T2: 1 lesion ≥2 but <5 cm, or as many as 3 lesions <3 cm each) received an additional 24 and 29 points, respectively (7). As a result, between 2004 and 2015 the ratio of registrants with HCC to those without increased 2.7–10.2 fold (8); in fact, HCC is currently the leading diagnosis on the 2-year liver transplant waitlist among all registrants, comprising 29% of diagnoses (2). However, this policy so heavily prioritized patients with HCC over those with chronic liver disease that the drop-out rate of the latter increased to 35.8% due to death or disease progression (7). Several revisions were then made such that under the current iteration patients with very early HCC (single HCC <2 cm) no longer receive exception points and those with T2 disease are initially listed with their natural MELD score and receive points only after a mandatory 6-month waiting period; thereafter they are awarded 28 points, which increases every 3 months to a maximum of 34 points (4). Therefore, in order to increase the odds of surviving to OLT for adult patients with cirrhosis and HCC, management with one of several locoregional therapies (LRT) has been proposed: (I) T1: LRT or observation, (II) T2: bridging LRT to prevent tumor progression beyond T2, and (III) T3: downstaging LRT for HCC beyond Milan criteria.
T1: LRT or watchful observation
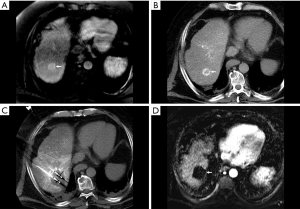
Patients with T1 disease can only be listed for transplantation for indications other than the tumor itself. Among those who are listed, those with good liver function can avoid transplantation while maintaining a good prognosis by undergoing alternative therapies, such as ablation or surgical resection (9) (Figure 1). The American Association for the Study of Liver Diseases (AASLD) currently recommends only observation with serial imaging for these patients (10). This “watchful waiting” recommendation stems from the UNOS policy in which patients with T1 HCC do not receive MELD exception points and can be abandoned depending on anticipated wait time to OLT, tumor growth rate, severity of hepatic decompensation, current allocation policy, and patient preference. This conservative approach is not without risk, however, which derives predominantly from the potential for disease progression, either from tumor enlargement or development of extrahepatic metastases. Two studies have attempted to address the frequency with which these outcomes occur (10).
Mehta et al. retrospectively evaluated 114 patients with T1 HCC measuring 1.0–1.9 cm who were followed with imaging every 3 months (11). After a median follow-up of 2.4 years, the median growth rate was 0.14 cm/month. Of the 114 patients, 6 (5.3%) remained within T1 at the end of the follow-up period, 100 (87.7%) progressed from T1 to T2 at a median of 6.9 months, and 6 (5.3%) progressed directly from T1 to beyond T2 at a median of 5.1 months. In this latter group, the progression was due to tumor burden alone in four, new portal vein tumor thrombus in one, and new adrenal metastasis in one. Huo et al. conducted a retrospective study evaluating the rate of dropout from the transplant list in patients with T1 and T2 disease who underwent LRT (12). Three hundred and ninety patients met inclusion criteria, 94 with T1 disease and 296 with T2 disease. Three- and six-month waitlist dropout rates were 2.1% and 5.3% for patients with T1 disease, respectively, and 3.0% and 6.8% for patients with T2 disease, respectively. Thus, the overall risk of loss-of-candidacy for patients with T1 disease approximates one in twenty at 6 months. Whether this risk is worth taking depends on the remainder of the clinical picture as well at patient and provider preference.
