Challenges and insights of early oncology drug development in the Asia-Pacific region: introduction of phase I oncology clinical trial center and experience sharing for early clinical trials in Seoul National University Hospital, Korea
Clinical trial in Korea
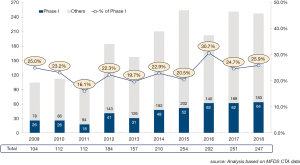
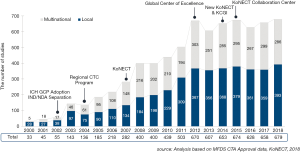
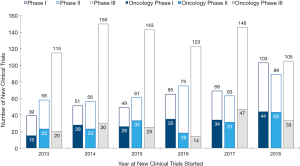
Korea has become the hub of clinical trials in the world. Korea Good Clinical Practice (KGCP) was legislated in 1995, with an amendment in 2001 to adopt International Council of Harmonization (ICH)-Good Clinical Practive (GCP). When choosing a site for clinical trials, Korea was not the first choice for many global pharmaceutical companies and Contract Research Organizations (CROs) in early 2000s. In the early 2000s, the annual number of clinical trials approved by the Ministry of Food and Drug Safety (MFDS) [a former Korea Food and Drug Administration (KFDA) which is the Korean regulatory agency in charge of drug marketing approval] was less than 50. Following the introduction of a new Clinical Trial Authorization (CTA) process in 2002, the number of multinational trials began to increase rapidly. The number accelerated once more in 2004, when the Korean government began funding for programs promoting regional clinical trials centers. In 2008, the total number of clinical trials approved by KFDA reached over 400 (Figures 1,2,3). The accelerating growth in clinical trial activity over the past decade initially centered on late-phase clinical development. However, an emerging new trend for early phase research has recently been driven by a surge in new drug development by domestic companies, as well as notable increases in Phase I oncology trials by multinational companies. Following the foundation of the Korea National Enterprise for Clinical Trials (KoNECT), which is a government-funded organization that promotes clinical trials in Korea, the number of clinical trials increased to 679 per year by 2018. Seoul National University Hospital Clinical Trials Center (SNUH CTC) is the leading clinical research facility in the country. In 2018, total number of clinical trials received authorization by MFDS in SNUH was 297. Among them, 120 clinical trials were newly started in oncology field (solid tumor) including 44 phase I oncology trials (Figure 4).
SNUH CTC and Seoul National University Bundang Hospital Clinical Trials Center (SNUBH CTC)
SNUH was established as government hospital Jejungwon (“house of universal helpfulness”) in 1885, which is one of the leading hospitals in Korea. SNUH is composed of the Main Hospital, the Children's Hospital, the Cancer Hospital, the Dental Hospital, and three satellite hospitals including the Bundang Hospital, the Boramae Hospital, and the Gangnam Healthcare Center. Main hospital has 1,800 in-patient hospital beds, over 530 faculty members, and 5,800 medical staff. They are fully devoted to the care and covers approximately 2,000 inpatients and 10,000 outpatients every day. As of 2017, 87,000 and 155,000 patients visited SNUH outpatient clinic and admitted for inpatient care, respectively. The main hospital is located in the capital city of Korea, Seoul, which makes SNUH even more attractive to global pharmaceutical companies for conducting clinical trials. The city is known for high population density, diverse ethnicity, and convenient public transportation system. These advantage makes subject recruitment easier in SNUH. In addition, Korea has a high literacy rate so there is no problem on patients following the protocols of the trials.
