Emerging technologies in stereotactic body radiotherapy
Historical developments leading to modern stereotactic body radiation therapy (SBRT) technologies
As the precursor to SBRT, stereotactic radiosurgery (SRS) was originally pioneered by the Swedish neurosurgeon Dr. Lars Leksell in the 1950s (1). The fundamental principle of SRS is to employ a rigid frame system to establish a common coordinate system by fixating the frame onto the patient’s skull (2,3). As a result, internal patient anatomy as well as external radiation beams can be independently referenced in a “stereotactic” manner based on single unified Cartesian coordinates. Assuming negligible motion between the frame and patient’s skull, the so-called Leksell SRS coordinate system has enabled high-precision localization and navigation of the brain anatomy including complex vascular structures and functional nerves etc. (2-8).
From the start, mechanical accuracy of a SRS radiation unit has also played an essential role in positioning and aligning external radiation beams toward a given coordinate system once established (9,10). The intracranial Gamma Knife system is a successful example of such an achievement in terms of overall physical and mechanical accuracy. The system weighs over a ton and yet produces a beam-focusing accuracy of less than half a millimeter anywhere inside the brain (10,11). Later on, integrations of stereo MR/CT imaging studies have closed the loop by allowing brain lesion to be accurately mapped and targeted within the Leksell coordinate system.
Following the success of frame-based intracranial SRS, investigators at the Karolinska institution proposed to apply the same principle of intracranial SRS to extracranial sites in the early 1990s (12). A stereotactic body frame (SBF) was built in attempt to fixate the patient’s torso and establish a common coordinate system for the whole body. The SBF consists of a rigid outer shell for the establishment of a reference coordinates for radiation beam alignment. It also consists of an inner soft shell (vacuum bags, vacuum pillows etc.) so that the patient can be positioned more comfortably while fully fixated in reference to the outer shell of the body frame.
Since the SBF can be easily and accurately positioned in reference to the beam axes of a modern linear accelerator system, setup uncertainties such as when trying to align the beam axes with regard to the patient’s skin marks as normally practiced in the conventional treatments have greatly reduced. However, investigators quickly noted the issues of internal organ motions such as breathing motion, heart beats, bowel movements etc. To manage such motions, passive means of applying abdominal compression devices to restrict diaphragmatic motions and/or heavy strapping have become standard accessories to the SBF. It has been reported that thousands of patients have been treated at the Karolinska institution since early 1990s with the SBF system (13).
Beginning in the early 2000s, investigators in the US started experimenting with alternative means of localizing internal targets and managing patient motions versus rigid body frame systems. One of the major developments was on-line imaging guidance based on utilization of kV X-ray systems either installed inside the treatment room or via an on-board-imager (OBI) device mounted on the C-arm of a standard linear accelerators (14-17). By aligning the imaging studies acquired prior to the treatment with the imaging studies acquired during the standard treatment planning process, patient setup coordinates are obtained by the image fusion and registration algorithms.
In essence, the online imaging system has served as a “virtual” body frame system. It elegantly combined the hardware/device alignment and patient setup into one single procedure that further reduced composite setup uncertainties for SBRT treatments. This technology has led to explosive applications of hypofractionated SBRT for multiple disease sites that include spine, lung, prostate and liver etc.
In this review article, we specifically focus on integrated image-guided systems relevant to SBRT applications. Emerging and future trends in SBRT technologies will be discussed in three categories: (I) C-arm S-band linear accelerator system; (II) robotic X-band CyberKnife® system; (III) specialized devices such as the image-guided Gamma Knife IconTM system and the upcoming integrated MRI-linear accelerator system.