T2: bridging therapy for OLT
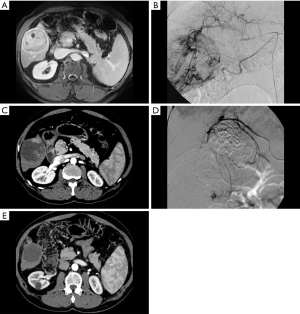
After one year without treatment, HCC will enlarge, invade vasculature, and spread to extrahepatic sites in 71%, 21%, and 9% of cases, respectively (13). The goal of bridging therapy is to reduce these rates and thereby decrease the likelihood of both pre-transplant dropout and post-transplant recurrence. Therapies available for this purpose include thermal and non-thermal ablation, conventional transarterial embolization (TAE), conventional or drug-eluting bead transarterial chemoembolization (cTACE or debTACE, respectively; Figure 2), transarterial radioembolization (TARE), or a combination of these. Currently, no one therapy has been established as superior to any other (10,14). Although the quality of evidence is low due to high risk of bias, technical inconsistency, and reporting imprecision, there is a trend towards lower dropout rates from all causes and from tumor progression in those who receive LRT (relative risk 0.38 and 0.32, respectively) (15). These therapies have also been shown to be effective in reducing dropout among patients with wait times of 6 months or longer (14,16).
Given the favorable risk-benefit ratio, both the AASLD and international consensus recommend LRT as bridging therapy for patients with T2 disease (10,14). Despite this, however, a recent metanalysis by Kulik et al. found that patients with T2 HCC who underwent bridging therapy with LRT showed no significant improvement in rates of post-transplant recurrence or recurrence-free survival (15,17). This finding was reiterated by Agopian et al. in a multicenter study which included 3,601 post-OLT patients; rates of 1-, 3-, and 5-year recurrence-free survival were not significantly different regardless of whether bridging therapy had been administered (17). In fact, the study found that the number of LRT sessions was positively correlated with likelihood of post-OLT recurrence, although the authors attributed this to the confounding factor of tumor aggression rather than the effect of the treatment itself.
T3: downstaging therapy
Downstaging refers to treatment administration with the intent of decreasing overall tumor burden such that patients whose disease previously precluded transplant candidacy now fall within established guidelines. Multiple forms of locoregional therapy have been used for this purpose, with TACE and radiofrequency ablation (RFA) accumulating the most experience (14). However, available evidence is limited with most studies being non-comparative and observational in nature; further, no study has directly compared post-transplantation outcomes in patients who initially met Milan criteria with those who underwent downstaging.
Despite this, the current evidence strongly suggests that outcomes are comparable between those who met criteria at initial evaluation and those who were successfully downstaged. For example, two prospective studies reported similar disease-free and OS between the two groups (18,19). Several retrospective studies have also supported this approach. Heckman et al. (20) retrospectively evaluated 123 patients who underwent OLT between 2000 and 2006 (spanning the pre- and post-MELD exception point eras), 50 of which were treated with pre-transplantation LRT and 73 of which were not. The 1-, 3-, and 5-year OS was not statistically different between the two groups (81%, 81%, and 81% vs. 81%, 71%, and 71%, respectively). Furthermore, the 12 patients who were successfully downstaged showed similar OS both to those who received LRT but did not respond and underwent OLT anyway and to those who did not undergo LRT. Kim et al. (21) showed that in patients meeting UNOS Region 4 T3 (R4T3) criteria (one lesion up to 6 cm, three lesions each up to 5 cm, total tumor burden up to 9 cm), those with expanded T3 disease who underwent pre-OLT LRT had lower 3-year recurrence rates (7% vs. 75%, P<0.001) and better 3-year OS rates (78% and 25%, P=0.02) than those who did not. Hołówko et al. (22) retrospectively analyzed 229 patients who underwent OLT, 75 of whom had undergone prior treatment with TACE. Again, there was no difference in 5-year recurrence-free survival between the groups.
Despite the absence of conclusive data, the AASLD currently supports listing for transplant after successful downstaging (10). As such, consideration should be given to allocating MELD exception points for patients who present with T3 disease but have the potential for successful downstaging to T2.
Surgical resection and pre-operative regenerative strategies
Overview
As an alternative to transplantation, patients with primary or secondary hepatic malignancies can be managed via partial hepatectomy. In patients with cirrhosis this does not remedy the underlying parenchymal milieu which promotes tumor development, but it obviates the need for organ procurement and long-term immunosuppression. When hepatic resection is being considered, specific attention must be paid to the size and functional status of the residual native organ, termed the future liver remnant (FLR). As surgical techniques have continued to advance allowing for more aggressive resections, the size of the FLR has become a limiting factor in determining patient candidacy (23,24). The FLR must retain enough functional capacity to support the needs of the body; thus, the more dysfunctional the underlying parenchyma, the greater the minimum FLR size necessary to prevent postoperative liver failure and death.
