Chemotherapy principles of managing stage IV breast cancer in the United States
Introduction
Breast cancer is the most common malignancy diagnosed in women worldwide and the second leading cause of cancer-related death in women, only after lung cancer (1). In the United States, 249,260 new breast cancer cases are expected in 2016 (2). Over the last decade, breast cancer death rates have decreased in large due to advances in early detection and improved therapies (2). The majority of breast cancer cases are diagnosed at an early localized stage and the five-year survival rate is close to 100%; however, 5–9% of women present with metastatic disease at the time of diagnosis with a five-year survival rate of 26% (2,3). Furthermore, up to 30% of women diagnosed with early-stage breast cancer will develop metastatic disease despite treatment (4). The majority of breast cancer-related deaths are due to complications from recurrent or metastatic disease (5). It is estimated that 40,890 women in the U.S. will die of breast cancer in 2016 (2).
Treatment options for metastatic breast cancer (MBC) have expanded significantly over the last two decades due to a better understanding of the heterogeneity of the disease. The identification of intrinsic molecular subtypes with prognostic and predictive biomarkers has offered therapeutic targets (6). Treatment options include endocrine therapies, monoclonal antibodies, antibody-drug conjugates, targeted therapies and different types of chemotherapy. Despite striking discoveries and a broad therapeutic armamentarium, MBC remains incurable. The goal of treatment of MBC is to prolong survival and to improve quality of life by mitigating cancer-related symptoms without increasing toxicity. In order to accomplish these goals, the treatment plan should be individualized. This review article addresses the general principles of cytotoxic chemotherapy in HER2 negative MBC.
Indications for cytotoxic chemotherapy in advanced breast cancer
Cytotoxic chemotherapy is a therapeutic option for many patients with MBC. Chemotherapy is generally recommended as first line treatment for patients with hormone receptor (HR)-negative breast cancer, patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive disease (in combination with HER-2 directed therapy), patients with HR-positive breast cancer with symptomatic visceral crisis or with endocrine resistance (i.e., patients who do not respond to three sequential endocrine therapy regimens) (7,8).
For patients with MBC in whom chemotherapy is recommended, the decision on a specific therapy should be individualized based on disease- and patient-related factors such as tumor biology, disease growth rate and presence of visceral metastases, menopausal status, comorbidities, prior therapies and patient preference. For instance, in patients with limited tumor burden or minimal cancer-related symptoms, single-agent treatment is less toxic and overall survival (OS) is similar when compared with combination chemotherapy. A meta-analysis by Dear et al., included 2,317 patients with MBC from 12 randomized clinical trials to assess the effect of combination chemotherapy (9) compared to the same drugs given sequentially. This study showed higher tumor response rates in the combination arm [RR 1.16; 95% confidence interval (CI), 1.06–1.28; P=0.001], however, there was no difference in OS between combination versus sequential monotherapy (HR 1.04, 95% CI, 0.93–1.16; P=0.45) and the risk of febrile neutropenia was higher in the combination arm (RR 1.32; 95% CI, 1.06–1.65; P=0.01) (9). The appropriate patients for combination chemotherapy are those in which a rapid response is needed due to symptomatic disease, large tumor burden and rapid progression.
In patients in whom a single agent is recommended, an evaluation of the overall health status, comorbidities, prior treatments and toxicities may guide the choice of therapy. There are a number of drugs with single-agent activity in MBC. Anthracyclines and taxanes are considered the most active and frequently recommended as the initial treatment for patients with metastatic disease (10,11). In addition, capecitabine, eribulin, gemcitabine, ixabepilone, vinorelbine and etoposide have demonstrated single agent activity and, therefore, constitute therapeutic options. The selection should be individualized based on the relative benefits and toxicities of the drug.
Anthracyclines
The anthracyclines alone or in combination with other agents are among the most active therapies for the treatment of breast cancer. In the 1990s, doxorubicin was the comparator arm in most clinical trials evaluating newer agents. More recently, the use of anthracyclines has been declining in the metastatic setting due to the extensive use in the adjuvant setting and its limitations of cumulative toxicity although anthracyclines can be considered in those who did not receive an anthracycline in the past. The anthracyclines approved for the treatment of MBC are doxorubicin (60 to 75 mg/m2 every 3 weeks or 20 mg/m2 weekly for three weeks/1 week off), epirubicin (75 to 100 mg/m2 every 3 weeks, or 20 to 30 mg/m2 weekly for three weeks/1 week off) and pegylated liposomal doxorubicin (40 mg/m2 every four weeks). Doxorubicin and epirubicin have similar efficacy and the choice between them is mainly driven by geographic location and institutional preference. The response rates (RR) for doxorubicin and epirubicin in the metastatic setting depend on prior chemotherapy exposure. In phase II studies of previously untreated patients (12-14), the RR of doxorubicin is approximately 50%, compared to approximately 30% in patients pretreated with chemotherapy (15-17).
The risk of cardiac toxicity is a major factor to consider when choosing an anthracycline in the treatment of MBC. Anthracyclines may cause acute and late cardiotoxicity especially in patients with preexisting cardiovascular disease, concomitant cardiotoxic agents, concurrent or prior chest irradiation and advanced age (18,19). Early cardiotoxicity peaks at three months after last anthracycline infusion and may present with arrhythmias, ECG changes, acute pericarditis or decline in left ventricular ejection fraction. Late cardiotoxicity may occur 10 to 15 years after anthracycline treatment and presents with clinical heart failure or subclinical decline in myocardial function (20). The cardiac toxicity of anthracyclines is strongly correlated with the cumulative dose with higher rates of symptomatic heart failure reported in patients who received more than 550 mg/m2 of doxorubicin (21,22). Therefore, it is generally recommended that cumulative doxorubicin and epirubicin doses be limited to 450 to 500 and 900 mg/m2, respectively. Dexrazoxane is an EDTA-like chelator that may prevent anthracycline-related cardiac damage (23), although there is concern of its association with decreased tumor response to anthracycline. For patients that are responding to anthracycline treatment but cumulative dose is a concern (e.g., >300 mg/m2 cumulative dose of doxorubicin), dexrazoxane may be used concurrently with the anthracycline to reduce the risk of cardiotoxicity.