SNUH CTC was established in 1997 with the vision of becoming the world’s leading early clinical trials center (http://en.ctc.snuh.org/). SNUH CTC Scheme of Organization was shown in Figure 5. It is the first clinical trial center opened in Korea and has the best and the most experienced clinical research facility in the country. In December 2004, SNUH CTC was designated as the first regional clinical center by the Korean Ministry of Health and Welfare. Under the leadership of Professor YJ Bang, the past director of SNUH CTC since 2009, who has also served as the President of Biomedical Research Institute (BRI), and current director of SNUH CTC Professor IJ Jang, SNUH CTC began to move towards the new goal to become one of the world's leading early clinical trials centers. In November 2012, SNUH CTC formed a consortium with the SNUBH CTC (http://www.snubhctc.org/eng/main) and Chonbuk National University Hospital Clinical Trials Center. The consortium was granted a research fund of 20 million US dollars for 5 years by a Global Center of Excellence by the Korean Ministry of Health and Welfare. This money, along with a 1:1 matching fund raised by the consortium, upgraded and globalized the infrastructure and management systems. Clinical Trials Center at Seoul National University Bundang Hospital (SNUBH CTC) was designated as agency for pharmaceutical clinical trials by the Ministry of Food and Drug (MFDS) in 2003. SNUBH CTC was selected as the “Global Center of Excellence in Geriatric Early Clinical Trials (GREATS)” by the Ministry of Health and Welfare in 2012 and took another step forward to perform as an internationally competitive institute.
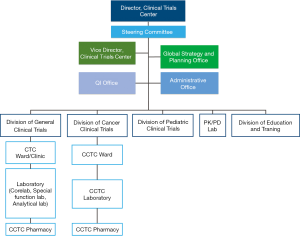
GREATS was focused in early phase clinical trials and created a clinical trials infrastructure according to global standards. SNUH CTC built and further expanded close relationships with many domestic and overseas pharmaceutical companies, CROs, and academic institutes. GREATS under SNUBH CTC aim to create new business model enabling to place geriatric early clinical trials from global and local sponsors. GREATS has established the Global Strategy and Planning (GSP), which is a business unit responsible for promoting SNUH CTC to sponsors, mostly global biopharmaceutical companies and CROs, and for coordinating pre-study activities between the sponsor and the investigator. GSP can assist a sponsor whenever they want a right principal investigator for clinical trials from the sponsor's perspective. GSP prepares meetings in a timely and appropriate place for legitimate people. Moreover, a subject recruitment team under GSP facilitates finding an eligible study participant in a short period of time.
Phase I clinical trial unit consists of 79 beds in one general research ward and three specialized research ward, injection rooms, 12 rooms for out-patient clinic, soundproof room, temperature humidity-controlled room, screening room, clinical trial pharmacy, and a core lab. We have ability to perform the First-in-Human (FIH) study, pharmacokinetic (PK) and pharmacodynamic (PD) bridging, bioequivalence and bioavailability studies, multi-ethnic PK/PD study, food-drug interaction study, drug-drug interaction, clinical trial for special population, and bioanalysis & biomarker studies. SNUH CTC established the first and only oncology clinical trials center in Korea in 2011. Ten fully monitored beds and 20 couches are available 24 hours a day only for oncology clinical trial. In 2012, a separate pediatric clinical trials unit was also opened for dedicated pediatric trial including phase I pediatric trial. Since the initiation of conducting phase I studies, patient enrollment has gradually increased from 1,626 patients in 2013 to 2,527 patients in 2018, a total of 11,981 patients were enrolled in phase I studies. The annual number of conducted clinical trials is on par with global hospitals, and SNUH CTC plays a pivotal role in the clinical development of new drugs in Korea. Our mission is “Through state-of-the-art clinical trials, we create knowledge that changes the standard of care to improve human health around the globe”.
SNUH Clinical Trial Center medical oncology statistics
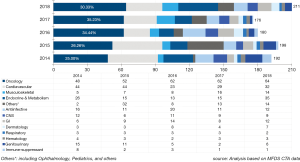
Medical oncology team is the leading group of the SNUH CTC. The number of newly initiated clinical trials in SNUH is shown in Figure 4. During 2013 to 2018, SNUH CTC conducted 1,560 clinical trials (376 phase I, 402 phase II, and 782 phase III clinical trials), which makes our institution one of the top recruiting sites worldwide. While the number of phase III trials remain similar, the number of phase I and phase II studies are increasing (Figure 4). During same period, 2013 to 2018, oncology team conducted or participated in 525 clinical trials (33.7% of SNUH trials) (182 phase I, 171 phase II, and 169 phase III), which makes our oncology team one of the top recruiting sites worldwide. Among 120 oncology study initiated in 2018, 44 (36.7%) studies were phase I trial. Oncology phase I clinical tails contributed 42.7% (44/103) of phase I newly started clinical trials in SNUH CTC (Figure 4).