Fortunately, the liver is unique in the human body for its prodigious regenerative capacity, which has been recognized at least since James Cantlie documented the concept of the atrophy-hypertrophy complex in 1897 (25). Compensatory hypertrophy of un-involved liver parenchyma following ligation of portal vein branches was described in animals in the first half of the 20th century (26), but was not leveraged for medical use until 1990 when Makuuchi et al. reported portal vein embolization (PVE) in 14 patients with hilar cholangiocarcinoma (27). PVE is now a well-established technique for inducing preoperative hypertrophy of the FLR in appropriately selected patients, and attempts have been made to iterate and expand upon it to improve outcomes, although often without definite benefit.
Physiology
A detailed discussion of the physiology of liver regeneration is beyond the scope of this article (28,29). In brief, in the absence of hepatic injury, approximately 0.01–0.1% of hepatocytes are actively engaged in mitosis while the remainder are quiescent (25,30). An injurious stimulus, iatrogenic or otherwise, releases growth factors which promote hepatocyte proliferation, the degree of which depends on both the extent of damage and the health of the underlying parenchyma (30). In cases of minor injury (<10% parenchymal involvement) the response is predominantly local, whereas larger degrees of damage result in an organ-wide response (30). Although myriad noxious stimuli can induce this process, in most cases the underlying cascade is similar with the bulk of the regenerative capacity resulting from cellular division and to a lesser extent from enlargement (31); as such, at a cellular level the response is better termed hyperplasia rather than hypertrophy. Because regeneration depends upon the proliferation of the remaining uninjured hepatocytes, chronically diseased livers have less regenerative capacity than healthy ones, a factor that must be considered when assessing patient candidacy for PVE (32-34).
Induction of hepatic hypertrophy has been specifically observed following occlusion of portal vein, biliary, and/or hepatic vein branches (29). In the first, occlusion of portal branches results in redistribution of portal flow into the uninjured parenchyma. The affected parenchyma undergoes atrophy predominantly via the controlled process of apoptosis, which differs from the necrotic response seen following TAE and helps to explain the absence of a post-embolization syndrome following the former (35). The increased portal flow experienced by the un-embolized liver produces several changes which promote the regenerative response: heightened shear stress which stimulates hepatocyte DNA synthesis, relatively decreased oxygen saturation resulting from increased deoxygenated portal inflow in the setting of uncharged oxygenated arterial inflow, and increased delivery of intestinally derived growth factors (29,36). The pace of hypertrophy is greatest in the first 2 weeks following injury, before a relative plateau sets in during which hypertrophy continues at a substantially reduced rate (37). Attempts to augment the rate and ultimate degree of hypertrophy have prompted several modifications to the standard PVE, which are discussed below.
Patient selection
Pre-operative PVE should be considered for any patient who is being evaluated for partial hepatectomy but has an anticipated FLR which is too small to sustain the needs of the body in the immediate postoperative period (Figure 3). Inadequate post-resection hepatic reserve can have dire consequences including liver failure, prolonged hospital stays, and/or death (23,38). Because the right hepatic lobe is anatomically larger than the left, PVE is commonly considered before right hepatectomy to pre-operatively augment the size of the left lobe; embolization of segment 4 can be included or excluded depending on the surgical approach.

Assessment of the FLR is typically done using cross-sectional imaging, most commonly computed tomography (CT). 3D volumetry is performed by tracing the hepatic contour on each slice, measuring the enclosed cross-sectional area, multiplying the area by the slice thickness to calculate volume, and then adding the volumes together across slices to get the total volume of the measured target (39). Although cumbersome, this process has been shown to be accurate to within ±5% (40,41). The calculated FLR must be interpreted in the context of the patient’s size, as larger patients have both bigger livers and a need for a larger FLR (40). As such, the FLR is often normalized relative to the total liver volume (TLV) and reported as the standardized future liver remnant (sFLR) (40,42). The TLV can be calculated either using CT volumetry, which can be time consuming given the need to measure and then subtract the tumor volume from the total liver volume, or a formula based on body surface area (BSA) (40,42-44). The comparative accuracy of each method remains debated, although at least one study suggested BSA outperforms CT volumetry (43). The minimum necessary size of the sFLR depends on both the health of the liver parenchyma and the expected treatment the patient will be receiving: 20% for patients with healthy underlying livers, 30% for those with healthy livers who are receiving extensive chemotherapy, and 40% for those with cirrhosis and/or advanced fibrosis (34,37,45-47).