State-of-the-Art SBRT Systems and the emerging developments
By definition, a stereotactic treatment needs to achieve stereotactic precision such that treatment uncertainties are substantially less than those of the conventional fractionated treatments. With tools such as near real-time stereotactic imaging (i.e., imaging systems that are capable of performing stereotactic coordinate mapping similar to the traditional stereotactic frame system), the fine line between the classic intracranial SRS technology and extracranial SBRT technology has started to blur. At present, on a modern SBRT delivery system, intracranial SRS can be also performed (2,3,18,19). The dedicated intracranial SRS Gamma Knife unit has also modernized with the introduction of an integrated kV cone-beam computed tomography (CBCT) system, that will also allow for treatments of head and neck and C-spine lesions and multi-fraction delivery (7,8,20-22). We will discuss these developments in separate sections to highlight common and unique features of these developments.
C-Arm S-band linear accelerator
C-arm S-band linear accelerators have dominated the field of conventional fractionated radiation therapy since 1970s. By design, the system possesses a single isocenter in space that is defined as the interception point of (I) the C-arm gantry rotational axis; (II) collimator rotation axis and (III) the couch rotation axis. Isocentric accuracy has been the hallmark parameter in determining whether a linear accelerator is satisfactory or not for performing SRS/SBRT treatments (17,23).
With the addition of kV OBI systems to the C-arm gantry, volumetric CBCT can be acquired in addition to the 2D kV planar imaging, both of which possess significantly higher contrast and resolution compared to the conventional MV portal imaging quality. Consequently, the isocenter of beam delivery system must coincide with the isocenter defined from the OBI system (Figure 1).
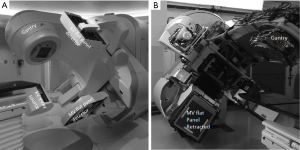
In the context of SBRT implementation, one of the major concerns has always been the integrity of the OBI spatial accuracy and imaging quality in relationship to the radiation beam isocenter. Strict quality assurance checks must therefore be implemented to ensure the isocenter of the imaging system coincide with that of the beam delivery system. Any distortion from one system to another due to mechanical factors such as irregular gantry rotations and hardware sagging etc. should be carefully calibrated and corrected (17,24).
With the introduction and developments of digitally controlled linear accelerator (DC-linac) such as that implemented in the latest Varian TrueBeam system, concerns on hardware misalignment and miscalibrations are by and large eliminated. New generation of DC-linac systems possess a multi-level feedback and sensor system that allows high definition control of the gantry speed, collimator angles, pulse rate and beam energy switches on the level of every few milliseconds. With such a level of hardware control, volumetric modulated arc therapy (VMAT) with a limited number of arcs has become an essential mode for SBRT treatments (25-29). An example of the beam arrangement for a pelvic SBRT case is illustrated in Figure 2. The VMAT technique is a significant departure from the early techniques of SBRT where a “beam bouquet” is commonly used where multiple fixed 3D conformal beams or intensity modulated beams on the order of 8–20 are commonly arranged toward a single isocenter for the purpose of minimizing the entrance/exit dose contribution from each beam thus minimizing the normal tissue dose near the target.
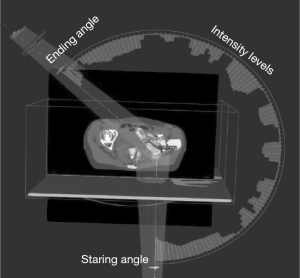
For the VMAT mode of delivery, the gantry rotates around the patient in an arc beam with the MLC modulating the field shapes at the same time. In addition, digitally controlled beam pulse rates also vary continuously to deliver different beam outputs at each gantry angle per each MLC-defined segment. One of the key developments of DC-linacs was the accurate steering of the beam in the so-called flattening-filter-free (FFF) mode. When operating in the FFF mode, the conventional flattening filter is shifted away on the target carousal leading to multiple folds increase in beam output over that of flattening-filter-attenuated beams. For example, a dose rate on the order of 10–20 Gy/min can be readily tuned for a treatment. This has significantly shortened the total beam-on time required to deliver a dose of 5 to 20 Gy per fraction as required of SBRT for either single-fractionated or hypofractionated treatments. For complex cases such as the spine SBRT treatments of 18 to 24 Gy per fraction, rapid beam-delivery not only shortens the treatment time, but also is essential in minimizing the potential for patient shifts during the treatment delivery. Stringent clinical constraints such as 1 degree rotation or 1 mm shift is usually required in order to prevent a high dose from accidentally placed onto the spinal cord leading to disastrous treatment complications (30,31).