KoNECT
Korea is rapidly becoming recognized as a global leader in clinical research, standing as a top 10 location worldwide in terms of the number of studies conducted annually. International sponsors cite numerous advantages within the country that consistently ensure speed and quality for the conduct of their clinical trials. In addition to a high population density, the Korean healthcare system provides universal coverage and is characterized by clusters of high-capacity hospitals concentrated in large cities like Seoul. Optimized recruitment practices combined with the large volumes of daily patient traffic in these institutions allow for rapid recruitment and accelerated study start-up times.
Korea has one of the world’s most efficient clinical trial approvals (30 working days), and its medical institutions and practices meet the highest international standards. Korea’s research-intensive ecosystem of collaborative innovation along with the rapid growth of Research and Development (R&D) expenditure has increased the diversity of global talent found within the country, complemented by some of the most advanced IT infrastructure and training. With a highly educated and motivated workforce, Korean sites have proven track records in quality and efficiency in clinical trials. Another significant advantage is the extensive support provided by the Korean government for the pharmaceutical R&D industry and clinical trials, including the establishment of the Korea National Enterprise for Clinical Trials (KoNECT) (https://www.koreaclinicaltrials.org) and designation and support of Research-driven Hospitals, Regional Clinical Trial Centers and Global Clinical trial centers of Excellence. The data generated by Korean sites are accepted for Japanese registration, which allows for a flexible regulatory strategy for Asian development, facilitating regional research networks and ultimately reducing the drug lag in this region.
Streamlined regulatory process & faster study start up
CTA process was introduced by the Korean government in 2002, which has continuously sought to improve the nation’s regulatory environment through careful analysis of the industry and global trends. After a submission, the MFDS issues either a response for trial approval or a request for supplementary information to the applicant within 30 working days. The CTA procedure in Korea allows for applications to be submitted in parallel to institutional review boards (IRBs)/Ethics Committees (ECs) and the MFDS. This has greatly reduced the time taken until clinical trial approval. In general, the entire process from submission of application until clinical trial approval takes between 4 to 8 weeks. Most sites regularly meet for IRB reviews at least monthly and hold expedited review meetings as necessary.
The Study Start Up (SSU) process is frequently noted as one of the most cumbersome and costly bottlenecks present in clinical research. Major milestones in the process include site selection and initiation, contract and budget execution, and other activities occurring prior to enrollment of the first patient. Korea has shown more competitive SSU timelines than other countries.
Strength and achievements of SNUH and SNUBH CTCs
Working together as a team
One of the important strengths of our team is that our team is working together in one team for all types of solid tumors and this collaborative work was very important to recruit patients for multi-cohort in early phase study including basket trial, umbrella trial and platform trials. In addition, SNUH and SNUBH also worked together for these studies. One of the representative examples was the pembrolizumab multi-cohort phase Ib study KENOTE-028 (1-8). Merck Sharp and Dohme (MSD), a pharmaceutical company, have a strict vendor selection and lengthy qualification process. In 2009, MSD decided to initiate their two first-in-human clinical trials in SNUH CTC. In fact, SNUH CTC is the first qualified phase I vendor for Merck in Korea. KEYNOTE-028 (ClinicalTrials.gov identifier: NCT02054806) is a nonrandomized, phase Ib trial that enrolled 475 patients with programmed death receptor ligang-1 (PD-L1)-positive advanced solid tumors. Patients were treated with pembrolizumab for 2 years or until confirmed disease progression or unacceptable toxicity occurred. During KENOTE-028 study, our oncology team contributed significantly to gastric cancer, non-small cell lung cancer, small cell lung cancer (1), breast cancer (2), colorectal cancer (3), endometrial cancer (4), pancreas and biliary cancer, nasopharyngeal cancer (5), papillary or follicular thyroid cancer (6), advanced salivary gland carcinoma (7), and others. Results of this phase Ib proof-of-concept study suggest that pembrolizumab has a manageable safety profile and demonstrate evidence of antitumor activity in many PDL-1 positive advanced cancers. Furthermore, our team have been working intimately with pathologist for translational research and have great enthusiasm for obtaining tissue from patients for accurate diagnosis of molecular subtypes. We contribute substantially for the KEYNOTE 028 study, where higher response rates and longer progression-free survival (PFS) were demonstrated in tumors with higher T-cell-inflamed gene expression profiling (GEP), PD-L1 expression, and/or tumor mutational burden (TMB) (8). Correlations between TMB, GEP, and PD-L1 were low. Response patterns were different according to tissue signature and tumors with high levels of both TMB and inflammatory markers (GEP or PD-L1) had a highest likelihood of response. As a result, 6 investigators from our institution contributed as a co-author for each representative cohort.