The only absolute contraindication to PVE is portal hypertension precluding surgery. However, in cases in which the target portal vein branch is already occluded, such as from tumor invasion, PVE should be avoided as it is unlikely to produce an effect given that the portal flow has already been redirected into the FLR (48).
Technical considerations
As the goal of PVE is to redirect portal flow into the FLR, embolization must be as complete as possible so as to avoid recanalization or the development of portaportal collaterals, either of which can limit the ultimate degree of hypertrophy (49). Segment 4 should be included in the embolized territory when a trisegmentectomy/extended right hepatectomy (removal of the right hepatic lobe as well as the medial segment of the left lobe) is planned (50). When performed, embolization of segment 4 produces greater and more rapid hypertrophy of the FLR without an increase in complication rates (51-53), although unintentional reflux of embolic material from segment 4 into the FLR has been described (54).
Access to the portal vein is typically obtained using a percutaneous transhepatic approach. Either ipsilateral or contralateral access points (in reference to the lobe to be resected) may be selected, noting that in most cases PVE is performed such that the left lobe, or part of it, comprises the FLR. The contralateral approach was described first and provides the advantage of relative technical ease at the potential risk of damage to the FLR (55). The ipsilateral approach, by contrast, avoids damage to the FLR but is technically more challenging secondary to extreme angulation between right portal vein branches, potentially necessitating the use of reverse curve catheters (56). Notably, however, access to segment 4 is typically easier from an ipsilateral than a contralateral approach (57). Also, as embolic is deposited in a retrograde manner with this approach, it carries the risk of embolic disruption secondary to instrumentation of the treated side; thus, care in catheter manipulation is essential, as it is treatment of segment 4 prior to treatment of the right lobe, when indicated (57). Current evidence suggests no clear superiority of one approach over the other, although several studies have suggested that the contralateral approach may have slightly lower complication rates (37,58,59).
The selection of embolic material is largely based on operator comfort and preference as nearly every commonly used embolic material has been tried and few direct comparisons are available. In general, permanent agents are believed to be more effective at inducing hypertrophy than are temporary ones (60). N-butyl-2-cyanoacetate (NBCA) glue has accrued the most experience and current evidence seems to suggest that it is the most effective overall (61,62). This material induces a potent inflammatory reaction in the liver when instilled secondary to the exothermic reaction it undergoes during polymerization, and it has been suggested that the resulting cytokine release directly stimulates liver hypertrophy (62). At least one direct comparison found NBCA to be both more effective and less expensive compared to combination coils and particles with equivalent complication rates (63). However, it can be challenging to use for those with less experience and the inflammatory reaction and scarring it produces may increase operative difficulty, although differing rates of operative success have not been reported (62).
Following embolization of the portal vein, tract embolization should be undertaken with coils, gelfoam, and/or glue to reduce the risk of hemorrhage. Bleeding from the access site is uncommon (<5%), although ipsilateral access appears to be lower risk than contralateral, likely as a result of stasis in the ipsilateral vein and increased post-embolization pressure in the contralateral vein (64).
Outcomes
Rates of technical success are typically reported at greater than 95% regardless of the procedural specifics. Mean FLR hypertrophy rates range between 37.9–49.4% with consequent successful hepatectomy undertaken in 75.9–96.1% (61,65,66). In patients with chronic liver disease hypertrophy is both slower and less exuberant, with hypertrophy rates reported to be 28–46% (67,68). Of note, kinetic growth rate (KGR), defined as degree of hypertrophy divided by time elapsed since PVE, has been reported as an alternative outcome measure to FLR (69). In one study comparing KGR to sFLR and degree of hypertrophy, a KGR of less than 2% per week was the most accurate predictor of postoperative hepatic insufficiency (69). When surgery is not ultimately performed, it is most commonly due to either inadequate FLR growth or progression of disease. The latter is of particular concern as it has been postulated that PVE accelerates tumor growth via release of trophic factors (70,71).
