
Current status and future perspective of neoadjuvant therapy in locally advanced and borderline resectable pancreatic adenocarcinoma: a narrative review
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly fatal malignant neoplasm arising from the exocrine pancreas. It is the second leading cause of cancer death in the United States, and the 5-year survival rate at the time of diagnosis is only 10% (1-3). PDAC can be classified according to its resectability, evaluated using multi-phase dynamic contrast-enhanced computed tomography (CT). The resectability criteria suggested by the National Comprehensive Cancer Network is widely accepted for PDAC resectability status evaluation (Table 1) (4,5). In brief, a normal tissue plane between the tumor and vessels indicates a resectable disease, contact with the adjacent artery (>180°) or unreconstructable superior mesenteric vein or portal vein involvement indicates a locally advanced disease, and contact with the adjacent artery (≤180°) or venous involvement, which can be surgically resected and reconstructed, indicates a borderline resectable disease. Only few patients with PDAC are diagnosed with resectable disease and may undergo surgical resection, which is the only curative treatment modality for patients with PDAC (6).
Table 1
| Resectability | Arterial | Venous |
|---|---|---|
| Resectable | No arterial contact of the tumor including CA, SMA, and CHA | (I) No venous contact of the tumor including SMV and PV; (II) contact with SMV or PV but ≤180° without distorting the vein contour |
| Borderline resectable | Pancreatic head or uncinate process tumor: (I) contact with the SMA ≤180°; (II) contact with the CHA but no extension to CA or to the bifurcation of the hepatic artery, allowing safe and complete resection and reconstruction; (III) contact with variant artery | (I) Contact with the SMV or PV more than 180°; (II) contact with the SMV or PV ≤180° and contour irregularity of the vein or venous thrombosis with suitable vessel proximal and distal to the site of involvement enough for safe and complete resection and reconstruction; (III) contact with the IVC |
| Pancreatic body or tail tumor: (I) contact with the CA ≤180°; (II) contact with the CA more than 180° but no involvement of the aorta and with intact and uninvolved gastroduodenal artery, which allows the modified Appleby procedure (some panel members of the NCCN prefer these criteria to be in the locally advanced) | ||
| Locally advanced | Pancreatic head or uncinate process tumor: contact with the SMA or CA more than 180° | Unreconstructable SMV or PV due to tumor involvement or occlusion by the tumor itself or bland thrombus |
| Pancreatic body or tail tumor: (I) contact with the SMA or CA of more than 180°; (II) contact with the CA and aortic involvement |
CA, celiac artery; SMA, superior mesenteric artery; CHA, common hepatic artery; SMV, superior mesenteric vein; PV, portal vein; IVC, inferior vena cava; NCCN, National Comprehensive Cancer Network.
For borderline resectable pancreatic cancer (BRPC), neoadjuvant treatment followed by surgical resection is the standard treatment (4). Neoadjuvant therapy is aimed at achieving a higher R0 resection rate, which is well correlated with better survival outcomes (7-9). Neoadjuvant therapy allows the early treatment of micrometastasis, and unnecessary surgery is avoided in patients with unfavorable biology who does not respond to the neoadjuvant therapy (7). The application of modern chemotherapeutic regimens approved for the treatment of metastatic PDAC has strengthened the use of the neoadjuvant approach in patients with BRPC with higher response rates and longer survival outcomes (10-12). The regimens include a combination of 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFIRINOX) and gemcitabine plus nab-paclitaxel (GA), which were developed for the treatment of metastatic disease (13-18).
For selected patients with locally advanced pancreatic cancer (LAPC), accounting for one-third of all patients with PDAC, the standard first-line treatment is systemic chemotherapy with additional locoregional radiotherapy in selected patients (4). The clinical outcomes of LAPC treated with old-fashioned chemotherapy and chemoradiotherapy (CRT) were extremely poor, with a median overall survival (OS) of approximately 12 months (19). Regarding BRPC, the application of modern-era regimens for the treatment of patients with LAPC has also improved clinical outcomes and increased the surgery conversion rate (11,12). The use of the neoadjuvant approach for the management of patients with LAPC has been increasingly investigated, with a higher proportion of patients with LAPC undergoing conversion surgery (11,12). Recently, several well-written review articles have discussed updates of neoadjuvant treatments for BRPC, LAPC and resectable PDAC (11,12,20). In this review, we will discuss recent updates of neoadjuvant approach focusing on LAPC and how FOLFIRINOX and GA regimen have improved outcomes of LAPC patients. Also, we will discuss the role of CRT in modern era chemotherapeutic regimens when compared to conventional chemotherapies. Moreover, we will discuss conversion surgery and adjuvant therapy options for LAPC patients following neoadjuvant treatment. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-21-166/rc) (21).