First in human (FIH) phase I trial
A key step in translational science is the move from preclinical studies to initial human clinical trial. Our team had an experience to develop poziotinib from in vitro to FIH and subsequent phase II study. SNUH and SNUBH worked together for these FIH studies. Researchers’ ability to predict antitumor effect of new agents in human is limited in FIH trial and there are significant uncertainties present. Participants in this form of research might face risks and can experience serious, even lethal adverse events. Therefore, for FIH trial, we need experienced oncologists who can manage adverse events appropriately and need 24-hour full monitoring system including dedicated nursing staff for oncology clinical trials. Ten fully monitored beds are available 24 hours a day only for oncology clinical trial in SNUH CTC oncology clinical trials center which was established in 2011 for this purpose.
Pan-human epidermal growth factor receptor (pan-HER) tyrosine kinase inhibitor HM781-36B (poziotinib) and HM61713 for T790Mmt NSCLC developed by Korean pharmaceutical companies Hanmi corporation performed their preclinical study using cell lines and FIH study with our team. In vitro, poziotinib has shown potent activity against wild type of epidermal growth factor receptor (EGFR) family kinases including EGFR, HER2, and HER4 and EGFR-mutant cells (9,10). Two phase I studies were conducted to determine the maximum tolerated dose (MTD), PK, safety, and antitumor activity against advanced solid tumors (11,12). The MTDs were determined as 24 mg/day in the intermittent dosing schedule and 18 mg/day in the continuous dosing schedule. Poziotinib showed an encouraging activity against EGFR-mutant and HER2-amplified cancers. Subsequently, phase II study for HER2-amplified breast cancer as monotherapy and for HER2-amplified stomach cancer as second line therapy combined with paclitaxel and trastuzumab were performed (12,13). Hanmi corporation made a contract with Spectrum in the United State of America (USA) and collaborate with US investigator and identified poziotinib as a potent, clinically active inhibitor of EGFR exon 20 mutations and illuminate the molecular features of TKIs that may circumvent steric changes induced by these mutations (14). SNUH and SNUBH oncology team are currently working together for novel Poly ADP-ribose polymerase (PARP) inhibitor phase I trial, novel FIH EGFR targeted antibody with chemotherapy combination trial (ClinicalTrials.gov identifier: NCT02352571) (15) and albumin-bound paclitaxel clinical trials (ClinicalTrials.gov identifier: NCT02979392) with Korean bio-pharmaceutical companies.
Macrogenics based on US California area visited our institution for FIH of MGAH22 (Margetuximab) in 2009. Margetuximab is an anti-HER2 antibody that binds to both the lower and higher affinity forms of CD16A, which is an Fc-receptor important for antibody dependent cell-mediated cytotoxicity (ADCC) against tumor cells. Among 66 patients, 46 patients from our institute received margetuximab. The MTD was not reached and treatment was well-tolerated, with mostly grade 1 and 2 toxicities consisting of constitutional symptoms such as pyrexia, nausea, anemia, diarrhea, and fatigue. Among 60 response-evaluable patients, confirmed partial responses were observed in 7 (12%) and stable disease in 30 (50%). Twenty-six (70%) of these patients were previously treated with HER2-targeted therapy. In breast cancer patients, over half (18/23, 78%) of response-evaluable patients had tumor reductions. Ex vivo analyses of peripheral blood mononuclear cell samples confirmed that margetuximab may have enhanced ADCC compared with trastuzumab (16). Early involvement in early phase clinical trial facilitate to know the efficacy and safety of the specific agents in many tumor types and this is one of the representative examples that we could work with pharmaceutical company to develop further phase II/III clinical trial which subsequently change the standard of care. From this FIH results, randomized phase III clinical trial, SOPHIA reported statistically significant PFS prolongation compared with trastuzumab (17) and phase II clinical trial using pembrolizumab and margetuximab for HER2 positive stomach cancer who failed 1st line trastuzumab with chemotherapy is currently ongoing (ClinicalTrials.gov identifier: NCT02689284). We currently have collaboration not only with Korean pharmaceutical company but also with global pharmaceutical company and CRO for early clinical trial development.
