
The effects of radiation therapy on the heart: implications for management
Introduction
Background
Radiotherapy is utilized for curative and palliative intent in the treatment of cancers of multiple disease sites, including the breast, esophagus, lung, and lymph nodes. Radiotherapy causes damage to the DNA of cells via both direct and indirect mechanisms (1). Given that cancer cells proliferate uncontrollably due to altered signaling and metabolism, such damage may lead to their demise (2).
Rationale and knowledge gap
Radiotherapy also affects normal cells within the treatment field, including those of the heart. Thus, the heart is recognized as an organ-at-risk (OAR) due to the potential for developing radiation-induced cardiac disease, which includes coronary artery disease (prevalence of up to 85%), valvular disease (12–60% at 20 years), cardiomyopathy (up to 10%), pericardial disease (6–30%), and conduction disorders (up to 5%) (3-5). Counterintuitively, radiotherapy may also be used to target select cardiac regions to treat recurrent in-stent restenosis and cardiac arrhythmias.
Objective
In this review, we provide an overview of radiotherapy-induced cardiac toxicities, with an emphasis on the clinical implications for therapeutic radiotherapy planning and treatment for cancers of the breast, esophagus, lung, and lymph nodes and for select cardiac-directed management.
Effects of radiotherapy on patients with baseline cardiac risk factors
In the United States, cardiovascular disease (CVD) is a main cause of morbidity and mortality (6). Non-modifiable risk factors, including a family history of CVD, hypertension, and older age, are consistently associated with an increased risk for the development of CVD (7-9). Modifiable risk factors, such as a high body mass index (BMI), tobacco use, and type II diabetes mellitus, have also been well-documented (10-13).
Patients with cancer are often at higher risk for cardiovascular events due to the cardiotoxic potential of systemic therapy and radiotherapy. Trastuzumab, for example, a human epidermal growth factor receptor 2 (HER2)-directed therapy that has revolutionized clinical outcomes for patients with HER2-positive breast cancer, is known to pose significant cardiac risks, including left ventricular contractile dysfunction (14). Used to treat breast cancer and lymphoma, anthracyclines have also been implicated in dose-dependent cardiomyocyte injury and heart failure (15).
There has been growing concern that multimodality treatment regimens may trigger synergistic cardiotoxic effects, especially in the context of baseline risk factors. Tjong et al. developed a cardiac event risk prediction model for patients with lung cancer who were treated with radiotherapy and found that pre-existing coronary heart disease and hypertension are predictors of cardiac events after radiotherapy (16). A systematic literature review conducted by Taylor et al. estimated that the absolute risk of cardiac mortality in patients with breast cancer who received radiotherapy was 1% for smokers as compared to 0.3% for nonsmokers (17). In a landmark study, Darby et al. found that an elevated rate of major coronary events (MCEs) among patients with breast cancer who had a prior history of ischemic heart disease as compared to those with no such history [rate ratio: 6.67, 95% confidence interval (CI): 4.37–10.18] (18). This increased cardiac risk was also observed in patients who possessed at least one of the following underlying risk factors: diabetes, chronic obstructive pulmonary disease, smoking, and high BMI (rate ratio: 1.96, 95% CI: 1.60–2.40). Merkx et al. found a higher frequency of abnormal left ventricular ejection fraction (LVEF) among childhood cancer survivors who received both an anthracycline and radiotherapy to the heart region as compared to their counterparts who received either anthracyclines or radiotherapy alone (19). Similarly, Zhang et al. found that radiotherapy to the left chest wall was independently associated with increased cardiotoxicity related to HER2-targeted therapy in patients with early-stage breast cancer (odds ratio: 1.98, 95% CI: 1.12–3.48) (20). Thus, understanding the potential interaction between radiotherapy-induced cardiotoxicity and the cardiac risk posed by pre-existing factors and/or systemic therapy may inform clinical management and mitigate treatment-related toxicity.
Pathophysiology of radiation-induced cardiac changes
Researchers have explored the various mechanisms of radiation-induced cardiotoxicity and their resulting consequences at both the molecular and cellular levels (21,22). Radiation exposure induces an inflammatory response and triggers the release of pro-inflammatory cytokines and growth factors (23). A persistent inflammatory response leads to intimal hyperplasia and the development of atherosclerotic plaques. Long-term consequences from chronic oxidative stress and inflammation in the setting of a high cholesterol burden can expedite lipid peroxidation and accelerate atherosclerosis (24). Additionally, monocyte and macrophage accumulation can increase intraplaque hemorrhage and plaque instability. In parallel, direct vascular endothelial damage and decreased endothelial barrier integrity causes albumin leakage, leading to an up-regulation of the aforementioned pro-inflammatory signals, an increase in platelet activation, and a loss of endothelial-derived vasodilatory factors (25). Myofibroblast activation increases fibrosis within the extracellular matrix and compromises vessel integrity, thereby resulting in arterial rigidity (26). The presence of worsening mechanical occlusion of these vessels in a pro-thrombotic, vessel-rigid microenvironment may result in microvascular thrombosis, hypoperfusion, and ischemic cell death (27,28). Myocardial fibrosis can also lead to alterations, such as ventricular remodeling and cardiac wall motion abnormalities, that increase the risk for ischemia, diastolic dysfunction, and conduction abnormalities. Thus, radiotherapy can damage adjacent and healthy cardiac tissue via both direct and indirect mechanisms.
Accelerated coronary artery disease is the most common cardiac toxicity after radiotherapy, and is most commonly observed as proximal coronary artery lesions of the left anterior descending (LAD) artery. Coronary artery disease can start as early as 5 years after treatment, but more commonly is seen 10 to 30 years after radiation therapy (22). Valvular heart disease, in contrast, is a result of fibrosis with or without calcium formation on the valves of the heart themselves. The formation of calcium deposits may be caused by the release of calcium from the endoplasmic reticulum and/or an increased concentration of calcium in the cytoplasm. Most commonly, the aortic and mitral valves, both on the left side of the heart, are affected. The majority of cases of radiation-induced valvular heart disease occur approximately 20 years after radiation therapy and manifest as stenosis or regurgitation (22). Conduction abnormalities most often present as right bundle branch blocks leading to subsequent arrhythmias approximately two months after the completion of radiotherapy (22,29). Lastly, pericarditis may transiently occur as early as days or weeks after treatment, while pericardial effusions may occur weeks or months after treatment, though most cases resolve spontaneously (22,30).
Risk factors for the development of cardiovascular toxicity include a cumulative radiation therapy dose of >30–35 Gy, a fractionated radiation therapy dose of >2 Gy, an increasing volume of irradiated heart, a younger age at the time radiotherapy is delivered, an increasing time since exposure to radiotherapy, the use of chemotherapy, and baseline CVD risk factors (31). To minimize the risks of radiation-induced cardiotoxicity, Qualitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) recommends that the volume of heart receiving ≥30 Gy be kept below 46%, and the mean heart dose to be kept to <15 Gy (30). A linear relationship between cardiac radiation dose and major adverse cardiac events (MACEs) has previously been demonstrated (18).