Methods
We have reviewed the recent literature and up-to-date evidence to discuss current perspective of neoadjuvant approach for LAPC and BRPC. Reviewed literatures were searched by systematic search of PubMed and Google Scholar, including articles published in English between January 1st, 2013, and October 31st, 2021 on November 1st, 2021. The search terms used included locally advanced pancreatic cancer or borderline resectable pancreatic cancer, pancreatic cancer AND neoadjuvant and pancreatic cancer for PubMed search and neoadjuvant therapy for locally advanced pancreatic cancer and neoadjuvant therapy for borderline resectable pancreatic cancer for Google Scholar (Table 2). Review articles and original research articles including prospective trials and observational studies of locally advanced and borderline resectable pancreatic cancer written in English were included in the review. Non-English articles and case reports and case series were excluded.
Table 2
| Items | Specifications |
|---|---|
| Date of search | November 1st, 2021 |
| Database searched | PubMed and Google Scholar |
| Search terms used | PubMed: (I) locally advanced pancreatic cancer OR borderline resectable pancreatic cancer; (II) pancreatic cancer AND neoadjuvant; (III) pancreatic cancer |
| Google Scholar: (I) neoadjuvant therapy for locally advanced pancreatic cancer; (II) neoadjuvant therapy for borderline resectable pancreatic cancer | |
| Timeframe | Between January 1st 2013 and October 31st 2021 |
| Inclusion and exclusion criteria | Inclusion criteria—(I) Articles language: English; (II) Article type: literature reviews and original studies including prospective clinical trials and observational studies of locally advanced and borderline resectable pancreatic cancer |
| Exclusion criteria—(I) Articles language: non-English; (II) Article type: case studies and case series | |
| Selection process | Jaewon Hyung did the study selection and reviewed by Changhoon Yoo |
| Additional considerations | None |
Neoadjuvant chemotherapy
The efficacy of FOLFIRINOX as treatment for BRPC and LAPC has been widely investigated. A systematic review of 13 studies including 689 patients with BRPC and LAPC treated with neoadjuvant FOLFIRINOX showed a median OS of 10–32.7 months (22). A Korean phase 2 clinical trial of 44 patients with BRPC treated with neoadjuvant modified FOLFIRINOX followed by surgery and adjuvant gemcitabine showed an objective response rate (ORR) of 34.1%, a resection rate of 61.4% (R0 resection in 81.5%), and a median OS of 24.7 months (23). Similar results were reported in several retrospective studies on patients with BRPC treated with neoadjuvant FOLFIRINOX, with resection rates ranging from 41.7% to 87% (24,25).
The conversion surgery rate and survival outcomes of patients of LAPC treated with FOLFIRINOX were lower than those of patients with BRPC (22,26). In a systematic review, only 91 patients among 325 patients with LAPC underwent conversion surgery (resection rate: 28%, R0 resection rate: 74%) (22). Another systematic review of 14 studies involving 365 patients with LAPC showed similar outcomes, with a median OS of 8.9–25.0 months, a pooled resection rate of 28%, and an R0 resection rate of 77% (26). Other observational studies showed a resection rate of 19–60.8% among patients with LAPC treated with FOLFIRINOX (24,27-30). A previous analysis of 22 patients with LAPC treated with FOLFIRINOX reported an ORR of 27.3%, and resection was performed only in 5 (22.7%) patients who underwent additional CRT after receiving FOLFIRINOX as neoadjuvant treatment (27).
The efficacy of gemcitabine-based chemotherapy regimens for the treatment of patients with LAPC was also broadly investigated (Table 3). In a phase 3 study comparing the efficacy of gemcitabine plus S-1 and gemcitabine or S-1 monotherapy in 834 patients with LAPC or metastatic PDAC, no significant differences were observed in the survival outcomes between groups; moreover, a higher incidence of gastrointestinal and hematologic toxicity was documented in the combination arm (33). On the other hand, in a pooled analysis comparing the efficacy and safety of gemcitabine plus S-1 and gemcitabine alone in patients with PDAC, patients with LAPC treated with gemcitabine plus S-1 had significantly longer survival than those treated with gemcitabine alone, with median OS times of 16.4 months and 11.8 months, respectively (P=0.0220) (46). In a phase 1B trial of nab-paclitaxel, gemcitabine, capecitabine, and cisplatin in 24 patients with BRPC or LAPC, the ORR was 67%, with a resection rate of 25% (R0 resection rate 50%) and median OS of 18.1 months (34).