Target identification through translational research and proof-of-concept
Identification of predictive biomarker for the targeted agent is a key step for the success of a new drug. Around 30–40% of Asian NSCLC have EGFR activation mutations. Our translational research team found EGFR activating sensitive mutation using early access program for gefitinib and subsequently studies have shown that 1st line EGFR tyrosine kinase inhibitor (TKI), erlotinib and gefitinib, is effective in NSCLC with EGFR activation mutation (18-20). However, most cancer eventually acquire resistance and one of the main mechanisms is through obtaining EGFR T790M mutation (21) As the incidence of EGFR mutation is higher in Asian compared to Western, SNUH CTC had an important role in developing new drugs. Our institute had a pivotal role in phase I study of HM61713, which is a 3rd generation EGFR TKI effective in EGFR T790M mutation (22). In addition, another phase I study of a potent EGFR TKI, AZD3759 was performed in SNUH CTC (23). The BLOOM trial was an open-label, multicenter, phase I study which evaluated the efficacy of AZD3759 in NSCLC with CNS metastases who had either never received a tyrosine kinase inhibitor or who had been pretreated with a tyrosine kinase inhibitor. This phase I study assessed the PKs [in both plasma and cerebrospinal fluid (CSF)] to prove equivalent drug concentrations in plasma and CSF, attributable to effective penetration of the blood–brain barrier. While the study required frequent plasma and CSF collection, the study was well performed in our institute. These are the examples to show the collaborative work with translational research, phase I PK/PD clinical pharmacology team with lung cancer trial group.
Our breast cancer team participated in FIH trial for a new oral selective estrogen receptor degrader (SERD) AZD9496, which is an oral nonsteroidal, small-molecule inhibitor of estrogen receptor alpha (ERα) and a potent antagonist and degrader of ERα. In this trial, only 5 centers (2 from UK, 2 from USA and 1 from Korea) participated to determine the safety and tolerability of ascending doses of oral AZD9496 in ER+/HER2− advanced breast cancer. This phase I study characterized its single-dose and multiple-dose PK profile and made preliminary assessment of antitumor activity (24). AZD9496 showing evidence of prolonged disease stabilization in heavily pretreated patients with ER+/HER2- advanced breast cancer. ER loss and acquired activating mutations in the ligand-binding domain of the ER gene (ESR1LBDm+) are common resistance mechanism for endocrine therapy. ESR1 mutation mediated resistance might be overcome by SERD. Early changes in circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) were also explored as potential noninvasive tools, with paired tumor biopsies, to assess PDs and early efficacy. We sent blood for CTC and ctDNA with paired fresh frozen tissue biopsies (baseline, on-treatment, and after progression) and paraffin-embedded tissue to UK for further analysis. Elevated CTC at baseline was a strong prognostic factor. Early on-treatment changes were observed in CTC−ER+ and ESR1LBDm+ ctDNA. Persistently elevated CTC and/or ESR1LBDm+ ctDNA at C1D15 was a worse predictive factor for PFS (25). Even though Korea is geographically distant from Western, there was no difficulty in conducting collaborative work between SNUH CTC oncology team and global oncology team. Incheon International Airport serves as a hub for international civilian air transportation and good air traffic in Korea is another advantage of SNUH CTC.
Target enrichment
Target enrichment using tests with faster turn-around time and good correlation with validated selection test is very helpful to recruit appropriate patients faster and select right patients for right drug.