The use of pegylated and nonpegylated liposomal anthracyclines allows higher cumulative doses with lower incidence of cardiotoxicity (24). Smith et al., conducted a meta-analysis of liposomal doxorubicin compared with conventional doxorubicin in patients with MBC. Liposomal doxorubicin decreased the risk of clinical cardiotoxicity (OR 0.18; P<0.0001), subclinical cardiotoxicity (RR 0.31; P<0.0001) and any cardiotoxic event (RR 0.30; P<0.0001) (25). In terms of efficacy, pegylated liposomal doxorubicin administered every 4 weeks appears to be equally effective and less toxic compared with doxorubicin administered every three weeks. O’Brien et al., randomized 509 patients with MBC (56% had previously received anthracyclines) to treatment with pegylated liposomal doxorubicin (50 mg/m2 every 4 weeks) or doxorubicin (60 mg/m2 every 3 weeks) (26). Compared with pegylated liposomal doxorubicin, doxorubicin resulted in slightly higher ORR (38% vs. 33%), similar progression free survival (PFS) (median, 7.8 vs. 6.9 months; HR 1.0; 95% CI, 0.82–1.22) and OS (median, 22 vs. 21 months; HR; 0.94, 95% CI, 0.74–1.19), an increase in the risk of cardiotoxicity (26% vs. 7%, HR 3.16; 95% CI, 1.58–6.31) and higher rates of alopecia (66% vs. 20%), nausea (53% vs. 37%), vomiting (31% vs. 19%), and neutropenia (10% vs. 4%). Pegylated liposomal doxorubicin was associated with a higher rate of palmar-plantar erythrodysesthesia (48% vs. 2%), stomatitis (22% vs. 15%), and mucositis (23% vs. 13%) (26). Because of its lower cardiac toxicity, pegylated liposomal doxorubicin is often preferred than conventional anthracyclines in the metastatic setting.
As first-line chemotherapy in MBC, anthracyclines have been compared with taxanes as single agents or both agents combined, and there is no evidence of superiority of either strategy in terms of OS. Paridaens et al., found higher response rates and median PFS with doxorubicin every 3 weeks compared to paclitaxel every 3 weeks. However, there was no significant difference in OS with a median survival of 18.3 months in the doxorubicin arm and 15.6 months in the paclitaxel arm (P=0.38) (27). A meta-analysis collected individual patient data on three single-agent trials comparing taxanes with anthracyclines (n=919 patients). Median survival was 19.3 months and median PFS was 7.1 months. The response rates were similar in the taxanes (38%) and in the anthracyclines (33%) arms (P=0.08), the PFS was better in the anthracycline group (median, 7 vs. 5 months, P=0.011), but there was no difference in OS (HR 1.01; 95% CI, 0.88–1.16; P=0.90). Of note, this meta-analysis published in 2008 did not include trials using the weekly paclitaxel schedule (28).
Taxanes
Docetaxel every 3 weeks; paclitaxel weekly
Taxanes are among the most widely used chemotherapy agents in the treatment of MBC. Several studies including a recent meta-analysis support the benefit of taxanes on clinical outcomes, including OS, time to progression (TTP) and response rates in MBC (29). Taxanes stabilize microtubules, leading to cell cycle arrest and, eventually, cell death (30-32). Paclitaxel (sb-paclitaxel, Taxol; Bristol-Myers Squibb Co., Princeton, NJ, USA), docetaxel (Taxotere; sanofi-aventis US LLC, Bridgewater, NJ, USA), and nab-paclitaxel (Abraxane; Celgene Corporation, Summit, NJ, USA) are approved drugs for the treatment of recurrent and MBC. Paclitaxel is approved to be administered weekly (80 to 100 mg/m2 on days 1, 8, and 15 of a 28-day cycle) or every three weeks (175 mg/m2). Docetaxel can be administered every three weeks (80 to 100 mg/m2) or weekly (30 to 40 mg/m2 on days 1, 8, and 15 of a 28-day cycle). Docetaxel is associated with a high risk of fluid retention, which is ameliorated by premedication with dexamethasone (33). Both paclitaxel and docetaxel require the use of solvents to enhance their solubility. Paclitaxel is mixed with the castor oil derivative Cremophor EL and docetaxel is formulated with the solvent polysorbated 80. These solvents have been associated with allergic reactions and peripheral neuropathy (34-36). To decrease the risk of allergic reactions, patients are pretreated with corticosteroids.
The optimal schedule of administration of a taxane has been extensively investigated. A meta-analysis conducted by Mauri et al. (37), included 11 randomized clinical trials (n=2,540 patients) comparing weekly- and three-weekly taxanes in patients with advanced breast cancer. The weekly administration of paclitaxel resulted in higher OS compared to the three-week schedule (HR 0.78; 95% CI, 0.67–0.89; P=0.001). There was no difference in PFS between the two schedules. In regard to docetaxel, there was no difference for the weekly compared to the three-week regimen for objective response rate, PFS and OS. However, due to the low number of patients included in the docetaxel studies, a firm conclusion might not be given. The incidence of serious adverse events, neutropenia, neutropenic fever, and peripheral neuropathy were significantly lower in weekly taxanes schedules. The incidence of nail changes and epiphora were significantly lower in the every three weeks docetaxel regimens (37). Furthermore, in an adjuvant randomized trial comparing the efficacy of paclitaxel versus docetaxel at different schedules, the group receiving weekly paclitaxel and the group receiving docetaxel every 3 weeks had significantly improved DFS and the group receiving weekly paclitaxel had improved OS (38). The results are consistent with other studies of MBC that demonstrated a benefit of weekly paclitaxel (39) or docetaxel every 3 weeks (40), as compared with paclitaxel every 3 weeks. Weekly paclitaxel has not been compared with every three-week docetaxel in the metastatic setting.
