
Bilateral mastectomy associated with higher breast cancer mortality among patients with estrogen receptor positive progesterone receptor negative localized breast cancer
Highlight box
Key findings
• We found that among patients with estrogen receptor positive, progesterone receptor negative disease, bilateral mastectomy was associated with significantly higher risk of breast cancer mortality than lumpectomy.
What is known and what is new?
• Breast conservation therapy has been shown to be equally effective as mastectomy for patients with localized disease. The influence of the effects of composite estrogen and progesterone receptor status on surgical outcomes has not been studied.
• This manuscript demonstrates that patients with estrogen receptor positive, progesterone receptor negative disease have increased risk of breast cancer mortality with bilateral mastectomy than with lumpectomy.
What is the implication, and what should change now?
• These results provide evidence that for localized estrogen receptor positive, progesterone negative receptor negative breast cancer, bilateral mastectomy should be recommended against relative to lumpectomy due to its association with increased breast cancer mortality.
Introduction
The surgical management of invasive breast cancer changed drastically in response to two randomized controlled trials initiated in the mid 1970’s which showed no significant difference in overall and cancer-specific survival for patients undergoing lumpectomy with radiation relative to unilateral mastectomy for localized disease (1,2). Neither study required determination of hormone receptor status as a criterion for enrollment, an important limitation as hormone receptor status has since been found to have prognostic implications both independently as well as when combined with human epidermal growth factor receptor 2 (HER2) status as a clinical surrogate for molecular phenotype (3-7). Numerous retrospective studies have attempted to address the question of whether hormone receptor status influences the impact of surgical extent on clinical outcomes but importantly they have not treated estrogen and progesterone receptor (ER and PR) statuses independently (8-13). To our knowledge, no studies have examined the significance of the combination of ER and PR statuses on clinical outcomes with respect to surgical extent.
The study of ER and PR statuses on surgical outcomes is of interest because the breast is an endocrine gland with 10–30% of cells in the luminal epithelium of normal healthy breast tissue expressing ER, and a smaller proportion expressing PR (14,15). It is possible that the removal of an entire breast or both breasts may lead to alterations in hormone levels or any associated feedback pathways which could affect proliferation of disease in the contralateral breast or circulating or disseminated tumor cells at distant locations. If such endocrine changes were induced by mastectomy in breast cancer patients, it may be reflected in their long-term outcomes relative to patients undergoing lumpectomy. If such a phenomenon existed, it could help shed light on the curious finding that most retrospective analyses since the previously-mentioned pioneering randomized controlled trials have found improved overall survival or disease-specific survival with lumpectomy and radiation relative to mastectomy (13,16-23). A 2013 retrospective analysis from the California Cancer Registry suggested that the discrepancy in overall survival may be related to a higher burden of non-fatal comorbidities among patients undergoing mastectomy relative to lumpectomy with radiation (13). However, an explanation for the discrepancy in disease-specific survival has remained elusive.
Herein we aim to use data from the Surveillance, Epidemiology and End Results (SEER) program to identify if for patients with ER positive localized breast cancer the PR status influences the impact of lumpectomy, lumpectomy with radiation, unilateral mastectomy, and bilateral mastectomy on risk of ultimate breast cancer mortality. We present the following study in accordance with the STROBE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-22-87/rc).
Methods
Study approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was also conducted in accordance with U.S. Common Rule. The UCSD HRPP/IRB deferred need for approval due to use of public, de-identified data. Informed consent was not possible due to exclusive use of de-identified data.
Data extraction
For this retrospective cohort study, the SEER program 18 Registries database (1975–2016) was queried for female patients aged 40–70 diagnosed with a known first breast cancer that was unilateral and invasive without metastasis to the lymph nodes and without distant metastases. Patients were required to have known tumor size and tumor grade and laterality. Only patients who underwent surgery were included, and patients were excluded if they had identification of a contralateral breast cancer within 2 months of diagnosis of the initial breast cancer. Patients were also required to have known cancer-specific survival with follow-up of at least 2 months. Patients undergoing unilateral or bilateral mastectomy who also received radiation were excluded. Only patients with ER positive status and PR positive or negative status were included, and selected patients were categorized by PR status into two groups, PR+ and PR−. Treatment types were divided into the following four categories: lumpectomy without radiation, lumpectomy with radiation, unilateral mastectomy, and bilateral mastectomy. Patients lost to follow up were right-censored.
