
Tumor treating fields with radiation for glioblastoma: a narrative review
Introduction
Glioblastoma (GBM) is the most common subtype of primary malignant brain tumors in adults (1). Despite several treatment advances over the past few decades, the overall prognosis remains poor and new strategies are currently under investigation, including the use of tumor treating fields (TTFields) concurrently with chemoradiation (2,3).
The current management paradigm for glioblastoma (GBM) includes maximal safe surgical resection followed by concurrent chemoradiation with further temozolomide (TMZ) and TTFields used as maintenance therapy as defined by several landmark, randomized clinical trials (4-7). TMZ, when given both concurrently and as part of maintenance therapy, showed improvements in both progression-free survival (PFS) and overall survival (OS) (4,5). Patients with and without O6-methylguanine-DNA-methyltransferase (MGMT) silencing, a prognostic indicator, benefit with receipt of TMZ, although with more improved results in the methylated subgroup (8). Although initially approved for the treatment of recurrent and refractory GBM, TTFields soon became a part of maintenance therapy based on the significant improvements in PFS and OS observed in patients receiving concurrent chemoradiation (6,7). TTFields uses low-intensity, intermediate frequency (200 kHz) alternating electric fields to disrupt cellular processes including proliferation and replication (9,10). Due to the significant benefit observed with maintenance TTFields, several preclinical and clinical studies have investigated whether concurrent treatment offers a synergistic and feasible strategy. Given the growing evidence showing safety of combining radiation with TTFields in patients with GBM, as well as the currently enrolling phase 3, randomizing clinical trial (EF-32; NCT04471844) aimed at determining the clinical benefit of this approach, we have put together the following narrative review to highlight the work that has been done in this space.
In this review, we will discuss the preclinical work which demonstrates the complexity of the mechanisms by which TTFields operates and the significant interplay between TTFields and radiotherapy. We will review the clinical studies that have been performed which demonstrate the safety and feasibility of utilizing a combination approach. Lastly, we will review treatment techniques, including scalp sparing methodology and modified computed tomography (CT) simulation workflow. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-22-90/rc).
Methods
PubMed, Medline, Embase, Cochrane Library, and various center-specific guidelines were searched for literature regarding concurrent chemoradiation with TTFields for patients with GBM on August 1, 2022 (Table 1). Databases were searched using combinations of TTFields, radiation, and GBM based on both MeSH headings and text words. MeSH terms used included, but were not limited to, “TTFields”, “glioblastoma”, “concurrent therapy”, “radiotherapy”, “preclinical”, “clinical”, “scalp”, “toxicity”, “feasibility”, and “safety” in various combinations. Titles and abstracts were screened for relevant articles and studies. References from full-text articles were screened for additional studies. All co-authors contributed, reviewed, and approved the selected literature for this review.
Table 1
| Items | Specification |
|---|---|
| Date of search | August 1, 2022 |
| Databases and other sources searched | PubMed, Medline, Embase, Cochrane Library, center-specific guidelines |
| Search terms used | TTFields, tumor treating fields, glioblastoma, concurrent therapy, radiotherapy, preclinical, clinical, scalp, toxicity, feasibility, safety |
| Timeframe | All studies obtained were from 2000–current |
| Inclusion and exclusion criteria | All studies included were available in English language. Clinical studies involving concurrent TTFields with radiation must have explicitly stated that both treatments were provided at the same time |
| Selection process | All authors contributed and reviewed the selected literature |
Preclinical data
A major target for TTFields, which is shared by radiotherapy, is DNA. DNA fragmentation resulting in reduced clonogenicity has been reported in pancreatic cells exposed to TTFields (11-13). Radiotherapy, like TTFields, also disrupts the cell division machinery (14). These commonalities set the stage for a synergistic interaction between the two treatment modalities.