Nab-paclitaxel—solvent free
Nab-Paclitaxel is a, solvent free, albumin-bound form of paclitaxel. Nab-paclitaxel has activity in MBC similar to other taxanes (41,42). In a phase III trial of patients with MBC, nab-paclitaxel at a dose of 260 mg/m2 every 3 weeks demonstrated superior antitumor activity compared with paclitaxel 175 mg/m2 every 3 weeks (41). In 2005, nab-paclitaxel was approved for the treatment of MBC after failure of combination chemotherapy for metastatic disease or relapse within six months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated. More recently, paclitaxel and nab-paclitaxel were evaluated as first line treatment in combination with bevacizumab in the phase III Cancer and Leukemia Group B 40502/NCCTG N063H Alliance trial (43). In this study, 799 patients were randomized to bevacizumab with either weekly treatment with paclitaxel (90 mg/m2) or nab-Paclitaxel (150 mg/m2) on a three week on, one week off schedule. A third arm including weekly ixabepilone (16 mg/m2) was closed for futility at the first interim analysis. At the second planned interim analysis, the nab-paclitaxel arm crossed the futility boundary for superiority and was closed as well. There was no significant difference in PFS and OS between paclitaxel and nab-paclitaxel. The median PFS was 11 months for paclitaxel and 9.3 months for nab-paclitaxel (HR 1.20; 95% CI, 1.00–1.45); median OS was 26.5 months for paclitaxel and 23.5 months for nab-paclitaxel (HR 1.17; 95% CI, 0.92–1.47). Compared with paclitaxel, nab-paclitaxel resulted in worse hematologic and nonhematologic toxicity (P<0.001 for both) and increased incidence of grade ≥2 sensory neuropathy (54% vs. 46%, P=0.031) (43). At this point the choice between taxanes is guided by patient comorbidities, schedule preference and toxicity profile. Due to the lower risk of hypersensitivity reactions with nab-paclitaxel compared to other taxanes, it may be preferable in patients with poor steroid tolerance such as diabetics.
Chemotherapy in patients with anthracycline- and taxane-resistant metastatic breast cancer (MBC)
Multiple chemotherapy agents, with diverse mechanisms of action, have been studied in patients that have progressed on an anthracycline- and taxane-based therapy. The key clinical trials investigating the most commonly used agents, as single therapy or in combination, are summarized in Table 1.
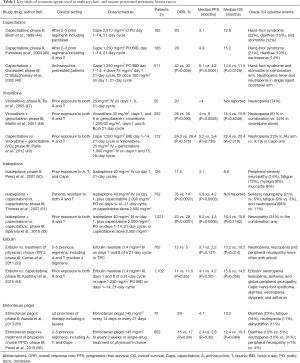
Full table
Capecitabine
Capecitabine is an oral prodrug of the antimetabolite fluorouracil (FU). Capecitabine is converted to FU by the enzyme thymidine phosphorylase. This enzyme is found in higher levels in breast tumors compared with healthy tissue which theoretically allows some tumor selectivity and less systemic toxicity compared with IV FU (57). The primary toxicities of capecitabine are hand-foot syndrome, mucositis and diarrhea. Capecitabine has been largely investigated as a single agent mostly in phase II clinical trials and in combination with other chemotherapy agents, most often a taxane (44,45,58-62).
Single agent capecitabine was compared with CMF (cyclophosphamide, methotrexate, and FU) as first line therapy in a randomized phase II study in patients with MBC (63). The overall response rate (ORR) in the capecitabine group was 30%, versus 16% in the CMF group. The median TTP was similar between the two groups (4.1 months for capecitabine and 3.0 months for CMF). However, OS was slightly longer in capecitabine treated patients compared to CMF (19.6 and 17.2 months, respectively). Diarrhea and hand-foot syndrome were more frequent with capecitabine, whereas the CMF treated patients had more alopecia and grade 3 or 4 neutropenia (63). First line combination therapy with capecitabine has also been investigated in MBC patients. Gradishar et al., investigated the combination of capecitabine with paclitaxel as first line in MBC (64). Capecitabine 1,650 mg/m2 per day divided in two doses for 14 days with paclitaxel 175 mg/m2 on day 1 was administered every 21 days. A total of 48 patients were enrolled, 77% of patients had received prior neoadjuvant and/or adjuvant chemotherapy. ORR was 51%, including 17 patients with CR. Median OS was 29.9 months (64). A non-inferiority phase III trial compared capecitabine plus paclitaxel versus epirubicin plus paclitaxel as first line treatment for MBC (65). Most patients were chemotherapy-naïve and without exposure to anthracyclines. The primary endpoint, to demonstrate the noninferiority of capecitabine plus paclitaxel to epirubicin plus paclitaxel, was not met. Therefore, despite similar response rates, PFS and OS between the two regimens, this trial could not confirm that the regimen of capecitabine plus paclitaxel is noninferior to epirubicin and paclitaxel in the first line setting (65).
In patients that have progressed on anthracyclines, capecitabine was compared to paclitaxel in a small phase II study (66). Patients were randomized to receive either capecitabine (2,510 mg/m2 per day in two divided doses for 14 days followed by seven days of rest) or paclitaxel (175 mg/m2 IV on day 1 of every 21 days). ORR was similar between the groups (36% for capecitabine and 26% for paclitaxel). Median TTP (3.0 vs. 3.1 months) and median OS (7.6 vs. 9.4 months) were similar between capecitabine and paclitaxel (66).
A systematic review of clinical trials published in 2011 included 1,494 patients from eight phase II trials and two phase III trials in which patients were treated with single agent capecitabine after progression on an anthracycline and taxane therapy. The response rate was 18%, median PFS 4.2 months, and OS 13.5 months (67). Most capecitabine trials in MBC are from molecularly-unselected populations. Interestingly, recent data suggest that capecitabine may be more active in patients with hormone-receptor positive breast cancer (68).