Furthermore, the latest DC-linac system also possesses the capability of rotating the collimator and couch simultaneously resulting in the so-called “4π” technique. Since 4π steradian is the maximum solid angle in the 3D space, the term 4π symbolically denotes an exhaustive beam angle search and beam parameter optimization in the beam-delivery searchable space of a C-arm linear accelerator (32-34). It is in fact not generally feasible for a C-arm S-band linear accelerator to access all the beam angles surrounding a patient, for example, delivering a coronal arc for a supine patient setup etc. Notwithstanding, 4π beam irradiation represents a powerful emergent SBRT tool with early studies promising excellent results in terms of dosimetric sparing for several disease sites (33,34). In terms of treatment planning optimization and robustness in treatment delivery, the intrinsic freedom offered by 4π SBRT delivery has provided ample opportunities for future developments on fast computation and adaptive treatments as well as automatic hardware collision detections etc. Future and on-going clinical studies are warranted to ensure enhanced technical complexities as offered by the emerging 4π echnologies will translate into measurable clinical benefits to the patients.
Robotic X-band linear accelerator
In contrast to standard C-arm S-band linear accelerators, where a single isocenter is the reference origin of all the treatment delivery, the X-band robotic CyberKnife® system has from its inception in the mid 1990’s heralded the concept of non-isocentric FFF-beam delivery (35). Due to higher X-band microwave power able to accelerate bunched electrons, the X-ray beam generation and transport system was constructed in a significantly more compact size as compare to the conventional S-band linear accelerator. As a result, the entire X-ray generation system (Figure 3) can be mounted onto a commercial-grade robotic manipulator that moves like an arm, with full 6 degree-of-freedom similar to the robotic arm adopted for assembling cars and heavy equipment (36-38).
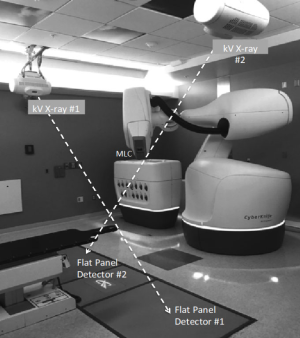
With nimble movements of the radiation beams, the “eyes” of the CyberKnife® system are a pair of stereoscopic in-room X-ray tubes and associated flat panel detectors, which enable for precise detection of target motion and tracking of the radiation beams (35). The X-ray tubes are mounted on the ceiling of the treatment room with two flat panel detectors under the floor (Figure 3). The X-ray tubes are usually synchronized to fire simultaneously to create a pair of near-real time X-ray images that are directly referenced to a library of digitally reconstructed radiographs (DDRs) that are created from the reference planning CT of the patient. Automatic imaging registration algorithms are implemented for instantaneous target detection for different disease sites. For treatment of bony targets such as spine or pelvis, distinctive landmark features are commonly used for detecting patient movements or setup shifts based on the stereoscopic X-ray imaging. For soft tissue targets such as the lung or the liver tumors, the system typically relies on metal fiducial markers implanted inside or near the target for online tracking to guide beam deliveries (37,38).
One of the distinct features of CyberKnife® SBRT is its near real time tracking and delivery capability. The kV X-rays frequently fire to track the patient motion and detect the target position, and the detected shifts are used to adjust robotic arm accordingly in near real time (39). Smart determinations of when or how frequently to fire stereoscopic X-rays are primarily influenced by the disease sites as well as patient-specific factors. For lung SBRT treatments, the real time tracking is achieved through a model based tracking mode called SynchronyTM, where a breathing model is developed before the delivery by correlating the position of the optical markers (light-emitting-diodes or LEDs) placed on the patient chest to the target position detected by the kV X-ray imaging. With an established breathing model, the robotic manipulator will move correspondingly to deliver radiation beams following the guidance of the optical markers on patient’s chest. With Synchrony TM, real time beam tracking can be realized for either regular or semi-irregular target motions on CyberKnife® (40).