Mitotic catastrophic rate is defined as the rate of microtubule polymerization and depolymerization during mitosis. Radiation therapy (RT) stresses target cells, causing an unbalanced catastrophic rate and an inappropriate entry of cells into mitosis, which consequently causes cell death (15). Such unbalanced catastrophic rates and mitotic abnormalities have also been ascribed to TTFields by various studies (9,10). These similarities in mechanisms of action led an in vivo study to apply TTFields for 24 hours followed immediately by RT to doses ranging between 2 and 6 Gy to human GBM cell lines (9). This resulted in cells with multi-nucleation phenotypes and mono- and multi-polar spindle structures, events that led to a catastrophic rate that was significantly greater than that achieved by either treatment modality on its own. In addition, they demonstrated a blockade of DNA repair and decreased glioma cell survival, thus suggesting that TTFields radiosensitizes cancer cells.
Such synergy was duplicated by another in vivo study that reversed the sequence of treatment, applying TTFields for 72 hours after RT to glioma cell lines (10). In contrast to the previous study, this particular treatment sequence did not impact the early steps of damage recognition and repair, as evidenced by unchanged levels of γ-H2AX between the combination treatment or treatment with TTFields or RT only. Moreover, the non-homologous end joining response with the sequential treatment was also unchanged from the response from RT alone. However, RAD51 foci formation was increased for 24 hours after irradiated cells were exposed to TTFields, which signals a disruption of the homologous recombination repair pathway. This consequently resulted in a significantly reduced cell survival with the combination treatment and showed that in vitro, TTFields improved radiation efficacy by inhibiting or delaying DNA damage repair
The synergy between RT and TTFields regardless of their application sequence could mean that subjecting GBM cells to TTFields while undergoing RT may similarly potentiate the desired cytotoxicity as compared to RT alone.
Clinical data
Several clinical trials have investigated the safety and efficacy of the concurrent tri-modality therapy. Currently, two pilot trials evaluated the safety and feasibility of concurrent TTFields and radiation (2,3). A pilot trial by Bokstein et al. demonstrated the feasibility and safety of TTFields administered concurrently with radiation and TMZ in GBM (2). In this study, patients received standard daily TMZ (75 mg/m2 daily) together with radiation at total dose of 60 Gy given in 30 fractions. TTFields were applied during the radiotherapy period (and continued use after for up to 24 months or second disease progression) starting within 1 week of the radiotherapy treatment start date. The electrode array in this study was removed during the actual radiation treatment. After clinical and radiological evaluation of treatment response was performed approximately 4 weeks after the end of RT with TTFields and TMZ, patients eligible for adjuvant treatment started monthly TMZ combined with TTFields treatment. 10 total patients were enrolled with a median Karnofsky performance score (KPS) of 90 and 5 (50%) underwent biopsy only. Adverse effects (AEs) with the combined therapies were evaluated. The most common AE related to TTFields treatment was skin toxicity, reported in 8 (80%) patients, and was of low severity (Common Terminology Criteria for Adverse Events, or CTCAE version 5.0, grade 1–2). Skin reactions included application site erythema, erosions, blisters, dermatitis, seborrheic keratosis, eczema, and pruritus in the skin areas covered by the transducer arrays. No delays in radiotherapy were related to TTFields treatment. No severe AEs (CTCAE grade ≥3) were attributed to TTFields treatment. Preliminary data from the study showed a median PFS of 8.9 months.
A separate pilot study was conducted which enrolled 30 patients (3). Patients received concurrent chemoradiotherapy (75 mg/m2 daily with 60 Gy in 30 fractions) and TTFields with radiation treatment delivered through the TTFields arrays. Median KPS was 90, 18 (60%) patients had a subtotal resection and 12 (40%) patients had multifocal disease at presentation. Skin adverse events were noted in 25 (83.3%) of patients and were limited to CTCAE grade 1–2. No grade 3 or higher adverse events were noted. The primary endpoint of the study was met and no TTFields discontinuation occurred due to high grade scalp toxicity. There was negligible change in mental status or quality of life between baseline and the concurrent phase of treatment as measured by a Mini-Mental State Examination (MMSE), EORTC Core Quality of Life questionnaire (QLQ-C30 version 3), and a brain cancer-specific health-related quality of life questionnaire (QLQ-BN20). The median PFS was 9.3 months.