Clinical impacts of radiotherapy to the heart
In November 2017, the National Cancer Institute (NCI) published the updated Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (32). There are more than 30 cardiac-related CTCAE terms listed, though they can be organized into a few major categories, such as arrhythmia/conduction disorders, pericardial disease, and valvular disease, that correlate with the known radiation-induced cardiac toxicities. In this section, we detail the clinical impacts of radiation-induced cardiac toxicities by disease sites and discuss evolving clinical practices to minimize these effects.
Breast cancer
Historical practices
The earliest evidence of radiation-induced cardiotoxicity in patients with breast cancer emerged from a retrospective study that showed that while there was no significant difference in all-cause mortality in patients treated with post-mastectomy radiation therapy (PMRT) or observed until recurrence for up to 15 years, the patients who received PMRT suffered higher mortality thereafter, a fact that was attributed to cardiac causes (33). This relationship was re-demonstrated in the largest meta-analysis of its kind, which compared 10- and 20-year outcomes in patients with early-stage breast cancer who had been randomized to +/− the addition of adjuvant radiation therapy (34). While radiotherapy decreased breast cancer mortality, it increased mortality from other causes, including vascular causes. Of note, both treatment techniques and target volumes employed in the 40 studies that comprised this meta-analysis differ from what are used in modern practice. All 40 studies started between 1961 and 1986, but trials initiated in 1975 or later did not have long-term data available for analysis. Thus, many patients were treated with older radiation techniques available prior to the widespread adoption of conformal, three-dimensional (3D) planning approaches. Additionally, in these patients, treatment fields typically included not just the chest wall or breast, but also the axillary, supraclavicular, and internal mammary lymph nodes (IMNs).
There has been a concerted effort to decrease cardiac risk from breast cancer radiotherapy as data have quantified the implications of this treatment. One such case-control study included 963 women from Denmark and Sweden who received radiotherapy for breast cancer from 1958 to 2001 and experienced MCEs (18). This study showed a linear association between the rates of MCEs and the mean heart dose. Specifically, for every 1 Gy increase in the mean cardiac dose, the rate of MCEs increased by 7.4%, with no apparent lower bound or “safe” cardiac dose. The effect began within 5 years of treatment and persisted past 20 years.
Current practices and reported cardiac toxicities
Per the National Comprehensive Cancer Network (NCCN), the standard of care for radiotherapy in patients with breast cancer includes a 3D, CT-based treatment planning approach and at least weekly imaging to assess treatment setup (35). Additional techniques, such as respiratory management and prone positioning, may also be utilized. Efforts to ameliorate cardiac doses in breast radiotherapy have focused on anatomy, laterality, target volumes, and treatment techniques. In patients with left-sided breast cancer, heart doses are typically significantly higher. For example, in the aforementioned Swedish/Danish study, the mean heart dose for patients with left-sided breast cancers was 6.6 Gy as compared to 2.9 Gy for those with right-sided cancers.
In patients with left-sided breast cancer, deep inspiration breast hold (DIBH) is routinely employed with the goal of increasing the separation between the treated breast and the heart and lungs (36,37). In appropriately selected patients, patient positioning can also have significant dose implications. A systematic review of heart doses published from 2003 to 2013 showed that prone or lateral decubitus positioning in certain patients can significantly decrease mean heart dose to a degree that is comparable to DIBH in patients with left-sided malignancies (2.4 Gy, P=0.004; 1.2 Gy, P<0.001; and 1.3 Gy, P<0.001, respectively) (38). Though the risks of treating the IMNs have decreased with the advent of modern treatment techniques, multiple studies have strongly associated treatment of the IMNs with cardiac toxicity, and efforts aiming to identify post-mastectomy patients who may safely omit IMN irradiation are underway (18,39-41). Finally, CALGB 9343 showed that a subset of patients, specifically those aged 70 or older with early-stage, estrogen receptor-positive cancer and on endocrine therapy, may forgo adjuvant radiation treatment without impacting their overall survival, breast cancer-specific survival, time to distant metastasis, and time to mastectomy, although they do have an increased risk of locoregional recurrence (42).
Evolving clinical practices and future directions
Ongoing research in breast radiotherapy seeks to decrease the target volume or alter fractionation regimens to better suit patients and the radiobiology of the tumor. For example, the NCT02400658 trial found that intraoperative radiation therapy (IORT), a type of brachytherapy that treats a smaller target volume than the traditional whole breast irradiation (WBI), offers good cosmetic outcomes with low toxicity at a follow up of 12 months, though no reports of cardiac toxicity have yet been published (43). In addition, the Danish Breast Cancer Group recently demonstrated that partial breast irradiation (PBI), which treats a smaller target volume and was delivered in 40 Gy over 15 fractions, has comparable 3-year breast induration with few disease recurrences as compared to WBI delivered in 40 Gy over 15 fractions (44). However, the patients only have a median follow-up of 5.0 years for morbidity evaluations and cardiac disease accounts for four (almost 10% of) non-cancer deaths: three in the WBI arm and one in the PBI arm. Furthermore, the accelerated PBI (APBI) intensity-modulated radiation therapy (IMRT) Florence Trial presented long-term results showing comparable 10-year cumulative ipsilateral breast tumor recurrence rates between the WBI arm (50 Gy in 25 fractions) and the APBI arm (30 Gy in 5 fractions), suggesting that a hypofractionated treatment regimen to a smaller target volume may be appropriate for certain patients (45). This trial has not yet published any information on cardiac toxicity or cardiac-related deaths.
Lastly, the 10-year results of UK FAST and the 5-year results of UK FAST-Forward have shown that hypofractionated WBI has similar oncologic and cardiac outcomes at 10 years as compared to conventional fractionation (46,47). Taken together with principles of radiation biology suggesting that hypofractionated approaches would preferentially protect late-responding tissues, such as the heart, the aforementioned data may favor hypofractionated breast treatments with respect to cardiotoxicity (48). However, the radiobiology and pathophysiology of heart toxicity as described above raise caution about the value of this 5–10-year follow-up data on cardiac toxicity profiles.
Hodgkin’s lymphoma
Historical practices
Radiotherapy has been a mainstay in the treatment of Hodgkin’s lymphoma since the early 1900s, when Dr. Pusey described the first cases treated with radiation (49). Continued advances in radiotherapy techniques and chemotherapeutic agents were made throughout much of the 20th century, resulting in improved disease outcomes. Even so, the radiotherapy techniques utilized during this time were generally far less sophisticated than what is available today and resulted in the treatment of large fields with relatively high doses of radiation. As such, these improvements in disease-specific outcomes came with an increased risk of long-term toxicities (50).