Because of the flexibility in the robotic beam movements, CyberKnife® is by design a 4π. The delivery space is composed of a group of discrete predefined delivery positions (nodes, normally 100–200 nodes depend on treatment site) spherically distributed around the patient. Direct posterior beams are not allowed due to collision to the ground. The dose delivery follows step and shoot fashion. At each node, the planning system typically allows the robotic arm to randomly pick several (up to 12 beams) beam directions for optimizations. This is the reason that sometimes the patient noted that during a CyberKnife® treatment, head of the robotic nozzle seems to “nod hello” intelligently several times at each angle throughout the beam delivery.
Given the fix mounting of the in-room kV imaging detector panels, all the treatments rely on a pair of orthogonal images. For this reason, multiple (n>3) fiducial markers are needed to determine rotational shifts from the fixed geometry and field of view of the detectors. Compared to the 3D CBCT system of the conventional S-band linear accelerator, the drawback of 2D planar imaging is its lack of volumetric information and soft-tissue contrast information. However, 2D planar imaging has a distinct advantage in its high-speed detection, processing and commanding of the robotic manipulator to move rapidly according to the detected target movements or any other required corrections.
Traditionally, the CyberKnife® system has been relying on circular fields such as those defined by tertiary cones or semi-circular fields defined by a motorized IRISTM collimator to shape a radiation beam. The maximum size for the circular field has been 6.0 cm in diameter at the source to axis distance (SAD) of 80 cm. While complicated dosimetric shape can be achieved with fixed or IRISTM cones, delivery efficiency is not optimal especially for irregular and larger tumors. To overcome this problem, CyberKnife® released a new M6TM model recently with an option of a high definition MLC (InCise TM) system that was specifically designed for large-field SBRT treatments (41-44). The newest version of the InCiseTM MLC system possesses 26 pairs of leaves with a leaf width of 0.38 cm at 80 cm SAD. The maximum field size has been augmented to 10 cm × 11.5 cm. Such a development has transformed the traditional CyberKnife® system from a small-field dedicated SRS/SBRT device to an adequate large-field IMRT/SBRT device as well.
In addition to treatment time reduction, the initial studies have shown that the MLC enhanced system significantly reduced the total MU required to achieve the same planning quality with cone or IRISTM-based collimators (42,45). Such a result may likely help to reduce distal peripheral dose and alleviate the concern regarding secondary malignancies associated with high-dose SBRT treatments. On-going studies will help define clinical benefits of combining InCise TM MLC with non-isocentric beam deliveries for CyberKnife®-based SBRT treatments.
Specialized emergent SBRT devices
In contrast to the conventional C-arm based linear accelerator and robotic CyberKnife® SBRT delivery, where treatments are primarily referenced to a single isocenter (i.e., C-arm linac) or no isocenter (i.e., CyberKnife®), the latest image-guided Gamma Knife Icon (GKI) system (Figure 4) employs multiple isocenters (for example n≥10) for complex tumors (21,22). Technical and clinical data with this new system have been rapidly accruing since its initial approval by the FDA for the US market in 2016.
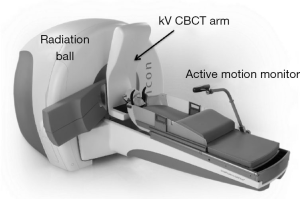
A major innovation of the GKI system is its compact integrated 3D CBCT system mounted on the current GK Perfexion unit, such that any voxel on the acquired scans for the online imaging studies are directly correlated with the stereotactic coordinates of the radiation unit. Due to the functionality of a translation-only couch, any detected rotational shifts via the 3D CBCT are corrected online by computing the resulting dose distributions rather than repositioning the patient. This is called on-line dose adaption and unique to the GKI-based treatments.