As evidenced by these two pilot studies, the most common toxicity of concurrent radiation with TTFields is scalp dermatitis, and therefore, caution must be considered with regard to skin dose. Scalp sparing techniques, as well as additional treatment considerations are discussed below.
Practical guide for concurrent TTFields with radiation
Previous work has demonstrated that compliance with TTFields and longer use lead to better outcomes, with >90% usage leading to a median survival of 24.9 months on an EF-14 subgroup analysis (16). Utilizing the device in the setting of 6 weeks of daily treatment creates a logistical challenge to achieve high level compliance (≥18 hours daily). In addition, there are barriers to accepting TTFields from a patient experience, with approximately 65% declining this treatment due to personal reasons or lack of social support when offered in both the primary and recurrent settings (17). Lastly, daily removal and reapplication of the electrodes to accommodate radiation treatments may increase skin irritation, thus confounding skin toxicity outcomes. In order to combat these challenges and provide an optimal duration for TTFields usage, alternative strategies have been suggested which allow patients to maintain electrode arrays in place during daily radiotherapy treatments. The benefits and consequences of radiation being delivered with TTFields arrays in place is summarized in Table 2.
Table 2
| Pros/cons | Radiation with TTFields arrays in place | Radiation with TTFields arrays off at time of treatment |
|---|---|---|
| Pros | Minimize interruption leads to better synergy with radiation | No bolus effect at time of treatment |
| Minimize physical scalp injury or irritation with daily array changes | No radiation dosimetry changes | |
| Compliance improved with less frequent changing | No radiation workflow changes | |
| IGRT more straightforward | ||
| Cons | Dosimetric considerations (bolus effect increases scalp dose, mild/negligible PTV dosimetry changes) | Daily array removal may lead to physical scalp injury |
| Need modification of standard radiation simulation workflow | Interruption of treatment leads to loss of optimal synergy with radiation | |
| Imaging artifact on CBCT image guidance, requiring kV-kV imaging guidance | Potential for poorer compliance and reduced daily usage |
TTFields, tumor treating fields; IGRT, image-guided radiation therapy; PTV, planning target volume; CBCT, cone beam computed tomography.
Dosimetric considerations
Removing and replacing the electrodes on a daily basis during radiotherapy decreases the total time TTFields can be used. One way to minimize this interruption, is to deliver radiation treatment through TTFields arrays, similar to what was performed in the aforementioned pilot study. However, transducer arrays placed on the surface of the patients receiving radiation can have dosimetric effects. Li et al. investigated the delivery of radiotherapy via volumetric-modulated-arc-therapy (VMAT) with and without TTFields electrodes on the Anderson RANDO phantom (18). The presence of the TTFields electrodes created additional buildup effect that increased surface dose (130–260%) directly underneath the probes. Attenuation by the electrodes on deep dose measurements was of much smaller magnitude, between 1–2%. A mean planning target volume (PTV) dose reduction of 0.5–1% was observed, as was a mean increase in scalp dose of 0.5–1 Gy. These results demonstrate that tumor dose is unlikely to be compromised due to blocking from the electrodes, however, care must be given when evaluating skin dose. Although no significant increase was observed in the treatment planning system using commonly evaluated dose volume histogram (DVH) parameters in this study, scalp dose physical measurements increased by a factor of 1.3–2.6 in open field and VMAT deliveries.
The results of the aforementioned study are further validated by additional work (19-21). Guberina et al. showed that dose deviations in the clinical target volume (CTV) were limited to a 2% underdosage due to transducer arrays in 7 patients evaluated on a combined modality pilot study (19). In addition, in comparison to a treatment plan created without electrodes, scalp overdosage was limited to 8.5% of the prescribed dose in the first 2 mm below and in deeper layers. Straube et al. utilized a RW3 slab phantom and compared megavoltage (MV)-CT based planned dose with measured dose (20). The group found attenuation due to TTFields arrays was 3.4%, 3.7%, and 2.7% for depths of 2, 3, and 4 cm, respectively. Dose conformity and homogeneity were not affected by the TTFields arrays and organs at risk (OAR) doses were only modestly affected (increase or decrease by <0.5 Gy). Stachelek et al. found that a slight bolus effect is observed with TTFields arrays (mean increase in skin D1 cc and D20 cc of 3.1%) but that PTV V97% and D97% were decreased by only 1.7% and 2.7% or less, respectively (21). When arrays were repositioned, dosimetric changes were minimized further.