The deleterious cardiac effects experienced by patients treated with radiotherapy for Hodgkin’s lymphoma have thus been well-documented. One study published by Aleman et al. evaluated causes of mortality in 1,261 patients treated before the age of 41 between 1965 and 1987 (51). Their analyses demonstrated that these patients were at significantly increased risk for death from CVD when compared to the general population (relative risk: 6.3). These risks were even more apparent in patients who underwent treatment prior to the age of 21, who were 13.6 times more likely to suffer a fatal cardiac event than the general population. Another study by Castellino et al. also examined the long-term toxicities experienced by survivors of Hodgkin’s lymphoma (52). Their study of 2,742 patients, of which 94% received radiotherapy as part of their treatment regimen, reported an excess adverse risk of 13.1 per 10,000 person-years for cardiac death and an 11.1% incidence of grade 3–5 CVD at 30 years.
The underlying causes of CVD following radiotherapy have also been studied extensively in these patients. A study of 2,232 patients treated between 1961 and 1990 found that the risk of death from acute myocardial infarction was 3.2 times higher than the general population, while a more recent study of 7,033 patients treated between 1967 and 2000 estimated the relative risk to be 2.5 (53,54). The latter study also noted the risk to be elevated for at least 25 years after treatment. In addition to coronary artery disease, Hodgkin’s survivors are also at increased risk for valvular heart disease. This is particularly pronounced for patients receiving higher doses of radiation, with one study reporting a cumulative risk for valvular heart disease of 12.4% for doses >40 Gy compared to just 3.0% for doses <30 Gy (55). Finally, it has been demonstrated that radiation dose is associated with an elevated risk of congestive heart failure in this population. A study by van Nimwegen et al. found a significant correlation between the degree of radiation exposure to the heart and the development of congestive heart failure, which was further exacerbated by treatment with anthracycline-based chemotherapy (56). In patients treated with radiation without anthracycline-based chemotherapy, a mean dose of ≥21 Gy to the left ventricle was associated with a 13.3% risk of heart failure at 25 years, which increased to 32.9% for patients who also received an anthracycline.
Current practices and reported cardiac toxicities
Due to the significant excess risks of cardiotoxicity associated with radiation techniques utilized throughout most of the 20th century, numerous contemporary attempts have been made to minimize the dose delivered to the heart of patients with Hodgkin’s lymphoma of the mediastinum. Per NCCN guidelines, 4D-CT scans with simulation and respiratory management are essential, and consideration should be given to advanced radiotherapy techniques, such as IMRT and proton beam therapy, to maximize cardiac sparing (57). Additionally, the size of the radiation fields used for treatment have been reduced. Involved-field radiotherapy, which uses anatomic landmarks to target an entire disease-containing nodal basin, replaced extended-field radiation (such as the mantle-field radiotherapy), which targets all disease-containing and the surrounding normal lymph node areas (58,59). Subsequent further reductions in treatment volume have been adopted with the development of involved-site and involved-node techniques, which use pre-chemotherapy imaging to delineate areas at risk (60).
In addition to reducing field sizes, radiotherapy dose reduction has also been pursued. With the publication of the HD10 trial, 20 Gy, rather than 30 Gy, is now typically employed for favorable-risk disease following chemotherapy, allowing for lower cardiac exposure (61). Finally, dose constraints for the heart have also been developed in order to optimize treatment planning, with a target mean heart dose of <5 Gy for cases with mediastinal involvement (62).
Evolving clinical practices and future directions
Ongoing cardioprotective research in patients with Hodgkin’s lymphoma focuses on adjusting systemic therapies and/or further reducing the use of radiotherapy. For example, RADAR (NCT04685616) is an international, multicenter, phase III trial where patients with stage IA/IIA Hodgkin’s lymphoma are randomized to either ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) chemotherapy or A2VD chemotherapy (doxorubicin, brentuximab vedotin, vinblastine and dacarbazine, with growth factor support) (63). After two cycles of systemic therapy, the patients in both arms will undergo a positron emission tomography (PET)/CT scan; those with a Deauville score of 4 will have two more cycles of systemic therapy followed by involved-site radiotherapy of 30 Gy, while those with a Deauville score of 1–3 will have one additional cycle of systemic therapy without radiotherapy. Additionally, LHGALOP2017 (NCT03500133) is a multicenter, non-randomized, phase III trial where therapy is tailored to the initial disease risk group and disease response to systemic therapy (64). Specifically, involved-node radiotherapy of 30 Gy is given to patients with a partial response at the end of systemic therapy. Given the low prevalence of Hodgkin’s lymphoma in comparison to the prevalence of breast, lung, and esophageal cancers, recruitment for the two aforementioned clinical trials is ongoing and the trial results may not be available for several years.
Lung cancer
Historical practices
Radiation therapy for the treatment of lung cancer has evolved significantly. A meta-analysis of nine European trials conducted between 1966 and 1995 with a median follow-up of 3.9 years revealed a 7% absolute increase in mortality linked to radiation therapy, despite a 24% reduction in local recurrences (65). The authors speculated that this rise in post-RT mortality might be linked to radiation-induced lung or heart toxicity. In 1999, Dautzenberg et al. found that major cardiac events accounted for 26% of non-cancer-related deaths following postoperative, cobalt-60-based radiotherapy in patients with non-small cell lung cancer (NSCLC) (66). The shift from cobalt-60-based radiotherapy to linear accelerator-based radiotherapy in the 1980s, along with the adoption of 3D-conformal radiotherapy (3D-CRT) in the 1990s, allowed for improved sparing of normal tissues (67,68).
Current practices and reported cardiac toxicities
Per the NCCN, the minimum technologic standard of radiotherapy for NSCLC and small cell lung cancer (SCLC) is 3D-CRT, though more advanced technologies, such as IMRT and respiratory motion management, may be appropriate (69,70). The initial trial to suggest that cardiac dose is associated with survival in patients treated for lung cancer was RTOG 0617, which allowed both 3D-CRT and IMRT for locally advanced NSCLC. The original report by Bradley et al. in 2015 revealed that the percentage of cardiac volume receiving ≥5 Gy (V5) or ≥30 Gy (V30) was associated with worse overall survival (71). Several subsequent studies of locally advanced NSCLC found that higher cardiac radiation doses were associated with increased risks of major cardiac events and mortality. In a secondary analysis of RTOG 0617, the use of IMRT resulted in lower heart doses (P<0.05) than 3D-CRT, and the heart V40 was significantly associated with overall survival on multivariable analysis (P<0.05) (72). Furthermore, IDEAL-CRT, which analyzed isotoxically escalated concurrent chemoradiation in patients with advanced-stage NSCLC, found that the “left atria wall principal component 6 scores”, which relate to the left atrial wall volumes receiving 63–69 Gy, were associated with higher all-cause death rate in a multivariable analysis (73).