A phase I clinical trial evaluating the efficacy of cMET/anaplastic lymphoma kinase (ALK) inhibitor, crizotinib, was performed in SNUH CTC. The ALK inhibitor crizotinib originally designed as cMET inhibitor and our institution participated in dose escalation phase and for cMET amplified gastric or gastro-esophageal cancer expansion cohort. During conduction of the study, Japanese translational research team found that crizotinib was effective for NSCLC with ALK fusion. During ALK inhibitor phase I enrollment, fluorescence in situ hybridization (FISH) was used to detect ALK gene rearrangement. However, FISH was not a practical method for screening ALK-positive patients in a large population due to its cost and long turn-around time and difficulty in interpretation. We had collaborative work with our pathology team to develop ALK immunohistochemistry (IHC) for screening, which allow us to enrich NSCLC with ALK fusion (4–5% of NSCLC) faster compared with using ALK break-apart FISH directly in other Western institution (26). During this trial, we collaborated with Asian centers and patients from Japan and China were enrolled in an expansion cohort. The results were presented in the plenary session of American Society of Clinical Oncology (ASCO) Annual meeting in 2010 by Professor YJ Bang (27). Based on the result from the phase I and II study, crizotinib received an accelerated approval from the US Food and Drug Administration (FDA) in 2011 and also approved in Korea. SNUH CTC also had a major role in a following phase III study of crizotinib (PROFILE 1007 and PROFILE 1014) (28,29). Moreover, next generation ALK inhibitors, ceritinib and brigatinib, was precisely studied in SNUH CTC. The lead author of the ASCEND-1 trial, which was a multicenter, open-label, phase I trial evaluating the efficacy of ceritinib in NSCLC with ALK Rearrangement, was professor Dong-Wan Kim of SNUH (22). Furthermore, we developed our targeted sequencing platform FiRST Lung Cancer Panel and SNUH FiRST cancer panel as a specific molecular screening tools which facilitate early phase clinical trial enrollment. SNU cancer research institute (http://cri.snu.ac.kr) has been collaborating with AstraZeneca former KuDOS pharmaceutical for antitumor effect of PARP inhibitor KU-0059436 (AZD2281; olaparib) and ATR inhibitor (AZD6783) with their action mechanism. When we started in vitro work in 2006, only limited knowledge existed that PARP inhibitor showed significant antitumor effect in germline BRCA-mutant breast and ovarian cancer cell lines. The ataxia-telangiectasia mutated (ATM) protein is one of several DNA repair proteins that are suggested to sensitize tumor cells to the PARP inhibitor when deficient. We found that low ATM (ataxia telangiectasia mutated) expression and RAD51C epigenetic silencing were a predictive factor for olaparib and PARP inhibitor sensitivity in gastric cancer cells (30,31). The prevalence of ATM negativity by immunohistochemistry (IHC) was 13.1% in gastric tissue biopsies (32), Identification of ATM as a potential predictive biomarker for PARP inhibitor, validated ATM IHC and growth inhibition by cisplatin correlated with olaparib sensitivity in gastric cancer cell lines led us to design an exploratory randomized phase II trial in gastric cancer. In this phase II, double-blind study (Study 39; NCT01063517), patients were randomly assigned to olaparib plus paclitaxel (olaparib/paclitaxel) or placebo plus paclitaxel (placebo/paclitaxel), followed by maintenance monotherapy with olaparib or placebo. The study population was enriched with 50% for patients having low or undetectable ATM levels (ATM-low). Compared to placebo/paclitaxel, olaparib/paclitaxel significantly prolonged overall survival (OS) in both the overall population and the ATM-low population (33). Unfortunately, the randomized phase III GOLD study did not meet its primary objective of showing a significant improvement in OS with olaparib/paclitaxel compared to placebo/paclitaxel in the overall (P=0.026) or ATM-negative population of Asian patients. This may due to shorter follow up for OS, since this trial was designed to have dual primary endpoint with PFS and OS at the same time (setting significant P value as 0.025), and lower ATM-low population than expected (34).