Capecitabine-based combinations have been investigated with mixed results. A multicenter, phase III trial that enrolled 511 patients with MBC compared single agent docetaxel with combination docetaxel and capecitabine in patients previously treated with an anthracycline (46). Approximately two thirds of both groups received the treatment as second- or third-line for metastatic disease. The primary end point of median TTP was 6.1 months for the combination, compared to 4.2 months for docetaxel alone (P<0.001). Median OS favored the combination group (14.5 vs. 11.5 months, P=0.013). Response rate was 32% in the combination arm, compared to 23% in the single arm (P=0.025), Median time to treatment failure was 4 months for the capecitabine/docetaxel group and 2.8 months for the docetaxel group (P<0.001). Overall, more patients in the combination group experienced grade 3 toxicity than in the single agent group (71% vs. 49%) (46). Despite the OS benefit, this combination has not been widely adopted in clinical practice due to the toxicity of the regimen. Nevertheless, in China, the combination of docetaxel and capecitabine is frequently used as first-line regimen for the treatment of patients with MBC who do not respond to anthracyclines.
Newer agents including ixabepilone and eribulin have been investigated in combination with capecitabine. The addition of ixabepilone to capecitabine increased ORR and PFS, but not OS, and the combination had more toxicity including more neuropathy and neutropenia (52,69). Based on the above, capecitabine monotherapy is FDA-approved for the treatment of patients with MBC that have progressed on both paclitaxel and anthracycline-containing chemotherapy regimens or resistant to paclitaxel and for whom further anthracycline therapy is not indicated. Capecitabine in combination with docetaxel is approved for patients with MBC after progression on anthracycline-containing regimen.
Non-taxanes targeting microtubule dynamics
Eribulin
Eribulin mesylate is a nontaxane microtubule dynamics inhibitor belonging to the halichondrin class of antineoplastic agents (70,71). Eribulin binds to high affinity sites on the growing ends of microtubules which may decrease the effect of eribulin on normal physiologic microtubule functions (72,73). An important distinction of eribulin with other tubulin-targeted agents is that the mitotic blockade with eribulin is irreversible for which, intermittent drug exposure leads to long-term loss of cell viability (71). This novel mechanism of action may explain the activity of eribulin in patients previously treated with a taxane (70). The most common AEs with eribulin were neutropenia, alopecia, leukopenia, global peripheral neuropathy, and nausea.
The first phase III trial of eribulin EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus Eribulin) compared eribulin mesylate (1.4 mg/m2 on days 1 and 8 every 21 days) with treatment of physician’s choice (TPC) in patients with locally recurrent or MBC previously treated with 2–5 prior chemotherapy regimens, including an anthracycline and a taxane (53). In this trial, there was a significant improvement in OS for eribulin compared with TPC. The median OS was 13.2 months for eribulin versus 10.5 months for TPC (HR, 0.81; 95% CI, 0.67 to 0.96; P=0.01). Most common adverse events reported in the Eribulin group were fatigue and neutropenia (53). As a result, eribulin has been approved in more than 50 countries, as monotherapy for patients with MBC who have previously received at least two chemotherapeutic regimens with prior therapy having included an anthracycline and a taxane in the adjuvant or metastatic setting.
The second phase III trial of eribulin in MBC (Study 301) compared capecitabine versus eribulin in patients with MBC who had received up to two prior chemotherapy regimens including an anthracycline and taxane (54). A total of 1,102 patients were randomly assigned to receive eribulin mesylate 1.4 mg/m2 IV over 2 to 5 minutes on days 1 and 8, or capecitabine 1.25 g/m2 orally twice per day on days 1 to 14, both in 21-day cycles. More than half of the patients in Study 301 had received only one prior regimen for advanced disease. The median OS was 15.9 months for eribulin compared with 14.5 months for capecitabine (HR 0.88; 95% CI, 0.77–1.00; P=0.056). Median PFS was 4.1 months for eribulin and 4.2 months for capecitabine (HR 1.08; 95% CI, 0.93–1.25; P=0.30). This study did not demonstrate superiority of eribulin versus capecitabine with regard to either OS or PFS which contrast with the results of the EMBRACE trial. The authors suggest that the treatment earlier in the course of MBC (up to two lines of prior therapy for MBC) with eribulin is less likely to impact OS, since multiple subsequent lines of effective treatment are available (54). Importantly, in a pre-specified subgroup analysis of the Study 301, median OS was longer with eribulin than with capecitabine in patients with human epidermal growth factor receptor 2 (HER2)-negative or triple-negative breast cancer.
A pooled analysis of the two studies, EMBRACE and Study 301, requested by the European Medicines Agency (EMA), showed a significant benefit in favor of eribulin (74). Median OS was 15.2 months in the eribulin arm compared with 12.8 months in the control arm (HR 0.85; P=0.003). In addition, treatment with eribulin was associated with benefits in OS across all patient subgroups, but more significant in the triple-negative and HER-2 negative/ER-positive subgroups. In patients with HER2-negative disease (median OS, eribulin vs. control: 15.2 vs. 12.3 months, respectively; HR 0.82; P=0.002), although this effect did not reach statistical significance in patients with HER2-negative but ER-positive disease (P=0.060). The difference in OS for those with HER2-positive disease favored eribulin but did not reach statistical significance (13.5 vs. 12.2 months; HR 0.82; P=0.135). In patients with triple-negative disease, median survival was 4.7 months longer in patients treated with eribulin than in those who received control (median OS: 12.9 vs. 8.2 months; HR 0.74; P=0.006). Interaction analysis showed clearest evidence of a greater benefit for eribulin in the case of patients with a higher burden of disease, as indicated by more than two organs involved (P=0.023 vs. those with two or fewer organs involved) (74). Eribulin is being compared to weekly paclitaxel as first- or second-line chemotherapy for MBC (ACCRU protocol #RU0112011).
Ixabepilone
Ixabepilone is a semi-synthetic analog of epothilone B, a non-taxane class of microtubule inhibitors. The epothilones disrupt the dynamic instability of microtubules, promote microtubule polymerization, and arrest cells in the G2/M transition of the cell cycle leading to apoptotic cell death (75). A phase II trial of ixabepilone in patients previously treated with anthracycline, taxane, and capecitabine resulted in an ORR of 18.3% (95% CI, 11.9%–26.1%) with a median duration of response of 5.7 months and median PFS of 3.1 months, respectively (50). 50% of patients had stable disease with a median OS of 8.6 months. In this study 14% of patients treated with ixabepilone experienced Grade 3/4 peripheral sensory neuropathy. Grade 3/4 hematologic adverse effects occurring during use of ixabepilone included neutropenia (54%), leukopenia (49%), anemia (8%), and thrombocytopenia (7%) (50).