Complications following PVE are typically related either to the access, manifesting as bleeding, infection, or fistula formation, or to the embolization itself, including non-target embolization and extension of thrombosis to involve the left or main portal vein. In either case major complications are uncommon and reported in 2.2–3.1% of cases; mortality occurs in less than 0.1% (61,65,66). Of note, the risk of post-PVE portal vein thrombosis in particular is greater in patients with chronic liver disease than in patients with healthy livers, likely as a result of slower baseline portal flow (59).
Modifications
Although PVE has received widespread adoption, its recognized limitations have led to the development of several alternative or modified techniques for induction of FLR hypertrophy, albeit with varying levels of success. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a surgical procedure which consists of single session portal vein ligation and FLR partitioning (72). It was expected that the more complete portal occlusion combined with elimination of collateral flow would result in more extensive portal ischemia and thereby produce more rapid and extensive FLR hypertrophy. Indeed, this expectation was borne out in several studies showing greater KGR compared to PVE and one randomized controlled trial demonstrating significantly greater rates of subsequent resection (73,74). However, this dramatic growth is not accompanied by a concomitant increase in function, which likely results from the immaturity of the newly formed hepatocytes and supporting stroma (75). As a result, both morbidity and mortality are substantially higher than following PVE with analysis of an international ALPPS database showing 75% of 90-day mortality to be attributable to liver failure (76). Consequently, ALPPS is not considered a first-line procedure in most patients.
Liver venous deprivation (LVD) is an augmented form of PVE in which both the portal and hepatic veins are embolized (77) (Figure 4). Occlusion of hepatic venous outflow is thought to both further reduce portal vein inflow and attenuate the hepatic arterial buffer response, thereby producing a greater degree of ischemia than does PVE in isolation. However, the ischemia is not so severe that staging is necessary; rather concurrent PVE and hepatic vein embolization (HVE) has been shown to be safe and effective (78). The right hepatic vein is typically embolized; when the middle hepatic vein is also treated and the entire right hepatic outflow is obstructed, the procedure is termed extended liver venous deprivation (eLVD). Preliminary results are promising, with one direct comparison suggesting increased hypertrophy, decreased length of postoperative hospital stay, and decreased 90-day mortality compared to PVE (79). These results appear even more dramatic when eLVD is performed, with hypertrophy comparable to that induced by ALPPS but without the associated complications (80). Further studies are ongoing and will help to clarify the role of LVD/eLVD in preoperative hepatic regeneration moving forward.
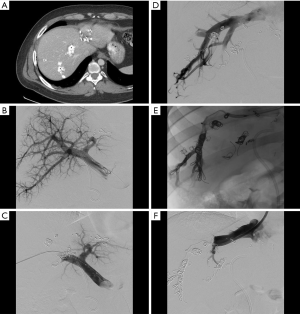
Radiation lobectomy (RL) is a transarterial approach to induction of FLR hypertrophy which developed as an outgrowth of hepatic radioembolization for treatment of malignancy (81,82) (Figure 5). Unlike its procedural parent, however, the goal is not simply to treat tumor but to radiate the entirety of the hepatic parenchyma planned for resection. Consequently, particles are administered from a more proximal location (likely a lobar rather than a segmental or subsegmental artery), and the administered radiation dose is greater (83). RL provides several advantages when compared to PVE and LVD. Because it is a trans-arterial therapy, portal vein thrombosis does not preclude performance and in fact may increase the overall efficacy (84). It requires only a single access, unlike the two that are commonly needed for LVD. In addition to producing FLR hypertrophy, it functions as a primary treatment in its own right and thereby helps to limit tumor progression in the interval between RL and surgical resection, and can additionally act as destination therapy should surgical resection ultimately not be performed (83). As with LVD, only preliminary data on efficacy are available, in part because of wide variance in procedural approach. Initial results are promising with a 2014 systematic review reporting degrees of FLR hypertrophy ranging between 26% and 47% at 44 days to 9 months (85). However, when compared with PVE, RL seems to produce a slower and ultimately smaller degree of hypertrophy (84,86,87).