A commonly used cardiac dose constraint is the mean heart dose, although the percentage of cardiac volume receiving a particular dose of radiotherapy has also been utilized as a treatment optimization constraint (74). A pooled analysis of four prospective multicenter trials revealed that among 125 eligible patients, the cumulative incidence of grade ≥3 cardiac events at 24 months was 11%, with higher mean heart doses and pre-existing cardiac disease associated with higher rates of such events (75). Along with disease progression, grade ≥3 cardiac events were linked to reduced overall survival on multivariable analysis. Additionally, another study found that higher mean heart doses were significantly associated with an increased risk of all-cause mortality (HR: 1.02/Gy; P=0.007) and MACEs (HR: 1.05/Gy; P<0.001) (76). Notably, a mean heart dose ≥10 Gy was associated with a higher all-cause mortality risk only in patients without pre-existing coronary heart disease (CHD). Dose escalation studies have further demonstrated this association. For example, Wang et al. published a pooled analysis of six prospective trials that evaluated dose-escalated radiotherapy in the treatment of 127 patients with stage III NSCLC (77). The mean heart dose was significantly associated with cardiac events (HR: 1.03/Gy; P=0.002), along with baseline cardiac risk. The aforementioned studies highlighted that minimizing heart dose is important to reduce radiotherapy-associated cardiac toxicities in patients with lung cancer.
Evolving clinical practices and future directions
Recently, proton therapy has been investigated for its potential to spare normal tissue in the treatment of lung cancer. In a phase II study of IMRT versus proton therapy for locally advanced NSCLC, pericardial effusions occurred in 31.4% of patients at 1 year, increasing to 45.4% at 2 years (78). More than 10% of heart volume receiving ≥35 Gy (V35) was a significant predictor of the development of a pericardial effusion (hazard ratio: 2.14; P=0.002). The optimal radiotherapy technique is being further investigated in RTOG 1308, an ongoing phase III clinical trial comparing cardiac toxicity after photon and proton radiotherapy for NSCLC (79). Other ongoing trials like NCT04305613 are utilizing biological and imaging markers for cardiovascular phenotyping to unravel the intricate functional and physiological changes that arise during radiation therapy (80). Additionally, subregions of the heart may be susceptible to radiotherapy, thus protective strategies for them could be warranted (81). Cardiac substructures and their associations with major cardiac events and cardiac-related mortality are therefore being studied, which will be elaborated upon in a latter section.
Esophageal cancer
Historical practices
Radiation-induced cardiac toxicity, as noted, has been well-documented in patients with breast cancer, Hodgkin’s lymphoma, and lung cancer. Yet, data on cardiac outcomes after radiation therapy for esophageal cancer remains sparse. This scarcity of information stems from the longstanding perception that radiation-induced cardiac toxicity manifests as a delayed side effect, and is further compounded by the historically and relatively low incidence and cure rates of esophageal cancer. Additionally, due to the high prescribed dose, commonly between 41.4 and 50.4 Gy, and the heart’s close proximity to the target volume in patients with esophageal cancer, administered cardiac doses tend to be higher for patients with esophageal cancer than for those with Hodgkin’s lymphoma. Additionally, the volume of the heart exposed to radiation in patients with esophageal cancer is larger than in those with breast cancer (82).
In examining the Surveillance, Epidemiology, and End Results (SEER) database of 5,630 patients with esophageal cancer diagnosed between 1973 and 2012 and with at least five years of follow-up, researchers identified a significantly increased risk of cardiac death in patients who underwent radiotherapy as compared to those who had not (HR: 1.961; P<0.0001) (83). A literature review of studies published between 1970 and 2013 found a symptomatic cardiotoxicity incidence of 10.8%, with most cases occurring within two years of treatment, whereas a different study of 55 patients published in 2015 revealed that 24% experienced cardiovascular events within a 90-day follow-up period (82,84). A more recent SEER analysis of 63,560 patients with esophageal cancer treated between 2000 and 2018 highlighted that for patients surviving more than ten years post-diagnosis, 59% succumbed to non-cancer-related causes, 43% of which were secondary to CVD (85). Together, these studies suggest that cardiovascular events in patients with esophageal cancer may begin within 90 days of completing radiotherapy, with an increase in cardiac-related deaths starting within a few years and persisting for more than ten years post-diagnosis.
The shift from two-dimensional (2D) to CT-based 3D treatment planning has significantly enhanced anatomical visualization, leading to better delineation of target areas, especially for OARs like the heart. Prior to the introduction of 3D-CRT, grade ≥3 cardiopulmonary complications were observed in 29% of elderly patients with esophageal cancer treated using the older 2D radiation therapy plans (86). Additionally, with these older techniques, the incidence of symptomatic cardiac disease was reported as high as 13.8% 5-year post-treatment, with the likelihood of developing symptomatic cardiac disease contingent upon the heart volume receiving doses exceeding 45, 50, and 55 Gy (87).
Current practices and reported cardiac toxicities
The majority of patients with locally advanced esophageal cancer undergo neoadjuvant chemoradiotherapy (CRT) followed by surgery or definitive CRT if they are not surgical candidates. However, their prognosis remains bleak, with a ten-year overall survival rate of just 38% (88). Notably, a significant fraction of patients with esophageal cancer are frail and have undiagnosed or inadequately managed CVD (84). Given the central anatomical position of the esophagus, radiation therapy introduces an increased risk of cardiovascular complications in these patients.
While esophageal cancers were treated for years with 3D-CRT, the advent of IMRT offered the potential to reduce exposure to surrounding tissues without compromising the dose directed at the tumor. A comprehensive retrospective analysis of 676 esophageal cancer patients who had received 3D-CRT or IMRT revealed a higher cumulative incidence of deaths attributable to cardiac causes in the 3D-CRT group as compared to the IMRT group (89). Furthermore, the majority of the deaths within the 3D-CRT group occurred within the first two years after radiotherapy. Despite IMRT’s dosimetric advantages, both IMRT and 3D-CRT are endorsed in the latest NCCN guidelines (90).
Despite concerns regarding cardiac dysfunction in patients with esophageal cancer who undergo radiotherapy, the explicit relationship between dosimetric parameters and radiation-induced cardiotoxicity remains a matter of ongoing investigation. A study of 80 patients with esophageal cancer treated with CRT found that there was a strikingly high risk ratio of 16.8 (95% CI: 4.94–53.07) for cardiac events when 280 ml of the heart received radiation doses exceeding 50 Gy (V50) (91). Another investigation involving 346 such patients found that a higher heart V5 was significantly correlated with decreased overall survival (92). Finally, a retrospective evaluation of 716 patients found that the dosimetric parameters of the left circumflex (LCX) and LAD coronary arteries were better predictors of significant cardiac events than those of the whole heart (93). Ongoing studies are seeking to further elucidate the relationships between radiation-induced cardiotoxicity and dosimetric parameters for patients with esophageal cancer.