Simulation and treatment delivery
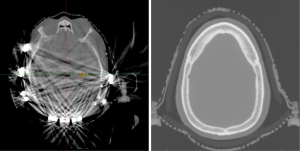
When patients are planned for treatment through the TTFields arrays, they are first simulated without these arrays, in the supine position, utilizing a thermoplastic mask. We recommend patients be simulated without TTFields arrays for several reasons: (I) arrays can cause significant CT artifact, and thus, radiation calculation is inaccurate (Figure 1); (II) arrays will be shifted slightly throughout the radiation course; (III) it avoids delay of radiation planning due to waiting for array mapping and equipment delivery.
At the time of simulation, a custom low-density foam, such as latex free open cell styrene butadiene rubber (SBR) foam or 3M Reston self-adhering foam, is created for the patient (Figure 2). For each patient, anterior and posterior foam pads are created. The posterior foam pad is first prepared by cutting 20 cm of length and adhering to the head rest for support. Next, the anterior foam pad is prepared by cutting 45–50 cm of length (can be adjusted based on patient anatomy). Four separate triangles are then cut in the anterior foam pad to allow for proper folding and fitting over the head. The foam pad is then placed over the patient’s head and adjusted for fit. The foam pad should be kept above the ears. Once the foam is placed, the thermoplastic mask should then be fitted to the patient and allowed to conform over the foam. Once the mask is cooled and firm, the foam is then adhered to the inside of the mask for use during daily treatments. Since the foam has a low density closely approximating that of air, there is minimum bolus effect when used and no increase in scalp dose as a result. The main purpose of the foam is to provide a space between the head mask and patient, in order to better accommodate the TTFields arrays.

With regard to treatment, the power supply to TTFields is discontinued before radiation and the device is left outside treatment room. Each day of radiation treatment, patients receive image-guided radiation therapy (IGRT) to ensure proper positioning. Array shifts are possible during the 6-week course of radiotherapy, however with IGRT, the setup is reproducible and accurate, regardless of the absence or presence of arrays, or with array position change. As a result, for the days that patients are not wearing their TTFields device, the setup can still be done correctly. Once radiotherapy treatments are finished, the TTFields device is reconnected and resumed promptly.
Scalp management considerations
As demonstrated in global post-marketing safety surveillance, the most common side effect of TTFields is scalp irritation (22). For newly diagnosed GBM and recurrent GBM, array-associated skin reaction occurred in 38% and 29% of patients, respectively. Other device-related skin AEs include heat or electric sensations, pruritus, hyperhidrosis, and in more severe cases, skin erosions/ulcers (each ≤1%) and wound complications including dehiscence and infection (≤1%). Given the known association with radiation and skin dermatitis, it is important to minimize overlapping scalp toxicity for the successful completion of concurrent treatment.
Prophylactic interventions to minimize skin toxicity prior to placement of TTFields arrays include optimal shaving to maximize transducer-skin contact, removal of natural oils or moisture from the scalp, regular transducer array changes (at least 2 times per week), and regular array repositioning to minimize direct pressure on the scalp and avoid surgical scar lines (23). With regard to array shifts, they should be shifted back to their original position to ensure optimal targeting of the tumor bed (24). At every array change, the skin and scalp should be assessed for signs and symptoms of irritation and proper counseling should be provided in order to maximize risk reduction.
If skin toxicities, including hyperhidrosis, dermatitis, or ulcers, do develop despite these prophylactic measures, then various management strategies can be performed. In general, topical corticosteroids (included betamethasone or clobetasol) can be used for irritant or contact dermatitis with topic antibiotics (such as clindamycin or gentamicin) reserved for skin ulcers and infection (25-27). Efforts should be made to limit petroleum-based formulations of these agents as they can affect electrical impedance of TTFields (28-30). Attempts should be made to minimize treatment breaks at the risk of compromising treatment outcomes.