The fundamental rationale behind such a procedure is its clever leverage of 192 radiation sources distributed on a conical surfaced surrounding the patient’s skull (7). As a result, small rotations (such as <5 degree) are equivalent to rotating the entire sources in the opposite direction, which produces little or negligible perturbation to the composite dose distributions for most clinical targets. As a result, on-line dose adaption or any adjustments via even manual replanning, is an easy and efficient process.
Evidently, the new GKI system has successfully taken advantage of simultaneous beam irradiation from wide solid angles to make a hypofractionated delivery more robust against rotational shifts. Such a feature has inherently rendered on-line dose adaption a viable approach for multiple isocentric cross-firing GKI dose distributions. On-going and future studies will continue to validate such an emerging technology for future SRS and SBRT applications.
Another ground-breaking device is the expected clinical release of the first high-field strength integrated MR linear accelerator (46). Multiple sites from North America and Europe are actively pursuing the installation and the commissioning of the system at the time of this report (47). It is expected that high-quality real time MR imaging studies can be acquired simultaneously during the delivery of intensity-modulated beams (48,49). The technological innovation of the system lies in its design that minimizes the magnetic and radio frequency (RF) interferences between the MRI and the linac (Figure 5). This is achieved via active magnetic shielding and a redesigned Faraday cage, which positions the accelerator outside of the cage. However, the incorporation of a 1.5 Tesla magnet with its associated magnetic field oriented perpendicular to the incident radiation results in magnetic return effects on the Compton electrons that need to be carefully monitored with respect to dose deposition (48,50,51). It has been demonstrated that the dosimetric impact can be mitigated effectively using multi-beam arrangements (52-54).
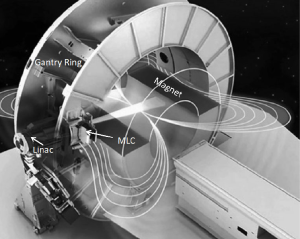
The primary benefits of an MR-guided radiotherapy system with online MR imaging capabilities are superior soft tissue contrast, improved direct monitoring of anatomical changes in real time per fraction and throughout the entire treatment course, and avoidance of additional dose exposure to the patient associated with CBCT imaging. These will allow a shift in workflow with respect to online or potentially even real time adaptive radiotherapy. To this end, A GPU-based Montel Carlo engine has been developed to achieve fast dose calculation and replanning in the presence of a magnetic field (55).
It is anticipated that volumetric non-ionizing imaging combined with fast FFF beam delivery is likely to emerge as a new paradigm for SBRT treatments spanning multiple disease sites. The intent is to create a new level of normal tissue sparing, unprecedented real-time targeting accuracy, potential for margin reduction and functional imaging based dose delivery. Ongoing research and upcoming studies are expected to further address the challenges and validate clinical gains made possible by this revolutionary technology.
Future trends of SBRT technologies
With the rapid global dissemination of SBRT, technical standards and clinical protocols are maturing. Multiple guidelines are available from professional organizations such as American Association of Physicists in Medicine (AAPM) and Canadian Association of Radiation Oncology etc. (23,56,57). It is worth mentioning, however, that initiating an SBRT program is heavily dependent on regional practices including user trainings and local resources. Without adequate vetting of the technology, it is dangerous to experiment SBRT for the sake of treating more cases in a shorter time. Future developments and standardization of SBRT solutions likely minimize such a problem. It is possible that a simple turnkey solution for SBRT would be available in the future with advancement of highly integrated systems similar to the GKI and emerging MRI linear accelerators.