Evolving clinical practices and future directions
Given the potential risk of treatment-related cardiac complications after photon-based radiotherapy in patients with esophageal cancer, there is much interest in proton beam therapy. Numerous retrospective dosimetric studies tout the benefits of proton beam therapy as compared to IMRT for the treatment of esophageal cancer, including lower administered radiation doses to the whole heart, cardiac substructures, and coronary arteries (94-96). While dosimetric findings underline the potential benefits of proton beam therapy in treating esophageal cancer, the real-world implications of these results in terms of improved patient outcomes remains a point of contention, chiefly due to limited prospective clinical data. A notable phase IIB study comparing clinical outcomes of proton beam therapy with IMRT in patients with esophageal cancer found that proton beam therapy consistently delivered lower mean heart doses (11.3 vs. 19.8 Gy; P<0.01) and fewer cardiopulmonary toxicities than IMRT (97). However, comprehensive phase III randomized clinical trials, such as NRG GI-006, that compare proton beam therapy and IMRT for patients with esophageal cancer are still in their infancy (98).
Cardiac-directed therapy
Intravascular brachytherapy (IVBT) for recurrent in-stent restenosis
In the United States, coronary artery disease is a major cause of morbidity and mortality and affects over 20 million adults annually (99). For patients presenting with an ST-segment elevation myocardial infarction (STEMI) secondary to coronary artery stenosis, the standard of care for treatment remains the placement of a percutaneous drug-eluting stent in the stenotic area(s) in order to reestablish coronary blood flow (100). Although the rate of stent restenosis is relatively low with modern drug-eluting stents, a large number of patients still experience this complication given the high prevalence of stenting (101). The management of patients with bare metal stents who experience in-stent restenosis entails the placement of a drug-eluting stent (102,103).
However, in the setting of recurrent in-stent restenosis, treatment with a drug-eluting stent may result in luminal narrowing from the multiple layered stents. Thus, in such patients, IVBT remains a valuable tool (100,104). Historically, radiation has been utilized in the management of uncontrolled fibroproliferative processes, such as keloids (105). This treatment has been extrapolated to the setting of in-stent luminal restenosis by using IVBT (106). Despite a lack of prospective trials, many retrospective studies suggest favorable outcomes post-IVBT for recurrent in-stent restenosis, as demonstrated in a recent literature review by Detloff et al. (100). Despite its effectiveness and relative safety, IVBT has inherent risks, such as thrombosis secondary to endothelial damage from angioplasty and delayed healing (107,108). Nonetheless, IVBT is deemed to be a safe treatment modality, with less than a 5% risk of complications (109).
Ablative stereotactic body radiotherapy (SBRT) for cardiac arrhythmias
In recent years, advances in the mapping of cardiac arrhythmias have enabled the delivery of ablative SBRT to treat refractory cardiac arrhythmias (110). Sharma et al. demonstrated the feasibility of using stereotactic radiosurgery in an animal model to create cardiac lesions in order to alter cardiac conductivity (111). This was further studied in a prospective study in which patients received a single SBRT fraction of 25 Gy to the ventricular tachycardia circuits targeted by noninvasive electrocardiographic imaging (112). This study showed that SBRT resulted in a marked reduction in ventricular tachyarrhythmias. Of note, however, one out of the five enrolled patients developed a fatal stroke, although the association with the treatment remains unclear due to patient’s underlying risk factors. Given the small cohort, the authors warned about using this technique outside of clinical studies given the non-negligible risks of thromboembolic events and radiation-induced myocardial injury, with the resulting potential decrease in the functionality of specialized cardiac structures. While the results of ENCORE-VT, a prospective phase I/II study assessing the impacts of noninvasive radioablation, appear promising, additional studies are needed (113).
General attempts to lower administered cardiac doses and implications for therapy
The main aim of radiotherapy is to maximize the administered dose to the tumor and to minimize the dose delivered to the surrounding tissue. Based on this principle, there are past and ongoing attempts to lower the radiotherapy dose delivered to the heart, some of which are mentioned in prior sections. Applicable to some or all of the aforementioned disease sites in proximity to the heart, these approaches are characterized and grouped accordingly in Figure 1.
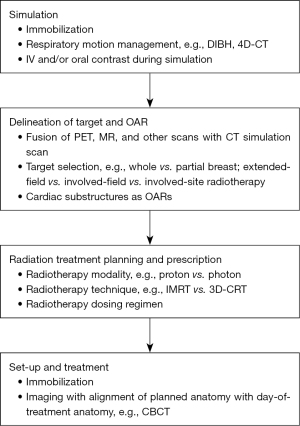
An approach to minimize cardiac radiotoxicity recognizes the differing radiosensitivity of various cardiac substructures, resulting in updated, anatomically-defined contouring guidelines. Bergom et al. explored the relationship between the radiation dose delivered to various heart regions, including the atriums (lung cancer and Hodgkin lymphoma treatments), ventricles (lung cancer, breast cancer, and Hodgkin lymphoma treatments), valves (Hodgkin lymphoma treatments), blood vessels (lung cancer and Hodgkin lymphoma treatments), and pericardium (esophageal cancer treatment), with cardiac toxicities in 2021 (114). Since then, van den Bogaard et al. have noted that the mean radiation dose to atherosclerotic plaques already present in the LAD of patients with breast cancer is the strongest predictor of acute coronary events (ACEs) (115). In addition, Tjong et al. published a model in 2022 that linked the volume of LAD receiving ≥15 Gy with the risk of MACE in patients with lung cancer (16). Also in 2022, Wang et al. first reported on the relationship between the radiation dose to coronary substructures and MCEs in patients with esophageal cancer (116). Specifically, the volume of LAD receiving ≥30 Gy was associated with the incidence of MCEs. Furthermore, an increased mean dose to the left main coronary artery (LMA) was associated with an increased relative risk of death (HR: 1.014; P=0.002). Three atlases have included cardiac substructures for radiotherapy planning and an auto-segmentation model of twelve cardio-pulmonary substructures has been created, though additional investigations are warranted to validate them prior to their widespread adoption in clinical practice (117-120).