Statistical analysis
Within both the PR+ and PR− groupings, one-to-one nearest neighbor matching was performed from the bilateral mastectomy group (the smallest treatment group in both categories) to each of the other treatment groups with matching for age at diagnosis, tumor size, race, receipt of chemotherapy, and tumor grade. Competing risks analysis with non-cancer death as the competing event was performed to estimate cumulative incidence for each of these four treatment groups within both the PR+ and PR− categories. Confidence intervals for the cumulative incidence estimates were calculated using the method described by Marubini and Valsecchi (24). Multivariate competing risks regressions were then performed including each of the treatment groups using the same covariates used in matching for adjustment. The proportional hazards assumption for each covariate in both regressions was tested graphically. P values were calculated as two-sided and statistical significance was declared for P less than 0.05. All statistical analysis was performed in R (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria) using RStudio (Version 1.1.463) and packages “tidyverse” (Version 1.3.0), “survival” (Version 3.1-7), “survminer” (Version 0.4.6), and “cmprsk” (Version 2.2-9) (25,26).
Results
Cohort description
From an initial 763,873 patients with a first breast neoplasm documented in SEER, 83,520 patients met inclusion criteria (Figure 1). After one-to-one matching from the bilateral mastectomy group to each other treatment group for both the PR+ and PR− categories, 23,080 patients remained. The median follow-up time was 7.6 years (interquartile range, 4.0–8.3 years). Years of diagnosis were from 1998 to 2015. Median age at diagnosis was 52 years (interquartile range, 47–59 years). Median tumor size was 14.0 cm (interquartile range, 9.0–20.0 cm). Among patients, 7,066 (30.6%) received chemotherapy and 16,014 (69.4%) did not. There were 1,202 black patients (5.2%) and 21,878 (94.8%) non-black patients. The tumor was high grade or undifferentiated in 4,781 (20.7%) of cases, intermediate grade in 11,183 (48.5%) of cases, and low grade in 7,116 (30.8%) of cases. Among patients, 19,996 (86.6%) had PR+ disease and 3,084 (13.4%) of patients had PR− disease.
Bilateral mastectomy associated with higher breast cancer mortality among patients with PR− disease
Among patients with PR− disease, bilateral mastectomy was associated with a higher cumulative incidence of breast cancer mortality relative to patients undergoing lumpectomy with radiation, with a 10-year cumulative incidence of 9.2% [95% confidence interval (CI): 6.6–12.7%] for patients undergoing bilateral mastectomy and 4.4% (95% CI: 3.0–6.6%) for patients undergoing lumpectomy with radiation (Figure 2). This difference was significant in the adjusted multivariate model [hazard ratio (HR) =1.77; 95% CI: 1.12–2.82; P=0.02] (Table 1). The only other factor in the multivariate model that was significant was tumor size (HR =1.06; 95% CI: 1.04–1.07; P<0.001).