It is our expectation that future SBRT technology will make significant advancements not only in the treatment quality but also in the overall workflow of complex delivery, so that entire treatment process will be highly labor-efficient for the clinical users. It is also our expectation that future treatment planning and treatment delivery process will be highly automated given on-line dose adaption and fast 3D imaging process as afforded by the integration of on-line CT/MR and other imaging capabilities for the SBRT treatments.
In conclusion, volumetric imaging, volumetric modulated beams, and real-time motion management with real-time dose adaption will continue to drive future SBRT technologies to make the treatment more user-friendly, more automated and more accessible to all cancer patients in the world.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 1951;102:316-9. [PubMed]
- Andrews DW, Bednarz G, Evans JJ, et al. A review of 3 current radiosurgery systems. Surg Neurol 2006;66:559-64. [Crossref] [PubMed]
- Sahgal A, Ma L, Chang E, et al. Advances in technology for intracranial stereotactic radiosurgery. Technol Cancer Res Treat 2009;8:271-80. [Crossref] [PubMed]
- Grandhi R, Kondziolka D, Panczykowski D, et al. Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg 2012;117:237-45. [Crossref] [PubMed]
- Lindquist C, Paddick I. The Leksell Gamma Knife Perfexion and comparisons with its predecessors. Neurosurgery 2007;61:130-40; discussion 140-1. [PubMed]
- Ma L, Mason E, Sneed PK, et al. Clinical realization of sector beam intensity modulation for Gamma Knife radiosurgery: a pilot treatment planning study. Int J Radiat Oncol Biol Phys 2015;91:661-8. [Crossref] [PubMed]
- Régis J, Tamura M, Guillot C, et al. Radiosurgery with the world's first fully robotized Leksell Gamma Knife PerfeXion in clinical use: a 200-patient prospective, randomized, controlled comparison with the Gamma Knife 4C. Neurosurgery 2009;64:346-55; discussion 355-6. [Crossref] [PubMed]
- Régis J, Tuleasca C, Resseguier N, et al. Long-term safety and efficacy of Gamma Knife surgery in classical trigeminal neuralgia: a 497-patient historical cohort study. J Neurosurg 2016;124:1079-87. [Crossref] [PubMed]
- Schlesinger D, Xu Z, Taylor F, et al. Interfraction and intrafraction performance of the Gamma Knife Extend system for patient positioning and immobilization. J Neurosurg 2012;117 Suppl:217-24. [PubMed]
- Wu A, Lindner G, Maitz AH, et al. Physics of gamma knife approach on convergent beams in stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 1990;18:941-9. [Crossref] [PubMed]
- Ma L, Pinnaduwage D, McDermott M, et al. Whole-procedural Radiological Accuracy for Delivering Multi-session Gamma Knife Radiosurgery With a Relocatable Frame System. Technol Cancer Res Treat 2014;13:403-8. [PubMed]
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [Crossref] [PubMed]
- Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol 2006;45:787-95. [Crossref] [PubMed]
- Jaffray DA, Siewerdsen JH. Cone-beam computed tomography with a flat-panel imager: initial performance characterization. Med Phys 2000;27:1311-23. [Crossref] [PubMed]
- Siewerdsen JH, Jaffray DA. Optimization of x-ray imaging geometry (with specific application to flat-panel cone-beam computed tomography). Med Phys 2000;27:1903-14. [Crossref] [PubMed]
- Yin FF, Guan H, Lu W. A technique for on-board CT reconstruction using both kilovoltage and megavoltage beam projections for 3D treatment verification. Med Phys 2005;32:2819-26. [Crossref] [PubMed]
- Yoo S, Kim GY, Hammoud R, et al. A quality assurance program for the on-board imagers. Med Phys 2006;33:4431-47. [Crossref] [PubMed]
- Snell M, Bova F, Larson D, et al. Stereotactic Radiosurgery, Report of TG42. Wisconsin: Medical Physics Publishing, 1995.