Ixabepilone in combination with capecitabine was evaluated in a phase III clinical trial in women with MBC previously treated with anthracyclines and a taxanes (51). Patients were randomized to receive ixabepilone 40 mg/m2 IV on day 1 of a 21-day cycle plus capecitabine 2,000 mg/m2 orally (n=375) on days 1 to 14 of a 21-day cycle or capecitabine alone 2,500 mg/m2 orally on the same schedule (n=377). The trial was positive with ixabepilone plus capecitabine showing an improved median PFS relative to capecitabine (5.8 vs. 4.2 months; HR 0.75; 95% CI, 0.64–0.88; P=0.0003), and higher ORR (35% vs. 14%; P<0.0001). Grade 3 and 4 adverse events were more common in patients receiving combination therapy and included sensory neuropathy (21% vs. 0%), fatigue (9% vs. 3%), and neutropenia (68% vs. 11%) (51). The favorable efficacy of ixabepilone plus capecitabine over capecitabine alone led to regulatory approval of this regimen in 2007 for MBC after failure of an anthracycline and a taxane.
In the first line setting, a randomized phase III trial comparing ixabepilone to weekly paclitaxel and weekly nab-paclitaxel, each combined with bevacizumab, closed recruitment to the ixabepilone arm at the first interim analysis when the comparison of ixabepilone versus paclitaxel crossed the boundary for futility (43). Weekly paclitaxel was superior to ixabepilone (median PFS 10.6 vs. 7.6 months, respectively; P<0.0010) and caused less peripheral neuropathy (16% and 25%, respectively).
Ixabepilone at a dose of 40 mg/m2 every 21 days is approved for use as monotherapy in MBC after failure of an anthracycline, taxane and capecitabine, or in combination with capecitabine in patients with locally advanced or MBC that has failed to respond to therapy with a taxane and an anthracycline.
Other active drugs in heavily pre-treated patients with metastatic breast cancer (MBC)
Vinorelbine
Vinorelbine is a semisynthetic vinca alkaloid with activity in MBC patients. Vinorelbine is widely used particularly because of its favorable side effect profile. Vinorelbine is administered IV at 30 mg/m2 on days 1 and 8 of a 21-day schedule (76). Vinorelbine causes less toxicity compared to other chemotherapy agents and is active as a single agent (ORR 20% to 45%), even in heavily pretreated patients (48,77,78). A randomized phase III trial compared single-agent vinorelbine and the combination of gemcitabine plus vinorelbine (gemcitabine 1,200 mg/m2 + vinorelbine 30 mg/m2, days 1 and 8) (48). A total of 252 patients with MBC previously treated with anthracyclines and taxanes were enrolled. The median PFS (primary end point) was higher in the combined treatment arm (6 vs. 4 months; HR 0.66; P=0.0028). However, OS was similar (15.9 vs. 16.4 months). The ORR was higher for patients receiving gemcitabine plus vinorelbine (36% vs. 26%). Some adverse events were more frequent in the combined treatment arm (grade 3 or 4 neutropenia: 61% vs. 44%; febrile neutropenia: 11% vs. 6%). Incidences of grade 3 or 4 nonhematological toxic effects were similar between both treatment groups (48).
The combination of vinorelbine-gemcitabine was compared to capecitabine single agent in a small phase III trial with only 74 patients in each arm (49). The results did not show a benefit for the combination arm. The median PFS in the combined therapy arm was 5.4 months compared with 5.2 months for capecitabine. Also, median OS (20.4 vs. 22.4 months) and ORR (28.4% vs. 24.3%) were not significantly improved. Neutropenia and fatigue were more common with vinorelbine-gemcitabine and hand-foot syndrome in patients receiving capecitabine (49).
Gemcitabine
Gemcitabine is a pyrimidine nucleoside analogue. Gemcitabine is commonly used as a single agent as salvage chemotherapy after several lines of treatment administered over a large dose range (800–1,250 mg/m2) (79). Response rates to gemcitabine have ranged from 14% to 42%, depending on the gemcitabine dose and schedule and the extent of prior treatment (79). In a study investigating gemcitabine in patients previously treated with anthracycline- and taxane-based regimen, Rha et al., found a RR of 20%, with median response duration of 9 months and OS 11 months (80). The most common grade 3 and 4 adverse event is myelosuppression. Thrombocytopenia can be a dose-limiting toxicity. Alopecia, neuropathy and gastrointestinal toxicity are less frequent.
It is important to note that gemcitabine, as a single agent, is inferior to epirubicin in women with MBC not previously treated with an anthracycline. Epirubicin demonstrated statistically significant superiority in a phase III study compared to gemcitabine in TTP (6.1 and 3.4 months, P=0.0001), OS (19.1 and 11.8 months, P=0.0004), and response rate (40.3% and 16.4%, P<0.001) (81). For patients with MBC that require a rapid response to decrease tumor burden, a combination of gemcitabine and paclitaxel, has shown effective results with improvements of ORRs, PFS and OS compared to paclitaxel alone (82).
Platinum agents
The platinum agents, cisplatin and carboplatin, induce DNA interstrand cross-links. Both drugs undergo renal elimination. They are rarely used as single agents in MBC; however, emerging data suggest these agents may be more effective in patients with MBC associated with germline BRCA mutations and in triple negative breast cancer (TNBC) (83). TNBC is a heterogeneous group in which subgroups such as BRCA1 mutation carriers may have particular sensitivity to platinum agents and relatively less sensitivity to taxanes (84). Platinum in combination with other agents such as gemcitabine, have shown promising results in tumors with defects in DNA repair pathways. A randomized phase II trial included 116 patients with TNBC receiving first or second-third line therapy which included carboplatin (AUC 2 IV) plus gemcitabine (1,000 mg/m2 IV on days 1 and 8 every 3 weeks) alone or in combination with the PARP inhibitor iniparib (85). The iniparib arm was associated with a higher response rate, median PFS, and OS, providing some information about the effectiveness of the carboplatin-gemcitabine combination in this population (85). However, a confirmatory phase III trial failed to show a benefit for the addition of iniparib to the same carboplatin/gemcitabine regimen in 519 patients with metastatic TNBC (86). Importantly, iniparib was subsequently found to be a relatively weak PARP inhibitor, and induced its antitumor effects by inducing cell cycle arrest in the G2-M phase, promoting double strand DNA damage, and potentiating cell cycle arrest induced by carboplatin and gemcitabine (86). Definitive data is needed to support the use of platinum agents in TNBC (87).