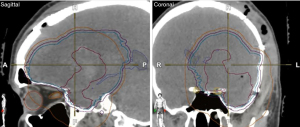
In addition to these preventative and pharmacologic interventions, another important measure is use advanced radiation planning techniques to minimize scalp dose. We recommend use VMAT scalp sparing planning. As a practical means of taking scalp dose into account, total scalp is defined as 5 mm thickness from skin surface above the level of foramen magnum (Figure 3). The area between lateral canthus, and below the orbital roof may be eliminated. The scalp is then used as an avoidance structure for planning, with the following dose constraints: mean <20 Gy (acceptable <30 Gy), D20 cc <50 Gy (acceptable <55 Gy), and D30 cc <40 Gy (acceptable <50 Gy) (18). Scalp constraints may be violated to ensure adequate PTV coverage. On the 30 patients pilot trial discussed previously, the mean dose median was 8.3 Gy, the D20 cc median was 25.9 Gy, and the D30 cc median was 23.5 Gy (3). From a practicality perspective, these are minimum goals and lower doses are frequently achievable. In order to assist with these goals, the authors recommend identification of the bone plate on CT. A 3 cm radial expansion of the bone plate should be made with the overlying scalp from the outer bone plate to skin surface defined as spared scalp. In addition, the CTV should be modified to the inner plate of the calvarium to make scalp sparing more feasible (Figure 4).
Conclusions
TTFields has led to significant improvements in outcome for patients diagnosed with GBM. Previous work has demonstrated the clinical benefit of administering this therapy as maintenance and more recent work has shown that concurrent administration is both feasible and offers biologic synergy. Although tumor dosing does not appear to be significantly affected by electrode array placement, significant attention needs to be given to scalp dose measurements to minimize toxicity. As discussed in this review, a challenge of concurrent TTFields with radiation is scalp dermatitis. Strategies including scalp structure implementation in the planning process, as well as foam cutouts at the time of CT simulation provide a means of safely delivering treatment and future work should look to further reduce toxicity through dosimetric means and medical interventions during therapy. With the feasibility of combined therapy established, current work is underway to establish whether a clinical benefit exists. EF-32 is a phase 3, randomized study (ClinicalTrials.gov: NCT04471844) currently enrolling that is comparing chemoradiation with TTFields vs. standard chemoradiation with a primary endpoint of median OS and secondary endpoints including median PFS and overall radiological response. With the results of this trial, the potential clinical benefit of tri-modality therapy will be established in patients with GBM.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Chinese Clinical Oncology for the series “Recent Advances in Neuro-Oncology”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-22-90/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-90/coif). The series “Recent Advances in Neuro-Oncology” was commissioned by the editorial office without any funding or sponsorship. WS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Chinese Clinical Oncology from October 2019 to September 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gandhi S, Tayebi Meybodi A, Belykh E, et al. Survival Outcomes Among Patients With High-Grade Glioma Treated With 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis. Front Oncol 2019;9:620. [Crossref] [PubMed]
- Bokstein F, Blumenthal D, Limon D, et al. Concurrent Tumor Treating Fields (TTFields) and Radiation Therapy for Newly Diagnosed Glioblastoma: A Prospective Safety and Feasibility Study. Front Oncol 2020;10:411. [Crossref] [PubMed]
- Miller R, Song A, Ali A, et al. Scalp-Sparing Radiation With Concurrent Temozolomide and Tumor Treating Fields (SPARE) for Patients With Newly Diagnosed Glioblastoma. Front Oncol 2022;12:896246. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [Crossref] [PubMed]