- Winston KR, Lutz W. Linear accelerator as a neurosurgical tool for stereotactic radiosurgery. Neurosurgery 1988;22:454-64. [Crossref] [PubMed]
- Stieler F, Wenz F, Abo-Madyan Y, et al. Adaptive fractionated stereotactic Gamma Knife radiotherapy of meningioma using integrated stereotactic cone-beam-CT and adaptive re-planning (a-gkFSRT). Strahlenther Onkol 2016;192:815-9. [Crossref] [PubMed]
- Tuleasca C, Leroy HA, Regis J, et al. Gamma Knife radiosurgery for cervical spine lesions: expanding the indications in the new era of Icon. Acta Neurochir (Wien) 2016;158:2235-6. [Crossref] [PubMed]
- Zeverino M, Jaccard M, Patin D, et al. Commissioning of the Leksell Gamma Knife(R) Icon. Med Phys 2017;44:355-63. [Crossref] [PubMed]
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [Crossref] [PubMed]
- Narayanasamy G, Saenz D, Cruz W, et al. Commissioning an Elekta Versa HD linear accelerator. J Appl Clin Med Phys 2016;17:179-91. [Crossref]
- Hossain S, Keeling V, Hildebrand K, et al. Normal Brain Sparing With Increasing Number of Beams and Isocenters in Volumetric-Modulated Arc Beam Radiosurgery of Multiple Brain Metastases. Technol Cancer Res Treat 2016;15:766-71. [Crossref] [PubMed]
- Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 2008;35:310-7. [Crossref] [PubMed]
- Thomas EM, Popple RA, Prendergast BM, et al. Effects of flattening filter-free and volumetric-modulated arc therapy delivery on treatment efficiency. J Appl Clin Med Phys 2013;14:4328. [Crossref] [PubMed]
- Wolff D, Stieler F, Welzel G, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol 2009;93:226-33. [Crossref] [PubMed]
- Yu CX, Tang G. Intensity-modulated arc therapy: principles, technologies and clinical implementation. Phys Med Biol 2011;56:R31-54. [Crossref] [PubMed]
- Sahgal A, Ma L, Fowler J, et al. Impact of dose hot spots on spinal cord tolerance following stereotactic body radiotherapy: a generalized biological effective dose analysis. Technol Cancer Res Treat 2012;11:35-40. [Crossref] [PubMed]
- Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:107-16. [Crossref] [PubMed]
- Dong P, Lee P, Ruan D, et al. 4pi noncoplanar stereotactic body radiation therapy for centrally located or larger lung tumors. Int J Radiat Oncol Biol Phys 2013;86:407-13. [Crossref] [PubMed]
- Tran A, Zhang J, Woods K, et al. Treatment planning comparison of IMPT, VMAT and 4pi radiotherapy for prostate cases. Radiat Oncol 2017;12:10. [Crossref] [PubMed]
- Woods K, Nguyen D, Tran A, et al. Viability of Non-Coplanar VMAT for Liver SBRT as Compared to Coplanar VMAT and Beam Orientation Optimized 4pi IMRT. Adv Radiat Oncol 2016;1:67-75. [Crossref] [PubMed]
- Adler JR Jr, Murphy MJ, Chang SD, et al. Image-guided robotic radiosurgery. Neurosurgery 1999;44:1299-306; discussion 1306-7. [PubMed]
- Chang SD, Main W, Martin DP, et al. An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery 2003;52:140-6; discussion 6-7. [PubMed]
- Kilby W, Dooley JR, Kuduvalli G, et al. The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat 2010;9:433-52. [Crossref] [PubMed]
- Schlaefer A, Schweikard A. Stepwise multi-criteria optimization for robotic radiosurgery. Med Phys 2008;35:2094-103. [Crossref] [PubMed]
- Ernst F, Bruder R, Schlaefer A, et al. Correlation between external and internal respiratory motion: a validation study. Int J Comput Assist Radiol Surg 2012;7:483-92. [Crossref] [PubMed]