Strengths and limitations
This review has several limitations. First, some of the clinical studies referenced are from an older era in which the heart was not considered to be an OAR and thus attempts to spare it of radiation dose were relatively minimal. Overall, there are fewer studies reporting cardiac toxicity under the newer radiotherapy modalities that comprise the current standards of care. In addition, given the temporal relationship between radiation exposure and the clinical manifestation of cardiac toxicity, the most recent clinical studies may have insufficient long-term data to fully evaluate the risks of cardiac toxicity and its resulting morbidity and mortality (121). Lastly, more research into the possibility of synergistic effects between newer systemic therapies and radiotherapy on the heart are needed.
Conclusions
Radiation-induced cardiac toxicity has historically been prevalent among patients with cancer who have received radiotherapy, delivered via older techniques, to treat tumors in close proximity with the heart. In recent decades, the attempts to lower the administered cardiac radiation dose during cancer-directed treatments have broadly been successful. Recently, cardiac-directed radiotherapy has been utilized to treat recurrent in-stent restenosis and cardiac arrhythmias by leveraging the effect of radiotherapy on abnormally functioning, non-cancerous cells. More work remains to be done to further minimize the risks to patients while maximizing the benefits of radiotherapy.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-125/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-125/coif). K.H. is the 2023 ASTRO-AstraZeneca Radiation Oncology Research Training Fellow. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reuvers TGA, Kanaar R, Nonnekens J. DNA Damage-Inducing Anticancer Therapies: From Global to Precision Damage. Cancers (Basel) 2020;12:2098. [Crossref] [PubMed]
- Adjemian S, Oltean T, Martens S, et al. Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis 2020;11:1003. [Crossref] [PubMed]
- Mir R, Kelly SM, Xiao Y, et al. Organ at risk delineation for radiation therapy clinical trials: Global Harmonization Group consensus guidelines. Radiother Oncol 2020;150:30-9. [Crossref] [PubMed]
- Belzile-Dugas E, Eisenberg MJ. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J Am Heart Assoc 2021;10:e021686. [Crossref] [PubMed]
- Chang HM, Okwuosa TM, Scarabelli T, et al. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J Am Coll Cardiol 2017;70:2552-65. [Crossref] [PubMed]
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56-e528. [Crossref] [PubMed]
- Mehta A, Virani SS, Ayers CR, et al. Lipoprotein(a) and Family History Predict Cardiovascular Disease Risk. J Am Coll Cardiol 2020;76:781-93. [Crossref] [PubMed]
- Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 2021;397:1625-36. [Crossref] [PubMed]
- North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097-108. [Crossref] [PubMed]
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021;143:e984-e1010. [Crossref] [PubMed]
- Keto J, Ventola H, Jokelainen J, et al. Cardiovascular disease risk factors in relation to smoking behaviour and history: a population-based cohort study. Open Heart 2016;3:e000358. [Crossref] [PubMed]
- Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep 2019;21:21. [Crossref] [PubMed]
- Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci 2009;84:705-12. [Crossref] [PubMed]
- Dempsey N, Rosenthal A, Dabas N, et al. Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res Treat 2021;188:21-36. [Crossref] [PubMed]
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 2018;104:971-7. [Crossref] [PubMed]
- Tjong MC, Bitterman DS, Brantley K, et al. Major adverse cardiac event risk prediction model incorporating baseline Cardiac disease, Hypertension, and Logarithmic Left anterior descending coronary artery radiation dose in lung cancer (CHyLL). Radiother Oncol 2022;169:105-13. [Crossref] [PubMed]
- Taylor C, Correa C, Duane FK, et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol 2017;35:1641-9. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Merkx R, Leerink JM, Feijen ELAM, et al. Extensive Cardiac Function Analyses Using Contemporary Echocardiography in Childhood Cancer Survivors: A DCCSS LATER Study. JACC CardioOncol 2023;5:472-85. [Crossref] [PubMed]
- Zhang L, Wang Y, Meng W, et al. Cardiac safety analysis of anti-HER2-targeted therapy in early breast cancer. Sci Rep 2022;12:14312. [Crossref] [PubMed]
- Koutroumpakis E, Deswal A, Yusuf SW, et al. Radiation-Induced Cardiovascular Disease: Mechanisms, Prevention, and Treatment. Curr Oncol Rep 2022;24:543-53. [Crossref] [PubMed]
- Siaravas KC, Katsouras CS, Sioka C. Radiation Treatment Mechanisms of Cardiotoxicity: A Systematic Review. Int J Mol Sci 2023;24:6272. [Crossref] [PubMed]
- Ping Z, Peng Y, Lang H, et al. Oxidative Stress in Radiation-Induced Cardiotoxicity. Oxid Med Cell Longev 2020;2020:3579143. [Crossref] [PubMed]
- Rygiel K. Cardiotoxic effects of radiotherapy and strategies to reduce them in patients with breast cancer: An overview. J Cancer Res Ther 2017;13:186-92. [Crossref] [PubMed]
- Banfill K, Giuliani M, Aznar M, et al. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J Thorac Oncol 2021;16:216-27. [Crossref] [PubMed]
- Díaz-Gavela AA, Figueiras-Graillet L, Luis ÁM, et al. Breast Radiotherapy-Related Cardiotoxicity. When, How, Why. Risk Prevention and Control Strategies. Cancers (Basel) 2021;13:1712. [Crossref] [PubMed]
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010;55:1227-36. [Crossref] [PubMed]
- Fajardo LF. The unique physiology of endothelial cells and its implications in radiobiology. Front Radiat Ther Oncol 1989;23:96-112. [Crossref] [PubMed]
- Kirova Y, Tallet A, Aznar MC, et al. Radio-induced cardiotoxicity: From physiopathology and risk factors to adaptation of radiotherapy treatment planning and recommended cardiac follow-up. Cancer Radiother 2020;24:576-85. [Crossref] [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [Crossref] [PubMed]
- Toste JC. Cardio-oncology: Understanding the different mechanisms of cardiovascular toxicity. Rev Port Cardiol 2022;41:587-97. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 [Internet]. U.S. Department of Health and Human Services: National Institutes of Health and National Cancer Institute; 2017 [cited 2023 Sep 21]. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Jones JM, Ribeiro GG. Mortality patterns over 34 years of breast cancer patients in a clinical trial of post-operative radiotherapy. Clin Radiol 1989;40:204-8. [Crossref] [PubMed]
- Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 2000;355:1757-70. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version 4.2023) [Internet]. 2023 [cited 2023 Sep 25]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Conway JL, Conroy L, Harper L, et al. Deep inspiration breath-hold produces a clinically meaningful reduction in ipsilateral lung dose during locoregional radiation therapy for some women with right-sided breast cancer. Pract Radiat Oncol 2017;7:147-53. [Crossref] [PubMed]
- Pandeli C, Smyth LML, David S, et al. Dose reduction to organs at risk with deep-inspiration breath-hold during right breast radiotherapy: a treatment planning study. Radiat Oncol 2019;14:223. [Crossref] [PubMed]
- Taylor CW, Wang Z, Macaulay E, et al. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int J Radiat Oncol Biol Phys 2015;93:845-53. [Crossref] [PubMed]
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75. [Crossref] [PubMed]
- Poortmans PM, Struikmans H, Bartelink H. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:1879-80. [PubMed]
- Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382-7. [Crossref] [PubMed]
- Meneveau MO, Petroni GR, Varhegyi NE, et al. Toxicity and cosmetic outcomes after treatment with a novel form of breast IORT. Brachytherapy 2020;19:679-84. [Crossref] [PubMed]
- Offersen BV, Alsner J, Nielsen HM, et al. Partial Breast Irradiation Versus Whole Breast Irradiation for Early Breast Cancer Patients in a Randomized Phase III Trial: The Danish Breast Cancer Group Partial Breast Irradiation Trial. J Clin Oncol 2022;40:4189-97. [Crossref] [PubMed]
- Meattini I, Marrazzo L, Saieva C, et al. Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J Clin Oncol 2020;38:4175-83. [Crossref] [PubMed]
- Brunt AM, Haviland JS, Sydenham M, et al. Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer. J Clin Oncol 2020;38:3261-72. [Crossref] [PubMed]
- Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020;395:1613-26. [Crossref] [PubMed]
- Appelt AL, Vogelius IR, Bentzen SM. Modern hypofractionation schedules for tangential whole breast irradiation decrease the fraction size-corrected dose to the heart. Clin Oncol (R Coll Radiol) 2013;25:147-52. [Crossref] [PubMed]
- Pusey WA. CASES OF SARCOMA AND OF HODGKIN’S DISEASE TREATED BY EXPOSURES TO X-RAYS—A PRELIMINARY REPORT. JAMA 1902;XXXVIII:166. [Crossref]
- Koh ES, Tran TH, Heydarian M, et al. A comparison of mantle versus involved-field radiotherapy for Hodgkin's lymphoma: reduction in normal tissue dose and second cancer risk. Radiat Oncol 2007;2:13. [Crossref] [PubMed]
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 2003;21:3431-9. [Crossref] [PubMed]
- Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 2011;117:1806-16. [Crossref] [PubMed]
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 1993;270:1949-55. [Crossref] [PubMed]
- Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 2007;99:206-14. [Crossref] [PubMed]
- Cutter DJ, Schaapveld M, Darby SC, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst 2015;107:djv008. [Crossref] [PubMed]
- van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 2017;129:2257-65. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Hodgkin Lymphoma (Version 2.2023) [Internet]. 2022 [cited 2023 Sep 25]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf
- Kumar PP, Good RR, Jones EO, et al. Extended-field isocentric irradiation for Hodgkin's disease. J Natl Med Assoc 1987;79:969-80. [PubMed]
- Aleman BM, Raemaekers JM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin's lymphoma. N Engl J Med 2003;348:2396-406. [Crossref] [PubMed]
- Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys 2014;89:854-62. [Crossref] [PubMed]
- Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med 2010;363:640-52. [Crossref] [PubMed]
- Wirth A, Mikhaeel NG, Aleman BMP, et al. Involved Site Radiation Therapy in Adult Lymphomas: An Overview of International Lymphoma Radiation Oncology Group Guidelines. Int J Radiat Oncol Biol Phys 2020;107:909-33. [Crossref] [PubMed]
- ClinicalTrials.gov Identifier: NCT04685616 [Internet]. 2023 [cited 2023 Sep 29]. Brentuximab Vedotin in Early Stage Hodgkin Lymphoma (RADAR). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04685616
- ClinicalTrials.gov Identifier: NCT03500133 [Internet]. 2019 [cited 2023 Sep 29]. Pediatric Hodgkin Lymphoma Treatment Trial With Low Cumulative Doses of Chemotherapy Agents and Reduced Radiation. (LHGALOP2017). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03500133
- Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet 1998;352:257-63. [Crossref] [PubMed]
- Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d'Etude et de Traitement des Cancers Bronchiques. Cancer 1999;86:265-73. [Crossref] [PubMed]
- Healy BJ, van der Merwe D, Christaki KE, et al. Cobalt-60 Machines and Medical Linear Accelerators: Competing Technologies for External Beam Radiotherapy. Clin Oncol (R Coll Radiol) 2017;29:110-5. [Crossref] [PubMed]
- Chandarana H, Wang H, Tijssen RHN, et al. Emerging role of MRI in radiation therapy. J Magn Reson Imaging 2018;48:1468-78. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 3.2023) [Internet]. 2023 [cited 2023 Apr 26]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- National Comprehensive Cancer Network. Small Cell Lung Cancer (Version 1.2024) [Internet]. 2023 [cited 2023 Sep 24]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Vivekanandan S, Landau DB, Counsell N, et al. The Impact of Cardiac Radiation Dosimetry on Survival After Radiation Therapy for Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;99:51-60. [Crossref] [PubMed]
- Niska JR, Hu J, Li J, et al. Using Novel Statistical Techniques to Accurately Determine the Predictive Dose Range in a Study of Overall Survival after Definitive Radiotherapy for Stage III Non-Small Cell Lung Cancer in Association with Heart Dose. J Cancer Ther 2021;12:505-29. [Crossref] [PubMed]
- Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:1395-402. [Crossref] [PubMed]
- Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. J Am Coll Cardiol 2019;73:2976-87. [Crossref] [PubMed]
- Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387-94. [Crossref] [PubMed]
- Ning MS, Tang L, Gomez DR, et al. Incidence and Predictors of Pericardial Effusion After Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;99:70-9. [Crossref] [PubMed]
- ClinicalTrials.gov Identifier: NCT01993810 [Internet]. 2023 [cited 2023 Sep 25]. Comparing Photon Therapy To Proton Therapy To Treat Patients With Lung Cance. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01993810
- ClinicalTrials.gov Identifier: NCT04305613 [Internet]. 2023 [cited 2023 Sep 25]. Cardiotoxicity in Locally Advanced Lung Cancer Patients Treated With Chemoradiation Therapy (CLARITY). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04305613
- Liu X, Fatyga M, Schild SE, et al. Detecting spatial susceptibility to cardiac toxicity of radiation therapy for lung cancer. IISE Trans Healthc Syst Eng 2020;10:243-50. [Crossref] [PubMed]