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015;314:2535-43. [Crossref] [PubMed]
- Stupp R, Taillibert S, Kanner A, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017;318:2306-16. [Crossref] [PubMed]
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997-1003. [Crossref] [PubMed]
- Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res 2004;64:3288-95. [Crossref] [PubMed]
- Kirson ED, Dbalý V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A 2007;104:10152-7. [Crossref] [PubMed]
- Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Sci Rep 2015;5:18046. [Crossref] [PubMed]
- Neuhaus E, Zirjacks L, Ganser K, et al. Alternating Electric Fields (TTFields) Activate Cav1.2 Channels in Human Glioblastoma Cells. Cancers (Basel) 2019;11:110. [Crossref] [PubMed]
- Giladi M, Schneiderman RS, Porat Y, et al. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014;14:54-63. [Crossref] [PubMed]
- Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys 2004;59:928-42. [Crossref] [PubMed]
- Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ 2008;15:1153-62. [Crossref] [PubMed]
- Toms SA, Kim CY, Nicholas G, et al. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol 2019;141:467-73. [Crossref] [PubMed]
- Onken J, Staub-Bartelt F, Vajkoczy P, et al. Acceptance and compliance of TTFields treatment among high grade glioma patients. J Neurooncol 2018;139:177-84. [Crossref] [PubMed]
- Li T, Shukla G, Peng C, et al. Dosimetric Impact of a Tumor Treating Fields Device for Glioblastoma Patients Undergoing Simultaneous Radiation Therapy. Front Oncol 2018;8:51. [Crossref] [PubMed]
- Guberina N, Pöttgen C, Kebir S, et al. Combined radiotherapy and concurrent tumor treating fields (TTFields) for glioblastoma: Dosimetric consequences on non-coplanar IMRT as initial results from a phase I trial. Radiat Oncol 2020;15:83. [Crossref] [PubMed]
- Straube C, Oechsner M, Kampfer S, et al. Dosimetric impact of tumor treating field (TTField) transducer arrays onto treatment plans for glioblastomas - a planning study. Radiat Oncol 2018;13:31. [Crossref] [PubMed]
- Stachelek GC, Grimm J, Moore J, et al. Tumor-Treating Field Arrays Do Not Reduce Target Volume Coverage for Glioblastoma Radiation Therapy. Adv Radiat Oncol 2019;5:62-9. [Crossref] [PubMed]
- Shi W, Blumenthal DT, Oberheim Bush NA, et al. Global post-marketing safety surveillance of Tumor Treating Fields (TTFields) in patients with high-grade glioma in clinical practice. J Neurooncol 2020;148:489-500. [Crossref] [PubMed]
- Lacouture ME, Anadkat MJ, Ballo MT, et al. Prevention and Management of Dermatologic Adverse Events Associated With Tumor Treating Fields in Patients With Glioblastoma. Front Oncol 2020;10:1045. [Crossref] [PubMed]
- Novocure. Optune®: Patient Information and Operation Manual. 2019. (Accessed August 2, 2022). Available online: https://www.optune.com/content/pdfs/Optune_PIOM_8.5x11.pdf
- Lacouture ME, Davis ME, Elzinga G, et al. Characterization and management of dermatologic adverse events with the NovoTTF-100A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol 2014;41:S1-14. [Crossref] [PubMed]
- Lacouture ME, DeNigris J, Kanner AA. Supportive care in patients using tumor treating fields therapy. In: Alternating Electric Fields Therapy in Oncology. Cham: Springer, 2016:103-16.
- Lukas RV, Ratermann KL, Wong ET, et al. Skin toxicities associated with tumor treating fields: case based review. J Neurooncol 2017;135:593-9. [Crossref] [PubMed]
- Blat R, Giladi M, Wasserman Y, et al. QOL-09 Effect of antiperspirants and skin barriers on electrical resistance during TTFields application. Neuro Oncol 2015;17:v189-90. [Crossref]
- Lacouture M, Hershkovich H, Giladi M, et al. P04.59 Modeling the safety of topical agents for skin toxicity associated with tumor treating fields therapy in glioblastoma. Neuro Oncol 2018;20:iii293. [Crossref]
- Lacouture M, Iwamoto F, Armentano F, et al. Mitigating skin irritations with Tumor Treating Fields therapy in glioblastoma. In: Washington, DC: Oncology Nursing Society’s Annual Conference 2018.