Etirinotecan pegol
Etirinotecan pegol (NKTR-102) is a long-acting inhibitor of topoisomerase-I, an enzyme essential for DNA replication. Etirinotecan pegol consists of the topoisomerase-I inhibitor irinotecan bound to a proprietary polyethylene glycol (PEG) core that prolongs exposure to, but reduces the toxicity of, SN38 the active metabolite of irinotecan (88). The linker slowly hydrolyses in vivo to release irinotecan, which is subsequently converted to SN38. The high molecular weight of etirinotecan pegol, restricts its ability to cross intact vasculature into healthy tissues, but promotes extravasation through leaky tumor vessels potentially resulting in fewer side effects (88,89). A Phase II study in patients with advanced taxane-resistant breast cancer showed that etirinotecan pegol at a dose of 145 mg/m2 every 21 days had an ORR of 29%, and resulted in clinical benefit (CR, PR, SD for ≥6 months) to 48% of patients (55). Median PFS was 5.6 months and median OS was 13.1 months. The most common grade 3 or worse adverse events were diarrhea, fatigue, neutropenia, dehydration and vomiting. Myelosupression, febrile neutropenia and alopecia were rare events. No cardiotoxicity or neurotoxicity was reported in this study (55).
These encouraging results led to the phase III BEACON study (Breast Cancer Outcomes With NKTR-102): a Phase III open-label, randomized, multicenter study of etirinotecan pegol versus treatment of physician’s choice (TPC) in patients with locally recurrent or MBC previously treated with an anthracycline, a taxane, and capecitabine (56). All patients had received two to five prior cytotoxic chemotherapy regimens for MBC. The primary endpoint was OS in the intention-to-treat population. The study recruited a total of 852 patients randomly assigned to etirinotecan pegol (n=429) and to TPC (n=423). Ninety percent of the patients enrolled in the BEACON study had HER2-negative disease. Patients in both treatment groups received a median of three treatment cycles. Eribulin was the most frequent treatment of physician's choice, followed by vinorelbine, gemcitabine, nab-paclitaxel, paclitaxel, ixabepilone, and docetaxel. There was no statistically significant difference in OS between etirinotecan pegol and TPC (median OS 12.4 vs. 10.3 months; respectively, HR 0.87; 95% CI, 0.75–1.02; P=0.084). The incidence of grade 3 and higher adverse events was lower in the etirinotecan pegol (48%) compared with the TPC arm (63%) (P<0.0001). The group treated with etirinotecan pegol had more grade 3 or worse diarrhea (10% vs. 1% in the control group) and less neutropenia (10% vs. 31%), and less peripheral neuropathy (<1% vs. 4%). Three patients in the etirinotecan pegol group died of treatment-related adverse events (pneumonia, myelodysplastic syndrome, and acute renal failure) and two in the TPC group (neutropenic sepsis and septic shock). The health-related quality of life analysis showed better results for both global health status and physical functioning in the etirinotecan pegol group compared with TPC (56).
Interestingly, pre-specified subgroup analyses suggest that etirinotecan pegol significantly prolonged OS in patients with a history of brain metastases, with liver metastases, and with two or more sites of disease. In the subgroup analysis of 67 patients with brain metastases, etirinotecan pegol improved the median OS compared to TPC (10.0 vs. 4.8 months; HR 0.51; 95% CI, 0.30–0.86). Etirinotecan pegol was also superior to TPC in the subgroup of 456 patients with baseline liver metastases (median OS 10.9 vs. 8.3 months; HR 0.73; 95% CI, 0.59–0.89) (56). The potential benefit of etirinotecan pegol in patients with a history of brain and liver metastases warrants further investigation.
Conclusions
Cytotoxic chemotherapy remains pivotal in the treatment of MBC. The choice of an agent, as a single therapy or in combination, requires an individualized approach with a comprehensive balancing of efficacy, toxicity profile and individual preferences to achieve the goals of extending survival and improving quality of life by treating cancer-related symptoms. For the patient previously exposed to chemotherapy in the adjuvant or metastatic setting, there is no optimal sequence of administration of chemotherapy drugs since several drugs have proven antitumor activity as single agents. Combination regimens have generally shown higher response rates and improved PFS, but not OS benefit, compared with single agent therapy and carry higher toxicity. Combination regimens should be reserved for patients with symptomatic disease and large tumor burden in need of rapid disease control. Cumulative toxicity is a limiting factor for anthracyclines inducing cardiomyopathy and drugs frequently used (including taxanes, eribulin, ixabepilone and vinorelbine) that are associated with severe neuropathy. Following progression on anthracycline- and taxane-based therapies, capecitabine is frequently the first choice because of oral administration and low risk of alopecia and myelotoxicity. To date, eribulin is the only agent that has been shown, when administered as monotherapy, to prolong OS in heavily pretreated MBC. The development of novel chemotherapy agents with antitumor activity in MBC and diverse mechanisms of action is imperative in this patient population. Furthermore, predictive biomarkers are needed to personalize chemotherapy and identify for an individual patient the precise drug from which they are most likely to benefit.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Uk CR. Worldwide cancer statistics. Cancer Res UK, 2014.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2016.