- Ozhasoglu C, Saw CB, Chen H, et al. Synchrony--cyberknife respiratory compensation technology. Med Dosim 2008;33:117-23. [Crossref] [PubMed]
- Echner GG, Kilby W, Lee M, et al. The design, physical properties and clinical utility of an iris collimator for robotic radiosurgery. Phys Med Biol 2009;54:5359-80. [Crossref] [PubMed]
- Fürweger C, Prins P, Coskan H, et al. Characteristics and performance of the first commercial multileaf collimator for a robotic radiosurgery system. Med Phys 2016;43:2063. [Crossref] [PubMed]
- Jin L, Price RA, Wang L, et al. Dosimetric and delivery efficiency investigation for treating hepatic lesions with a MLC-equipped robotic radiosurgery-radiotherapy combined system. Med Phys 2016;43:727-33. [Crossref] [PubMed]
- Kathriarachchi V, Shang C, Evans G, et al. Dosimetric and radiobiological comparison of CyberKnife M6 InCise multileaf collimator over IRIS variable collimator in prostate stereotactic body radiation therapy. J Med Phys 2016;41:135-43. [Crossref] [PubMed]
- McGuinness CM, Gottschalk AR, Lessard E, et al. Investigating the clinical advantages of a robotic linac equipped with a multileaf collimator in the treatment of brain and prostate cancer patients. J Appl Clin Med Phys 2015;16:284-95. [Crossref]
- Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, et al. MRI/linac integration. Radiother Oncol 2008;86:25-9. [Crossref] [PubMed]
- Lagendijk JJ, van Vulpen M, Raaymakers BW. The development of the MRI linac system for online MRI-guided radiotherapy: a clinical update. J Intern Med 2016;280:203-8. [Crossref] [PubMed]
- Bol GH, Hissoiny S, Lagendijk JJ, et al. Fast online Monte Carlo-based IMRT planning for the MRI linear accelerator. Phys Med Biol 2012;57:1375-85. [Crossref] [PubMed]
- Crijns SP, Kok JG, Lagendijk JJ, et al. Towards MRI-guided linear accelerator control: gating on an MRI accelerator. Phys Med Biol 2011;56:4815-25. [Crossref] [PubMed]
- Ahmad SB, Sarfehnia A, Paudel MR, et al. Evaluation of a commercial MRI Linac based Monte Carlo dose calculation algorithm with GEANT4. Med Phys 2016;43:894-907. [Crossref] [PubMed]
- Raaijmakers AJ, Raaymakers BW, Lagendijk JJ. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose increase at tissue-air interfaces in a lateral magnetic field due to returning electrons. Phys Med Biol 2005;50:1363-76. [Crossref] [PubMed]
- Chen X, Prior P, Chen GP, et al. Technical Note: Dose effects of 1.5 T transverse magnetic field on tissue interfaces in MRI-guided radiotherapy. Med Phys 2016;43:4797. [Crossref] [PubMed]
- Raaijmakers AJ, Hardemark B, Raaymakers BW, et al. Dose optimization for the MRI-accelerator: IMRT in the presence of a magnetic field. Phys Med Biol 2007;52:7045-54. [Crossref] [PubMed]
- Tseng CL, Epinga W, Seravalli E, et al. Dosimetric Feasibility of the Hybrid Magnetic Resonance Imaging (MRI)-Linear Accelerator System for Brain Metastases: The Impact of the Magnetic Field. Int J Radiat Oncol Biol Phys 2016;96:E628. [Crossref]
- Kontaxis C, Bol GH, Lagendijk JJ, et al. Towards adaptive IMRT sequencing for the MR-linac. Phys Med Biol 2015;60:2493-509. [Crossref] [PubMed]
- Redmond KJ, Lo SS, Soltys SG, et al. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg Spine 2017;26:299-306. [Crossref] [PubMed]
- Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:629-39. [Crossref] [PubMed]