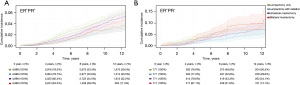
Table 1
| Treatment | ER+PR+ (N=19,996) | ER+PR− (N=3,084) | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Lumpectomy + RT | Reference | – | – | Reference | – | – | |
| Lumpectomy | 1.71 | 1.37–2.14 | <0.001 | 1.55 | 0.98–2.43 | 0.06 | |
| Unilateral mastectomy | 1.41 | 1.12–1.78 | 0.004 | 1.19 | 0.75–1.90 | 0.46 | |
| Bilateral mastectomy | 1.03 | 0.78–1.37 | 0.82 | 1.77 | 1.12–2.82 | 0.02 | |
| Race | |||||||
| Non-black | Reference | – | – | Reference | – | – | |
| Black | 1.73 | 1.28–2.33 | <0.001 | 1.44 | 0.88–2.35 | 0.15 | |
| Age | 1.02 | 1.01–1.03 | 0.004 | 1.00 | 0.98–1.02 | 0.72 | |
| Tumor size | 1.05 | 1.04–1.06 | <0.001 | 1.06 | 1.04–1.07 | <0.001 | |
| Chemotherapy | |||||||
| Not received | Reference | – | – | Reference | – | – | |
| Received | 1.10 | 0.91–1.33 | 0.32 | 0.79 | 0.55–1.12 | 0.19 | |
| Tumor grade | |||||||
| Low | Reference | – | – | Reference | – | – | |
| Intermediate | 1.54 | 1.22–1.95 | <0.001 | 1.52 | 0.90–2.56 | 0.12 | |
| High | 2.78 | 2.15–3.60 | <0.001 | 1.58 | 0.93–2.72 | 0.09 | |
ER, estrogen receptor; PR, progesterone receptor; N, number of patients; HR, hazard ratio; CI, confidence interval; RT, radiation therapy.
Lumpectomy and unilateral mastectomy associated with increased breast cancer mortality among patients with PR+ disease
Among patients with PR+ disease, the largest cohort (N=19,996), lumpectomy without radiation was associated with a higher cumulative incidence of breast cancer mortality, with a 10-year cumulative incidence of 4.5% (95% CI: 3.9–4.5%), as was unilateral mastectomy with a 10-year cumulative incidence of 4.0% (95% CI: 3.4–4.8%) relative to patients undergoing lumpectomy with radiation who had a 10-year cumulative incidence of 2.7% (95% CI: 2.1–3.3%). Patients undergoing bilateral mastectomy did not have a significantly different 10-year cumulative incidence of breast cancer mortality 2.9% (95% CI: 2.3–3.7%) relative to patients undergoing lumpectomy with radiation. In the adjusted multivariate model, lumpectomy without radiation (HR =1.71; 95% CI: 1.37–2.14; P<0.001) and unilateral mastectomy (HR =1.41; 95% CI: 1.12–1.78; P=0.004) were associated with increased hazard ratio for breast cancer mortality whereas bilateral mastectomy (HR =1.03; 95% CI: 0.78–1.37; P=0.82) was not significantly different from patients undergoing lumpectomy with radiation. Other factors that were significant in the multivariate model included black race (HR =1.73; 95% CI: 1.28–2.33; P<0.001), age at diagnosis (HR =1.02; 95% CI: 1.01–1.03; P=0.004), and intermediate grade (HR =1.54; 95% CI: 1.22–1.95; P<0.001) and high grade (HR =2.78; 95% CI: 2.15–3.60; P<0.001) disease.
Discussion
Here we demonstrate that the relative effect of surgical extent on breast cancer mortality for patients with localized ER positive disease is affected by the PR status of the associated cancer. The effect is most interesting for patients undergoing bilateral mastectomy. It would seem intuitive that patients undergoing bilateral mastectomy for localized unilateral disease should at worst have no difference from and at best gain a benefit over patients undergoing lumpectomy with radiation due to the elimination of any potential future sources of development of cancer. While this was the case in our study for patients with PR+ disease, for whom patients undergoing bilateral mastectomy did not have significantly different breast cancer mortality from patients undergoing lumpectomy with radiation, surprisingly among patients with PR− disease, bilateral mastectomy was associated with a significantly increased risk of breast cancer mortality relative to lumpectomy with radiation, and after 6 years even surpassed lumpectomy without radiation to have the highest cumulative incidence of breast cancer mortality among all the treatment groups.