- (EBCTCG) EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005;10 Suppl 3:20-9. [Crossref] [PubMed]
- Cadoo KA, Traina TA, King TA. Advances in molecular and clinical subtyping of breast cancer and their implications for therapy. Surg Oncol Clin N Am 2013;22:823-40. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer [Internet]. Version 3. 2015. 2015:187. Available online: www.NCCN.com
- Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev 2003.CD002747. [PubMed]
- Dear RF, McGeechan K, Jenkins MC, et al. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev 2013;12:CD008792. [PubMed]
- André F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol 2012;23 Suppl 6:vi46-51. [Crossref] [PubMed]
- Dranitsaris G, Gluck S, Faria C, et al. Comparative effectiveness analysis of monotherapy with cytotoxic agents in triple-negative metastatic breast cancer in a community setting. Clin Ther 2015;37:134-44. [Crossref] [PubMed]
- Ahmann DL, Bisel HF, Eagan RT, et al. Controlled evaluation of adriamycin (NSC-123127) in patients with disseminated breast cancer. Cancer Chemother Rep 1974;58:877-82. [PubMed]
- Richards MA, Hopwood P, Ramirez AJ, et al. Doxorubicin in advanced breast cancer: influence of schedule on response, survival and quality of life. Eur J Cancer 1992;28A:1023-8. [Crossref] [PubMed]
- Carmo-Pereira J, Costa FO, Henriques E, et al. A comparison of two doses of adriamycin in the primary chemotherapy of disseminated breast carcinoma. Br J Cancer 1987;56:471-3. [Crossref] [PubMed]
- Cowan JD, Neidhart J, McClure S, et al. Randomized trial of doxorubicin, bisantrene, and mitoxantrone in advanced breast cancer: a Southwest Oncology Group study. J Natl Cancer Inst 1991;83:1077-84. [Crossref] [PubMed]
- Henderson IC, Allegra JC, Woodcock T, et al. Randomized clinical trial comparing mitoxantrone with doxorubicin in previously treated patients with metastatic breast cancer. J Clin Oncol 1989;7:560-71. [PubMed]
- Ingle JN, Pfeifle DM, Green SJ, et al. Randomized clinical trial of doxorubicin alone or combined with mitolactol in women with advanced breast cancer and prior chemotherapy exposure. Am J Clin Oncol 1985;8:275-82. [Crossref] [PubMed]
- Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;91:710-7. [Crossref] [PubMed]
- Hershman DL, McBride RB, Eisenberger A, et al. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J Clin Oncol 2008;26:3159-65. [Crossref] [PubMed]
- Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol 2013;14:72-80. [Crossref] [PubMed]
- Bristow MR, Mason JW, Billingham ME, et al. Dose-effect and structure-function relationships in doxorubicin cardiomyopathy. Am Heart J 1981;102:709-18. [Crossref] [PubMed]
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003;97:2869-79. [Crossref] [PubMed]
- Marty M, Espié M, Llombart A, et al. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane®) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol 2006;17:614-22. [Crossref] [PubMed]
- Harris L, Batist G, Belt R, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002;94:25-36. [Crossref] [PubMed]
- Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010;10:337. [Crossref] [PubMed]
- O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004;15:440-9. [Crossref] [PubMed]
- Paridaens R, Biganzoli L, Bruning P, et al. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J Clin Oncol 2000;18:724-33. [PubMed]
- Piccart-Gebhart MJ, Burzykowski T, Buyse M, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol 2008;26:1980-6. [Crossref] [PubMed]
- Ghersi D, Willson Melina L, Chan Matthew Ming K, et al. Taxane-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev 2015;(6).
- Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A 1980;77:1561-5. [Crossref] [PubMed]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979;277:665-7. [Crossref] [PubMed]
- Rowinsky EK, Donehower RC. PACLITAXEL (TAXOL). N Engl J Med 1995;332:1004-14. [Crossref] [PubMed]
- Piccart MJ, Klijn J, Paridaens R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol 1997;15:3149-55. [PubMed]
- Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001;37:1590-8. [Crossref] [PubMed]
- Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol 1990;8:1263-8. [PubMed]
- ten Tije AJ, Verweij J, Loos WJ, et al. Pharmacological effects of formulation vehicles : implications for cancer chemotherapy. Clin Pharmacokinet 2003;42:665-85. [Crossref] [PubMed]
- Mauri D, Kamposioras K, Tsali L, et al. Overall survival benefit for weekly vs. three-weekly taxanes regimens in advanced breast cancer: A meta-analysis. Cancer Treat Rev 2010;36:69-74. [Crossref] [PubMed]
- Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008;358:1663-71. [Crossref] [PubMed]
- Jones SE, Erban J, Overmoyer B, et al. Randomized Phase III Study of Docetaxel Compared With Paclitaxel in Metastatic Breast Cancer. J Clin Oncol 2005;23:5542-51. [Crossref] [PubMed]
- Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leu. J Clin Oncol 2008;26:1642-9. [Crossref] [PubMed]
- Gradishar WJ. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil-Based Paclitaxel in Women With Breast Cancer. J Clin Oncol 2005;23:7794-803. [Crossref] [PubMed]
- Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 2009;27:3611-9. [Crossref] [PubMed]
- Rugo HS, Barry WT, Moreno-Aspitia A, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N0. J Clin Oncol 2015;33:2361-9. [Crossref] [PubMed]
- Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999;17:485-93. [PubMed]
- Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer 2004;40:536-42. [Crossref] [PubMed]
- O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: Phase III trial results. J Clin Oncol 2002;20:2812-23. [Crossref] [PubMed]
- Toi M, Saeki T, Aogi K, et al. Late phase II clinical study of vinorelbine monotherapy in advanced or recurrent breast cancer previously treated with anthracyclines and taxanes. Jpn J Clin Oncol 2005;35:310-5. [Crossref] [PubMed]
- Martín M, Ruiz A, Muñoz M, et al. Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: final results of the phase III Spanish Breast Cancer Research Group (GEICAM) trial. Lancet Oncol 2007;8:219-25. [Crossref] [PubMed]