Conclusions
Although surgery plays a major role in the management of primary and secondary hepatic malignancy, establishing and maintaining candidacy for these surgical interventions can be challenging. The IR, therefore, has the potential to broaden the candidacy for surgery with various minimally invasive locoregional therapies such as embolotherapy, thus contributing to an overall improvement in patient outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist 2007;12:1321-31. [Crossref] [PubMed]
- Goldberg D, French B, Newcomb C, et al. Patients with hepatocellular carcinoma have highest rates of wait-listing for liver transplantation among patients with end-stage liver disease. Clin Gastroenterol Hepatol 2016;14:1638-1646.e2. [Crossref] [PubMed]
- Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004;10:534-40. [Crossref] [PubMed]
- Kulik L. Criteria for liver transplantation in hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2015;6:100-2. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Zamora-Valdes D, Taner T, Nagorney DM. Surgical treatment of hepatocellular carcinoma. Cancer Control 2017;24:1073274817729258. [Crossref] [PubMed]
- Yang JD, Larson JJ, Watt KD, et al. Hepatocellular carcinoma is the most common indication for liver transplantation and placement on the waitlist in the United States. Clin Gastroenterol Hepatol 2017;15:767-775.e3. [Crossref] [PubMed]
- European Association for the Study of the Liver, European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Mehta N, Sarkar M, Dodge JL, et al. Intention to treat outcome of T1 hepatocellular carcinoma with the "wait and not ablate" approach until meeting T2 criteria for liver transplant listing. Liver Transpl 2016;22:178-87. [Crossref] [PubMed]
- Huo TI, Huang YH, Su CW, et al. Validation of the HCC-MELD for dropout probability in patients with small hepatocellular carcinoma undergoing locoregional therapy. Clin Transplant 2008;22:469-75. [Crossref] [PubMed]
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology 2018;67:381-400. [Crossref] [PubMed]
- Majno P, Lencioni R, Mornex F, et al. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011;17 Suppl 2:S98-108. [Crossref] [PubMed]
- Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multicenter HCC transplant consortium. Ann Surg 2017;266:525-35. [Crossref] [PubMed]
- Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant 2008;8:2547-57. [Crossref] [PubMed]
- Yao FY, Kerlan RK Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008;48:819-27. [Crossref] [PubMed]
- Heckman JT, Devera MB, Marsh JW, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol 2008;15:3169-77. [Crossref] [PubMed]
- Kim PT, Onaca N, Chinnakotla S, et al. Tumor biology and pre-transplant locoregional treatments determine outcomes in patients with T3 hepatocellular carcinoma undergoing liver transplantation. Clin Transplant 2013;27:311-8. [Crossref] [PubMed]
- Hołówko W, Wróblewski T, Wojtaszek M, et al. Transarterial chemoembolization prior to liver transplantation in patients with hepatocellular carcinoma. Ann Transplant 2015;20:764-8. [Crossref] [PubMed]
- Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999;188:304-9. [Crossref] [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. [Crossref] [PubMed]
- Black DM, Behrns KE. A scientist revisits the atrophy-hypertrophy complex: hepatic apoptosis and regeneration. Surg Oncol Clin N Am 2002;11:849-64. [Crossref] [PubMed]
- Rous P, Larimore LD. Relation of the portal blood to liver maintenance: a demonstration of liver atrophy conditional on compensation. J Exp Med 1920;31:609-32. [Crossref] [PubMed]
- Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7. [PubMed]
- Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017;65:1384-92. [Crossref] [PubMed]
- Kim RD, Kim JS, Watanabe G, et al. Liver regeneration and the atrophy-hypertrophy complex. Semin Intervent Radiol 2008;25:92-103. [Crossref] [PubMed]
- Koniaris LG, McKillop IH, Schwartz SI, et al. Liver regeneration. J Am Coll Surg 2003;197:634-59. [Crossref] [PubMed]