Proof-of-concept & pivotal phase III trial
Our team found that trastuzumab was effective to HER2 amplified gastric cancer cell lines in our laboratory using SNU-216 HER2 amplified cell line which was established in Korean Cell Line Bank (KCLB) from SNUH stomach cancer patients. Trastuzumab-mediated G1 arrest accompanied with increased expression of p27 (KIP1) and decreased cyclins. Phosphorylation of HER2 and downstream molecules (STAT3, AKT, and ERK), was also inhibited by trastuzumab. Treatment of SNU-216 cells with trastuzumab plus cisplatin resulted in a synergistic inhibitory effect, in addition, treatment of SNU-216 cells with trastuzumab plus 5-FU, or trastuzumab plus oxaliplatin produced an additive effect (35). Based on our preclinical in vitro finding and findings from HER2 positive breast cancer where HER2 positive breast cancer can get survival benefit with adding trastuzumab to standard of care chemotherapy (36), the ToGA (Trastuzumab for Gastric Cancer) study was designed as an open-label, international, phase III, randomised controlled trial. ToGA trial was conducted in 122 centers in 24 countries (37). Patients with HER2 positive gastric or gastro-oesophageal junction cancer were included. HER2+ was defined as overexpression of HER2 protein by immunohistochemistry or gene amplification by FISH. Trastuzumab plus chemotherapy had superior OS compared to chemotherapy alone (13.8 vs. 11.1months; hazard ratio 0.74; P=0.0046). Based on ToGA trial, trastuzumab plus chemotherapy is a new standard option for HER2-positve advanced gastric or gastro-oesophageal junction cancer. It was an important practice changing clinical trial led by Asian investigator and had great global impact.
Future prospects
SNUH and SNUBH oncology team is one of the top phase I clinical trials center in the world. More and more local and global biopharmaceutical companies and CROs are knocking the door of SNUH/SNUBH CTC to streamline and advance their clinical development projects. Collectively, these will provide our oncologists a chance to become an indispensable part of global drug development. Moreover, these experiences will encourage translational research in our institute to identify new target, develop methods for target enrichment, and to discover and overcome resistance. SNUH/SNUBH CTC will continue top-notch clinical trials research for the global drug development
Acknowledgments
The authors thank the Office of Policy Development and Research at Korea National Enterprise for Clinical Trials for statistical analyses and Youlee Baek for her assistance to refine figures.
Funding: This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), and funded by the Ministry of Health & Welfare, Korea (grant number: HI14C1277).
Footnote
Conflicts of Interest: SA Im received grants from AstraZeneca and Pfizer and have been working as consultant or advisory role for AstraZeneca, Amgen, Eisai, Hanmi, Ildong pharmaceutical, MediPactor, Novartis, Pfizer, Roche. JH Kim has received research grant from Ono Pharma Korea Co., Ltd. DW Kim reports travel expenses for advisory board participation from Novartis. IJ Jang has received grants from Hanmi, Sanofi-Aventis, Daewoong and CJ Healthcare. YJ Bang has received grants from AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Boston Biomedical, BMS, CKD, Curis, Eli Lilly, FivePrime, Genentech/Roche, Green Cross, GSK, Hanmi, MacroGenics, Merck Serono, MSD, Novartis, Pfizer, Ono, Otsuka, Taiho, and Takeda, and had a consulting/advisory role for ADC Therapeutics, AstraZeneca, Bayer, BMS, Eli Lilly, FivePrime, Genentech, Green Cross, Merck Serono, Merrimack, MSD, Novartis, Ono, Pfizer, Roche, Samyang Biopharm, and Taiho. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;35:3823-9. [Crossref] [PubMed]
- Rugo HS, Delord JP, Im SA, et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. Clin Cancer Res 2018;24:2804-11. [Crossref] [PubMed]
- O'Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One 2017;12:e0189848. [Crossref] [PubMed]
- Ott PA, Bang YJ, Berton-Rigaud D, et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J Clin Oncol 2017;35:2535-41. [Crossref] [PubMed]
- Hsu C, Lee SH, Ejadi S, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol 2017;35:4050-6. [Crossref] [PubMed]
- Mehnert JM, Varga A, Brose MS, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 2019;19:196. [Crossref] [PubMed]
- Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am J Clin Oncol 2018. [PubMed]
- Ott PA, Bang YJ, Piha-Paul SA, et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol 2019;37:318-27. [Crossref] [PubMed]
- Kim HJ, Kim HP, Yoon YK, et al. Antitumor activity of HM781-36B, a pan-HER tyrosine kinase inhibitor, in HER2-amplified breast cancer cells. Anticancer Drugs 2012;23:288-97. [Crossref] [PubMed]
- Cha MY, Lee KO, Kim M, et al. Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int J Cancer 2012;130:2445-54. [Crossref] [PubMed]
- Kim TM, Lee KW, Oh DY, et al. Phase 1 Studies of Poziotinib, an Irreversible Pan-HER Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Cancer Res Treat 2018;50:835-42. [Crossref] [PubMed]
- Kim TY, Han HS, Lee KW, et al. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2-positive advanced gastric cancer. Gastric Cancer 2019. [Crossref] [PubMed]
- Park YH, Lee KH, Sohn JH, et al. A phase II trial of the pan-HER inhibitor poziotinib, in patients with HER2-positive metastatic breast cancer who had received at least two prior HER2-directed regimens: results of the NOV120101-203 trial. Int J Cancer 2018;143:3240-7. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Oh DY, Lee KW, Han SW, et al. A First-in-Human Phase I Study of GC1118, a Novel Anti-Epidermal Growth Factor Receptor Antibody, in Patients with Advanced Solid Tumors. Oncologist 2019. [Crossref] [PubMed]
- Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol 2017;28:855-61. [PubMed]
- Rugo HS. SOPHIA primary analysis: A phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx). Journal of Clinical Oncology 2019;37:1000. [Crossref]
- Han SW, Kim TY, Hwang PG, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2005;23:2493-501. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Kim HP, Han SW, Kim SH, et al. Combined lapatinib and cetuximab enhance cytotoxicity against gefitinib-resistant lung cancer cells. Mol Cancer Ther 2008;7:607-15. [Crossref] [PubMed]
- Kim D-W, Lee DH, Kang JH, et al. Clinical activity and safety of HM61713, an EGFR-mutant selective inhibitor, in advanced non-small cell lung cancer (NSCLC) patients (pts) with EGFR mutations who had received EGFR tyrosine kinase inhibitors (TKIs). Journal of Clinical Oncology 2014;32:8011. [Crossref]
- Ahn MJ, Kim DW, Cho BC, et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med 2017;5:891-902. [Crossref] [PubMed]
- Hamilton EP, Patel MR, Armstrong AC, et al. A First-in-Human Study of the New Oral Selective Estrogen Receptor Degrader AZD9496 for ER(+)/HER2(-) Advanced Breast Cancer. Clin Cancer Res 2018;24:3510-8. [Crossref] [PubMed]
- Paoletti C, Schiavon G, Dolce EM, et al. Circulating Biomarkers and Resistance to Endocrine Therapy in Metastatic Breast Cancers: Correlative Results from AZD9496 Oral SERD Phase I Trial. Clin Cancer Res 2018;24:5860-72. [Crossref] [PubMed]
- Park HS, Lee JK, Kim DW, et al. Immunohistochemical screening for anaplastic lymphoma kinase (ALK) rearrangement in advanced non-small cell lung cancer patients. Lung Cancer 2012;77:288-92. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Min A, Im SA, Yoon YK, et al. RAD51C-deficient cancer cells are highly sensitive to the PARP inhibitor olaparib. Mol Cancer Ther 2013;12:865-77. [Crossref] [PubMed]
- Min A, Im SA, Jang H, et al. AZD6738, A Novel Oral Inhibitor of ATR, Induces Synthetic Lethality with ATM Deficiency in Gastric Cancer Cells. Mol Cancer Ther 2017;16:566-77. [Crossref] [PubMed]
- Kim HS, Kim MA, Hodgson D, et al. Concordance of ATM (ataxia telangiectasia mutated) immunohistochemistry between biopsy or metastatic tumor samples and primary tumors in gastric cancer patients. Pathobiology 2013;80:127-37. [Crossref] [PubMed]
- Bang YJ, Im SA, Lee KW, et al. Randomized, Double-Blind Phase II Trial With Prospective Classification by ATM Protein Level to Evaluate the Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients With Recurrent or Metastatic Gastric Cancer. J Clin Oncol 2015;33:3858-65. [Crossref] [PubMed]
- Bang YJ, Xu RH, Chin K, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1637-51. [Crossref] [PubMed]
- Kim SY, Kim HP, Kim YJ, et al. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol 2008;32:89-95. [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]