The finding of increased breast cancer mortality among patients undergoing bilateral mastectomy for PR− disease is important, because a rise in contralateral prophylactic mastectomies for both localized and advanced disease has been noted in North America during the past few decades in spite of a wealth of evidence demonstrating that breast conservation therapy is as effective as mastectomy for localized breast cancer (27-31). The factors contributing to selection of contralateral prophylactic mastectomy that have been identified in patient surveys include patient perceived decreased recurrence risk, patient perceived improved survival rate, desire to avoid frequent surveillance, and detection of occult disease on a preoperative magnetic resonance imaging (MRI) (28,32,33). While detection of occult disease may require more complex management decisions, for patients opting for contralateral prophylactic mastectomy due to perceived risk reduction or desire to avoid surveillance, the knowledge that they may be putting themselves at higher risk of ultimate breast cancer death may have the potential to change their decision. Moreover, if this knowledge were available to surgeons counseling them, and if those surgeons actively recommended against mastectomy when possible, it may deter patients from pursuing such over-aggressive surgery, as in one study 19% of average risk women underwent contralateral prophylactic mastectomy when their surgeon was ambivalent about it compared to 1.9% of women when the surgeon recommended against it (34).
One reason why surgeons may be ambivalent towards bilateral mastectomy versus breast conservation therapy is the perceived absence of additional oncologic risk by means of more extensive surgery. If this assumption were accurate, the only additional medical risk conferred would be those of operative complications, such that surgeons confident in their abilities and complication rates may not consider this adequate justification to recommend against mastectomy. But our results suggest that for patients with PR− disease, this is not the case, as bilateral mastectomy was associated with increased risk of ultimate breast cancer mortality.
The reason for the puzzling significant change in outcomes for patients undergoing bilateral mastectomy for ER positive disease depending on PR status cannot be addressed by this retrospective cohort study. On consideration of our results, however, we have generated an easily testable hypothesis which if confirmed could help to partially explain these findings. Estrogen exposure is a known risk factor for the development of breast cancer (35), so an increase in circulating estrogen could induce a proliferative response in any circulating or disseminated tumor cells expressing ER. PR, on the other hand, inhibits ER transcription activity through the PR A isoform, and in one study administration of progesterone to ERα+ cell line xenografts and human tumor explants inhibited estrogen-mediated growth of the xenografts and tumor explants (7,15). There is evidence of increased circulating estrogen and progesterone levels in bovine models after removal of all of the breast glandular tissue via mastectomy, with a 2002 study demonstrating significantly increased levels of circulating estrone, estradiol and progesterone in mastectomized cows relative to non-mastectomized cows (36). If human women had a similar rise in circulating estrogens after bilateral mastectomy, it is possible that the increased circulating estrogens may promote growth of any circulating or disseminated ER expressing tumor cells. Furthermore, in cases of PR− disease, the absence of an inhibitory effect of PR stimulation on ERα may lead to enhanced growth relative to PR+ tumor cells.
In contrast to bilateral mastectomy, both lumpectomy without radiation and unilateral mastectomy were associated with an increased risk of breast cancer mortality in patients with PR+ disease and an unchanged risk of breast cancer mortality in patients with PR− disease. It is possible, however, that the absence of a significantly elevated risk of breast cancer mortality in the PR− group was secondary to the much smaller cohort size (N=3,084) relative to the PR+ group (N=19,996) and not due to a true difference in outcomes, as the hazard ratios in the multivariate competing risks regressions for both PR+ and PR− disease were comparable. In both the PR+ and PR− groups, patients undergoing lumpectomy with radiation had the lowest cumulative incidence of breast cancer mortality. This treatment option therefore represents the best approach to reducing risk of ultimate breast cancer mortality for the majority of patients with ER positive disease.
The risk of development of a contralateral breast cancer would be interesting to compare between the PR+ and PR− groups by treatment type, but this unfortunately cannot be meaningfully studied in SEER. Ipsilateral invasive recurrences within 5 years of the initial cancer diagnosis are not given independent entries in the SEER database, so additional treatments that may be administered for an ipsilateral recurrence including greater surgical extent, radiation in a patient who has not previously received it, or systemic therapies preclude the meaningful comparison of development of contralateral cancers.