- Komori K, Nagino M, Nimura Y. Hepatocyte morphology and kinetics after portal vein embolization. Br J Surg 2006;93:745-51. [Crossref] [PubMed]
- Yamanaka N, Okamoto E, Kawamura E, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993;18:79-85. [Crossref] [PubMed]
- Lee KC, Kinoshita H, Hirohashi K, et al. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg 1993;17:109-15. [Crossref] [PubMed]
- Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208-17. [Crossref] [PubMed]
- Duncan JR, Hicks ME, Cai SR, et al. Embolization of portal vein branches induces hepatocyte replication in swine: a potential step in hepatic gene therapy. Radiology 1999;210:467-77. [Crossref] [PubMed]
- Hortelano S, Dewez B, Genaro AM, et al. Nitric oxide is released in regenerating liver after partial hepatectomy. Hepatology 1995;21:776-86. [PubMed]
- Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94. [Crossref] [PubMed]
- Tsao JI, Loftus JP, Nagorney DM, et al. Trends in morbidity and mortality of hepatic resection for malignancy. A matched comparative analysis. Ann Surg 1994;220:199-205. [Crossref] [PubMed]
- Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol 2005;16:779-90. [Crossref] [PubMed]
- Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512-9. [Crossref] [PubMed]
- Soyer P, Roche A, Elias D, et al. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology 1992;184:695-7. [Crossref] [PubMed]
- Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol 2008;25:104-9. [Crossref] [PubMed]
- Ribero D, Amisano M, Bertuzzo F, et al. Measured versus estimated total liver volume to preoperatively assess the adequacy of the future liver remnant: which method should we use? Ann Surg 2013;258:801-6; discussion 806-7. [Crossref] [PubMed]
- Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002;8:233-40. [Crossref] [PubMed]
- Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg 2002;137:675-80; discussion 680-1. [Crossref] [PubMed]
- Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540-8. [PubMed]
- Shindoh J, Tzeng CW, Aloia TA, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol 2013;20:2493-500. [Crossref] [PubMed]
- Madoff DC, Hicks ME, Vauthey JN, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics 2002;22:1063-76. [Crossref] [PubMed]
- Denys AL, Abehsera M, Sauvanet A, et al. Failure of right portal vein ligation to induce left lobe hypertrophy due to intrahepatic portoportal collaterals: successful treatment with portal vein embolization. AJR Am J Roentgenol 1999;173:633-5. [Crossref] [PubMed]
- Starzl TE, Bell RH, Beart RW, et al. Hepatic trisegmentectomy and other liver resections. Surg Gynecol Obstet 1975;141:429-37. [PubMed]
- Kishi Y, Madoff DC, Abdalla EK, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery 2008;144:744-51. [Crossref] [PubMed]
- Mise Y, Aloia TA, Conrad C, et al. Volume regeneration of segments 2 and 3 after right portal vein embolization in patients undergoing two-stage hepatectomy. J Gastrointest Surg 2015;19:133-41; discussion 141. [Crossref] [PubMed]
- Day RW, Conrad C, Vauthey JN, et al. Evaluating surgeon attitudes towards the safety and efficacy of portal vein occlusion and associating liver partition and portal vein ligation: a report of the MALINSA survey. HPB (Oxford) 2015;17:936-41. [Crossref] [PubMed]
- Capussotti L, Muratore A, Ferrero A, et al. Extension of right portal vein embolization to segment IV portal branches. Arch Surg 2005;140:1100-3. [Crossref] [PubMed]
- Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803-8. [Crossref] [PubMed]
- Nagino M, Nimura Y, Kamiya J, et al. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology 1996;200:559-63. [Crossref] [PubMed]
- Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 2005;16:215-25. [Crossref] [PubMed]
- Kodama Y, Shimizu T, Endo H, et al. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol 2002;13:1233-7. [Crossref] [PubMed]
- Di Stefano DR, de Baere T, Denys A, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology 2005;234:625-30. [Crossref] [PubMed]