- Pallis AG, Boukovinas I, Ardavanis A, et al. A multicenter randomized phase iii trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Ann Oncol 2012;23:1164-9. [Crossref] [PubMed]
- Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol 2007;25:3407-14. [Crossref] [PubMed]
- Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 2007;25:5210-7. [Crossref] [PubMed]
- Sparano JA, Vrdoljak E, Rixe O, et al. Randomized Phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2010;28:3256-63. [Crossref] [PubMed]
- Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011;377:914-23. [Crossref] [PubMed]
- Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015;33:594-601. [Crossref] [PubMed]
- Awada A, Garcia AA, Chan S, et al. Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: a randomised phase 2 study. Lancet Oncol 2013;14:1216-25. [Crossref] [PubMed]
- Perez EA, Awada A, O'Shaughnessy J, et al. Etirinotecan pegol (NKTR-102) versus treatment of physician's choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2015;16:1556-68. [Crossref] [PubMed]
- Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998;34:1274-81. [Crossref] [PubMed]
- Walko CM, Lindley C. Capecitabine: a review. Clin Ther 2005;27:23-44. [Crossref] [PubMed]
- Ghosn M, Kattan J, Farhat F, et al. Phase II trial of capecitabine and vinorelbine as first-line chemotherapy for metastatic breast cancer patients. Anticancer Res 2006;26:2451-6. [PubMed]
- Reichardt P, Von Minckwitz G, Thuss-Patience PC, et al. Multicenter phase II study of oral capecitabine (Xeloda(")) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol 2003;14:1227-33. [Crossref] [PubMed]
- Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 2001;92:1759-68. [Crossref] [PubMed]
- Wist EA, Sommer HH, Ostenstad B, et al. Oral capecitabine in anthracycline- and taxane-pretreated advanced/metastatic breast cancer. Acta Oncol 2004;43:186-9. [Crossref] [PubMed]
- Oshaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol 2001;12:1247-54. [Crossref] [PubMed]
- Gradishar WJ, Meza LA, Amin B, et al. Capecitabine plus paclitaxel as front-line combination therapy for metastatic breast cancer: a multicenter phase II study. J Clin Oncol 2004;22:2321-7. [Crossref] [PubMed]
- Lück HJ, Du Bois A, Loibl S, et al. Capecitabine plus paclitaxel versus epirubicin plus paclitaxel as first-line treatment for metastatic breast cancer: efficacy and safety results of a randomized, phase III trial by the AGO Breast Cancer Study Group. Breast Cancer Res Treat 2013;139:779-87. [Crossref] [PubMed]
- Talbot DC, Moiseyenko V, Van Belle S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer 2002;86:1367-72. [Crossref] [PubMed]
- Oostendorp LJ, Stalmeier PF, Donders AR, et al. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: a systematic review. Lancet Oncol 2011;12:1053-61. [Crossref] [PubMed]
- Glück S, Russell C, O’Shaughnessy J, et al. Treatment effect of capecitabine and docetaxel or docetaxel alone by oestrogen receptor status in patients with metastatic breast cancer: results of an exploratory analysis. Breast 2013;22:1087-93. [Crossref] [PubMed]
- Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol 2007;25:3399-406. [Crossref] [PubMed]
- Kuznetsov G, Towle MJ, Cheng H, et al. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res 2004;64:5760-6. [Crossref] [PubMed]
- Towle MJ, Salvato KA, Wels BF, et al. Eribulin induces irreversible mitotic blockade: implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res 2011;71:496-505. [Crossref] [PubMed]
- Wozniak KM, Nomoto K, Lapidus RG, et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Res 2011;71:3952-62. [Crossref] [PubMed]
- Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res 2012;72:4611-5. [Crossref] [PubMed]
- Twelves C, Cortes J, Vahdat L, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat 2014;148:553-61. [Crossref] [PubMed]
- Bollag DM, McQueney PA, Zhu J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 1995;55:2325-33. [PubMed]
- Vogel C, O’rourke M, Winer E. Vinorelbine as first-line chemotherapy for advanced breast cancer in women 60 years of age or older. Ann Oncol 1999;10:397-402. [Crossref] [PubMed]
- Fumoleau P, Delgado FM, Delozier T, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol 1993;11:1245-52. [PubMed]
- Jones S, Winer E, Vogel C, et al. Randomized comparison of vinorelbine and melphalan in anthracycline-refractory advanced breast cancer. J Clin Oncol 1995;13:2567-74. [PubMed]
- Heinemann V. Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology 2003;64:191-206. [Crossref] [PubMed]
- Rha SY, Moon YH, Jeung HC, et al. Gemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancer. Breast Cancer Res Treat 2005;90:215-21. [Crossref] [PubMed]
- Feher O, Vodvarka P, Jassem J, et al. First-line gemcitabine versus epirubicin in postmenopausal women aged 60 or older with metastatic breast cancer: a multicenter, randomized, phase III study. Ann Oncol 2005;16:899-908. [Crossref] [PubMed]
- Albain KS, Nag SM, Calderillo-Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 2008;26:3950-7. [Crossref] [PubMed]
- Narod SA. BRCA mutations in the management of breast cancer: the state of the art. Nat Rev Clin Oncol 2010;7:702-7. [Crossref] [PubMed]
- Isakoff SJ. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J 2010;16:53-61. [Crossref] [PubMed]
- O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 2011;364:205-14. [Crossref] [PubMed]
- Ledford H. Drug candidates derailed in case of mistaken identity. Nature 2012;483:519. [Crossref] [PubMed]
- Carrick S, Ghersi D, Wilcken N, et al. Platinum containing regimens for metastatic breast cancer. Cochrane database Syst Rev 2004.CD003374. [PubMed]
- Hoch U, Staschen CM, Johnson RK, et al. Nonclinical pharmacokinetics and activity of etirinotecan pegol (NKTR-102), a long-acting topoisomerase 1 inhibitor, in multiple cancer models. Cancer Chemother Pharmacol 2014;74:1125-37. [Crossref] [PubMed]
- Jameson GS, Hamm JT, Weiss GJ, et al. A multicenter, phase I, dose-escalation study to assess the safety, tolerability, and pharmacokinetics of etirinotecan pegol in patients with refractory solid tumors. Clin Cancer Res 2013;19:268-78. [Crossref] [PubMed]