There are several important limitations to our study. As a retrospective cohort study, in spite of our attempts to match and adjust for all of the important covariates available to us, there are many confounders that cannot be accounted for because they are not provided by SEER. For example, there are no measures of health status, as significant cardiopulmonary comorbidities may push a surgeon away from treatment plans including radiation or bilateral mastectomy. Family history and data on genetic testing is also absent, as such factors may account for some patients undergoing bilateral mastectomy. Also of note, SEER does not provide data on endocrine therapy, an important limitation in the analysis of the responses to the various treatment options for patient with ER positive disease. And finally, HER2 status is only available for patients in SEER diagnosed after 2010, so we were unable to include it in our analysis. While our interest was in the hormone receptor statuses, HER2 could have been used as an adjustment factor for the multivariate regressions if it were available. It is also for this reason that we chose not to evaluate patients with ER−PR− disease, as the HER2 status in this cohort has important prognostic implications which we would not be able to account for. We minimized auto-selection and no-participation biases by use of SEER as our source of data, as it is a national population-based registry. We also minimized selection bias with respect to the outcomes of interest by performing competing risks regression to account for confounding factors that may not be accountable in the covariates of interest toward the risk of non-breast cancer death, so as to more accurately assess risk for breast cancer mortality. Due to the use of retrospective cohort data we were unable to minimize information bias with respect to missing data, and opted to perform a complete-case analysis. We attempted to minimize confounding bias by performing a matched, multivariate analysis.
Conclusions
In conclusion, herein we have shown that among patients with ER positive localized breast cancer, PR status interacts with surgical extent to affect risk of ultimate breast cancer mortality. Lumpectomy with radiation was associated with the lowest breast cancer mortality among patients. Bilateral mastectomy was associated with significantly increased risk among patients with PR− disease, and no significant difference in breast cancer mortality for patients with PR+ disease. Lumpectomy without radiation and unilateral mastectomy were associated with increased breast cancer mortality risk for patients with PR+ disease, and unchanged risk for patients with PR− disease. Though limited by its retrospective nature, our results suggest that bilateral mastectomy should be avoided when possible for patients with localized PR− breast cancer, and that lumpectomy with radiation should be preferentially selected for patients with ER positive disease, regardless of PR status. Further studies are needed to identify whether changes in circulating hormone levels may account for the differential effect of PR status on patients with ER positive breast cancer undergoing bilateral mastectomy.
Acknowledgments
We acknowledge the National Cancer Institute, specifically the SEER team, for granting us access to SEER data.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-22-87/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-22-87/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-87/coif). OH has served as an expert witness in a patent law infringement case, owns stocks in Zentalis Pharmaceuticals, Novartis, Sanofi, and Abbvie, and is currently an employee of Zentalis pharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was also conducted in accordance with U.S. Common Rule.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Fisher B, Redmond C, Fisher ER, et al. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol 1988;6:1076-87. [Crossref] [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Parise CA, Caggiano V. Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immunohistochemical Biomarkers. J Cancer Epidemiol 2014;2014:469251. [Crossref] [PubMed]
- Mohammed H, Russell IA, Stark R, et al. Progesterone receptor modulates ERα action in breast cancer. Nature 2015;523:313-7. Erratum in: Nature 2015;526:144. [Crossref] [PubMed]
- Brewster AM, Bedrosian I, Parker PA, et al. Association between contralateral prophylactic mastectomy and breast cancer outcomes by hormone receptor status. Cancer 2012;118:5637-43. [Crossref] [PubMed]