- van den Esschert JW, van Lienden KP, Alles LK, et al. Liver regeneration after portal vein embolization using absorbable and permanent embolization materials in a rabbit model. Ann Surg 2012;255:311-8. [Crossref] [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Wajswol E, Jazmati T, Contractor S, et al. Portal vein embolization utilizing N-butyl cyanoacrylate for contralateral lobe hypertrophy prior to liver resection: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2018;41:1302-12. [Crossref] [PubMed]
- Guiu B, Bize P, Gunthern D, et al. Portal vein embolization before right hepatectomy: improved results using n-butyl-cyanoacrylate compared to microparticles plus coils. Cardiovasc Intervent Radiol 2013;36:1306-12. [Crossref] [PubMed]
- Saad WE, Madoff DC. Percutaneous portal vein access and transhepatic tract hemostasis. Semin Intervent Radiol 2012;29:71-80. [Crossref] [PubMed]
- Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49-57. [Crossref] [PubMed]
- Isfordink CJ, Samim M, Braat M, et al. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: a systematic review and meta-analysis. Surg Oncol 2017;26:257-67. [Crossref] [PubMed]
- Nagino M, Nimura Y, Kamiya J, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology 1995;21:434-9. [PubMed]
- Shimamura T, Nakajima Y, Une Y, et al. Efficacy and safety of preoperative percutaneous transhepatic portal embolization with absolute ethanol: a clinical study. Surgery 1997;121:135-41. [Crossref] [PubMed]
- Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201-9. [Crossref] [PubMed]
- Pamecha V, Levene A, Grillo F, et al. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer 2009;100:617-22. [Crossref] [PubMed]
- Hoekstra LT, van Lienden KP, Doets A, et al. Tumor progression after preoperative portal vein embolization. Ann Surg 2012;256:812-7; discussion 817-8. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg 2018;42:806-15. [Crossref] [PubMed]
- Sandström P, Røsok BI, Sparrelid E, et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian multicenter randomized controlled trial (LIGRO trial). Ann Surg 2018;267:833-40. [Crossref] [PubMed]
- Matsuo K, Murakami T, Kawaguchi D, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016;159:1289-98. [Crossref] [PubMed]
- Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg 2015;262:780-5; discussion 785-6. [Crossref] [PubMed]
- Hwang S, Lee SG, Ko GY, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg 2009;249:608-16. [Crossref] [PubMed]
- Guiu B, Chevallier P, Denys A, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol 2016;26:4259-67. [Crossref] [PubMed]
- Hocquelet A, Sotiriadis C, Duran R, et al. Preoperative portal vein embolization alone with biliary drainage compared to a combination of simultaneous portal vein, right hepatic vein embolization and biliary drainage in klatskin tumor. Cardiovasc Intervent Radiol 2018;41:1885-91. [Crossref] [PubMed]
- Guiu B, Quenet F, Escal L, et al. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol 2017;27:3343-52. [Crossref] [PubMed]
- Jakobs TF, Saleem S, Atassi B, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90yttrium microspheres. Dig Dis Sci 2008;53:2556-63. [Crossref] [PubMed]
- Gulec SA, Pennington K, Hall M, et al. Preoperative Y-90 microsphere selective internal radiation treatment for tumor downsizing and future liver remnant recruitment: a novel approach to improving the safety of major hepatic resections. World J Surg Oncol 2009;7:6. [Crossref] [PubMed]
- Malhotra A, Liu DM, Talenfeld AD. Radiation segmentectomy and radiation lobectomy: a practical review of techniques. Tech Vasc Interv Radiol 2019;22:49-57. [Crossref] [PubMed]
- Vouche M, Lewandowski RJ, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol 2013;59:1029-36. [Crossref] [PubMed]
- Teo JY, Allen JC Jr, Ng DC, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford) 2015. [Epub ahead of print].
- Fernández-Ros N, Silva N, Bilbao JI, et al. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB (Oxford) 2014;16:243-9. [Crossref] [PubMed]
- Garlipp B, de Baere T, Damm R, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology 2014;59:1864-73. [Crossref] [PubMed]