- Beukema JC, van Luijk P, Widder J, et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol 2015;114:85-90. [Crossref] [PubMed]
- Gharzai L, Verma V, Denniston KA, et al. Radiation Therapy and Cardiac Death in Long-Term Survivors of Esophageal Cancer: An Analysis of the Surveillance, Epidemiology, and End Result Database. PLoS One 2016;11:e0158916. [Crossref] [PubMed]
- Søndergaard MMA, Nordsmark M, Nielsen KM, et al. Cardiovascular Burden and Adverse Events in Patients With Esophageal Cancer Treated With Chemoradiation for Curative Intent. JACC CardioOncol 2021;3:711-21. [Crossref] [PubMed]
- Zheng X, Zhang A, Xiao Y, et al. What Causes Death in Esophageal Cancer Patients Other Than the Cancer Itself: A Large Population-Based Analysis. J Cancer 2022;13:3485-94. [Crossref] [PubMed]
- Morota M, Gomi K, Kozuka T, et al. Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 2009;75:122-8. [Crossref] [PubMed]
- Ogino I, Watanabe S, Iwahashi N, et al. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther Onkol 2016;192:359-67. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078-85. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers (Version 3.2023) [Internet]. 2023 [cited 2023 Jan 10]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Hayashi Y, Iijima H, Isohashi F, et al. The heart's exposure to radiation increases the risk of cardiac toxicity after chemoradiotherapy for superficial esophageal cancer: a retrospective cohort study. BMC Cancer 2019;19:195. [Crossref] [PubMed]
- Cai G, Li C, Yu J, et al. Heart Dosimetric Parameters Were Associated With Cardiac Events and Overall Survival for Patients With Locally Advanced Esophageal Cancer Receiving Definitive Radiotherapy. Front Oncol 2020;10:153. [Crossref] [PubMed]
- Cai G, Li C, Li J, et al. Cardiac Substructures Dosimetric Predictors for Cardiac Toxicity After Definitive Radiotherapy in Esophageal Cancer. Int J Radiat Oncol Biol Phys 2023;115:366-81. [Crossref] [PubMed]
- Xi M, Xu C, Liao Z, et al. Comparative Outcomes After Definitive Chemoradiotherapy Using Proton Beam Therapy Versus Intensity Modulated Radiation Therapy for Esophageal Cancer: A Retrospective, Single-Institutional Analysis. Int J Radiat Oncol Biol Phys 2017;99:667-76. [Crossref] [PubMed]
- Shiraishi Y, Xu C, Yang J, et al. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol 2017;125:48-54. [Crossref] [PubMed]
- Zhou P, Du Y, Zhang Y, et al. Efficacy and Safety in Proton Therapy and Photon Therapy for Patients With Esophageal Cancer: A Meta-Analysis. JAMA Netw Open 2023;6:e2328136. [Crossref] [PubMed]
- Lin SH, Hobbs BP, Verma V, et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J Clin Oncol 2020;38:1569-79. [Crossref] [PubMed]
- ClinicalTrials.gov ID NCT03801876 [Internet]. 2023 [cited 2023 Jan 10]. Comparing Proton Therapy to Photon Radiation Therapy for Esophageal Cancer. Available online: https://clinicaltrials.gov/study/NCT03801876
- Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021;143:e254-743. [Crossref] [PubMed]
- Detloff LR, Ho EC, Ellis SG, et al. Coronary intravascular brachytherapy for in-stent restenosis: A review of the contemporary literature. Brachytherapy 2022;21:692-702. [Crossref] [PubMed]
- Piccolo R, Stefanini GG, Franzone A, et al. Safety and efficacy of resolute zotarolimus-eluting stents compared with everolimus-eluting stents: a meta-analysis. Circ Cardiovasc Interv 2015;8:e002223. [Crossref] [PubMed]
- Holmes DR Jr, Teirstein P, Satler L, et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA 2006;295:1264-73. [Crossref] [PubMed]
- Stone GW, Ellis SG, O'Shaughnessy CD, et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA 2006;295:1253-63. [Crossref] [PubMed]
- Räber L, Jüni P, Löffel L, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol 2010;55:1178-88. [Crossref] [PubMed]
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Eighth edition. Philadelphia: Wolters Kluwer; 2019. 597 p.
- Wiedermann JG, Marboe C, Amols H, et al. Intracoronary irradiation markedly reduces restenosis after balloon angioplasty in a porcine model. J Am Coll Cardiol 1994;23:1491-8. [Crossref] [PubMed]
- Waksman R, Bhargava B, Mintz GS, et al. Late total occlusion after intracoronary brachytherapy for patients with in-stent restenosis. J Am Coll Cardiol 2000;36:65-8. [Crossref] [PubMed]
- Michael TT, Papayannis AC, Banerjee S, et al. Subintimal dissection/reentry strategies in coronary chronic total occlusion interventions. Circ Cardiovasc Interv 2012;5:729-38. [Crossref] [PubMed]
- Ohri N, Sharma S, Kini A, et al. Intracoronary brachytherapy for in-stent restenosis of drug-eluting stents. Adv Radiat Oncol 2015;1:4-9. [Crossref] [PubMed]
- Loo BW Jr, Soltys SG, Wang L, et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol 2015;8:748-50. [Crossref] [PubMed]
- Sharma A, Wong D, Weidlich G, et al. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm 2010;7:802-10. [Crossref] [PubMed]
- Cuculich PS, Schill MR, Kashani R, et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med 2017;377:2325-36. [Crossref] [PubMed]
- Robinson CG, Samson PP, Moore KMS, et al. Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation 2019;139:313-21. [Crossref] [PubMed]
- Bergom C, Bradley JA, Ng AK, et al. Past, Present, and Future of Radiation-Induced Cardiotoxicity: Refinements in Targeting, Surveillance, and Risk Stratification. JACC CardioOncol 2021;3:343-59. [Crossref] [PubMed]
- van den Bogaard VAB, Spoor DS, van der Schaaf A, et al. The Importance of Radiation Dose to the Atherosclerotic Plaque in the Left Anterior Descending Coronary Artery for Radiation-Induced Cardiac Toxicity of Breast Cancer Patients? Int J Radiat Oncol Biol Phys 2021;110:1350-9. [Crossref] [PubMed]
- Wang X, Palaskas NL, Hobbs BP, et al. The Impact of Radiation Dose to Heart Substructures on Major Coronary Events and Patient Survival after Chemoradiation Therapy for Esophageal Cancer. Cancers (Basel) 2022;14:1304. [Crossref] [PubMed]
- Duane F, Aznar MC, Bartlett F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol 2017;122:416-22. [Crossref] [PubMed]
- Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:10-8. [Crossref] [PubMed]
- Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys 2011;81:1442-57. [Crossref] [PubMed]
- Haq R, Hotca A, Apte A, et al. Cardio-pulmonary substructure segmentation of radiotherapy computed tomography images using convolutional neural networks for clinical outcomes analysis. Phys Imaging Radiat Oncol 2020;14:61-6. [Crossref] [PubMed]
- Højris I, Overgaard M, Christensen JJ, et al. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Radiotherapy Committee of the Danish Breast Cancer Cooperative Group. Lancet 1999;354:1425-30. [Crossref] [PubMed]