- Kurian AW, Canchola AJ, Ma CS, et al. Magnitude of reduction in risk of second contralateral breast cancer with bilateral mastectomy in patients with breast cancer: Data from California, 1998 through 2015. Cancer 2020;126:958-70. [Crossref] [PubMed]
- Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after contralateral prophylactic mastectomy: a decision analysis. J Natl Cancer Inst 2014;106:dju160. [Crossref] [PubMed]
- Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 2015;150:9-16. [Crossref] [PubMed]
- Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA 2014;312:902-14. [Crossref] [PubMed]
- Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013;119:1402-11. [Crossref] [PubMed]
- Clarke RB, Howell A, Potten CS, et al. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 1997;57:4987-91. [PubMed]
- Li Z, Wei H, Li S, et al. The Role of Progesterone Receptors in Breast Cancer. Drug Des Devel Ther 2022;16:305-14. [Crossref] [PubMed]
- Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014;149:267-74. [Crossref] [PubMed]
- Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: A systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 2015;24:175-81. [Crossref] [PubMed]
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158-70. [Crossref] [PubMed]
- Lagendijk M, van Maaren MC, Saadatmand S, et al. Breast conserving therapy and mastectomy revisited: Breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer 2018;142:165-75. [Crossref] [PubMed]
- Hartmann-Johnsen OJ, Kåresen R, Schlichting E, et al. Survival is Better After Breast Conserving Therapy than Mastectomy for Early Stage Breast Cancer: A Registry-Based Follow-up Study of Norwegian Women Primary Operated Between 1998 and 2008. Ann Surg Oncol 2015;22:3836-45. [Crossref] [PubMed]
- Corradini S, Reitz D, Pazos M, et al. Mastectomy or Breast-Conserving Therapy for Early Breast Cancer in Real-Life Clinical Practice: Outcome Comparison of 7565 Cases. Cancers (Basel) 2019;11:160. [Crossref] [PubMed]
- Ji J, Yuan S, He J, et al. Breast-conserving therapy is associated with better survival than mastectomy in Early-stage breast cancer: A propensity score analysis. Cancer Med 2022;11:1646-58. [Crossref] [PubMed]
- Ratosa I, Plavc G, Pislar N, et al. Improved Survival after Breast-Conserving Therapy Compared with Mastectomy in Stage I-IIA Breast Cancer. Cancers (Basel) 2021;13:4044. [Crossref] [PubMed]
- Marubini E, Valsecchi MG. Analysing Survival Data from Clinical Trials and Observational Studies. Chichester: J. Wiley, 2004.
- Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat 1988;16:1141-54. [Crossref]
- Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94:496-509. [Crossref]
- Findlay-Shirras L, Lima I, Smith G, et al. Canada follows the US in the rise of bilateral mastectomies for unilateral breast cancer: a 23-year population cohort study. Breast Cancer Res Treat 2021;185:517-25. [Crossref] [PubMed]
- Jerome-D'Emilia B, Suplee PD, D'Emilia I. Why Women Are Choosing Bilateral Mastectomy. Clin J Oncol Nurs 2015;19:764-8. [Crossref] [PubMed]
- Pollom EL, Qian Y, Chin AL, et al. Rising rates of bilateral mastectomy with reconstruction following neoadjuvant chemotherapy. Int J Cancer 2018;143:3262-72. [Crossref] [PubMed]
- Panchal H, Pilewskie ML, Sheckter CC, et al. National trends in contralateral prophylactic mastectomy in women with locally advanced breast cancer. J Surg Oncol 2019;119:79-87. [Crossref] [PubMed]
- Lim DW, Metcalfe KA, Narod SA. Bilateral Mastectomy in Women With Unilateral Breast Cancer: A Review. JAMA Surg 2021;156:569-76. [Crossref] [PubMed]
- Fisher CS, Martin-Dunlap T, Ruppel MB, et al. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol 2012;19:3246-50. [Crossref] [PubMed]
- Mukherjee SD, Hodgson N, Lovrics PJ, et al. Surgical attitudes toward preoperative breast magnetic resonance imaging in women with early-stage breast cancer. Curr Oncol 2019;26:e194-201. [Crossref] [PubMed]
- Tan MP, Silva E. Addressing the paradox of increasing mastectomy rates in an era of de-escalation of therapy: Communication strategies. Breast 2018;38:136-43. [Crossref] [PubMed]
- Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res 2003;5:239-47. [Crossref] [PubMed]
- Goff JP, Kimura K, Horst RL. Effect of mastectomy on milk fever, energy, and vitamins A, E, and beta-carotene status at parturition. J Dairy Sci 2002;85:1427-36. [Crossref] [PubMed]