Table 3
| Phase | Condition | Intervention | Primary endpoint | No. of patients | ORR | Resection rate | R0 resection rate | Median OS (months) | Toxicity | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | ||||||||||
| Phase 2 | LAPC | Arm A: GA; Arm B: GA followed by FOLFIRINOX | Surgery conversion rate | Total 130 (Arm A: 64; Arm B: 66) | Arm A: 22%;Arm B: 17% | Arm A: 35.9%;Arm B: 43.9%;(P=0.38) | Arm A: 65%;Arm B: 63% | Arm A: 18.5;Arm B: 20.7;(P=0.53) | AE grade 3–4—Arm A: 55%; Arm B: 53% | Kunzmann et al. (31) |
| Phase 2 | LAPC | GA | Time to treatment failure | 107 | 33.60% | 16% | 43.80% | 18.8 | Neutropenia grade 3–4: 33% | Philip et al. (32) |
| Phase 3 | LAPC and MPC | Arm A: Gemcitabine; Arm B: S-1; Arm C: Gemcitabine + S-1 | Overall survival | 834 (202 LAPC patients, 24.3%) | Arm A: 13.3%;Arm B: 21%;Arm C: 29.3% | – | – | Arm A: 8.8;Arm B: 9.7;Arm C: 10.1 | – | Ueno et al. (33) |
| Phase 1B | BRPC and LAPC | Nab-paclitaxel + Gemcitabine + Cisplatin + Capecitabine | RP2D of nab-paclitaxel | 24 (18 LAPC patients, 75%) | 67% | 25% | 50% | 18.1 | AE grade 3–4: 67% | Reni et al. (34) |
| Phase 2 | BRPC | FOLFIRINOX followed by adjuvant gemcitabine | 1-year PFS | 44 | 34.10% | 61.40% | 81.50% | 24.7 | Neutropenia grade 3–4: 54.5% | Yoo et al. (23) |
| Phase 2 | BRPC | GA + S-1 | OS | 47 | 43% | 96% | 87% | 41.0 | AE grade 3–4: 30% | Kondo et al. (35) |
ORR, objective response rate; OS, overall survival; LAPC, locally advanced pancreatic cancer; BRPC, borderline resectable pancreatic cancer; GA, gemcitabine plus nab-paclitaxel; FOLFIRINOX, 5-fluorouracil and leucovorin plus irinotecan and oxaliplatin; AE, adverse event; RT, radiation therapy; SBRT, stereotactic body radiation therapy; PFS, progression-free survival; CRT, chemoradiation therapy; RP2D, recommended phase 2 dose.
Recently, the evaluation of the GA regimen used for the treatment of LAPC showed comparable outcomes to those of FOLFIRINOX (Table 3). A phase 2 LAPACT trial evaluated the efficacy of neoadjuvant GA administered in six cycles to 107 patients with LAPC and showed an ORR of 33.6% and a median OS of 18.8 months, although conversion surgery was performed in only 27 patients (resection rate: 25.2%) (32). Another phase 2 trial evaluated the efficacy of adding S-1 to the GA regimen for the treatment of patients with BRPC and arterial contact (35). In 47 patients who received six cycles of GA plus S-1 regimen, the ORR was 46%, with an R0 resection rate of 86% and a median OS of 41.0 months (35).
Several studies comparing the outcomes of patients treated with FOLFIRINOX and GA have shown similar outcomes for the treatment of LAPC. A phase 2 trial compared the efficacy of sequential chemotherapy with two cycles of GA followed by four cycles of FOLFIRINOX and that of six cycles of the GA regimen for the treatment of LAPC or BRPC (31). Among the 130 patients, no significant difference was observed between groups in terms of resection rate (35.9% vs. 43.9%, P=0.38) and median OS (18.2 vs. 20.7 months, P=0.53), with similar toxicity profiles (31). Previous retrospective studies comparing the efficacy of GA and FOLFIRINOX as treatment for LAPC have shown similar clinical outcomes (47-49). In a study including 147 patients with LAPC treated with either GA (60 patients) or FOLFIRINOX (87 patients), the resection rates were 16.7% (R0 resection rate: 88.9%) and 16.1% (R0 resection rate: 88.9%), and no significant difference was found in terms of median OS (15.7 vs. 16.7 months, P=0.7) (49). However, there is lack of robust clinical trial data comparing the two regimens as treatment for LAPC.
Neoadjuvant chemoradiotherapy
There are relatively more randomized clinical trial data indicating the use of CRT as treatment for BRPC or LAPC than the trials which used chemotherapy alone (Table 3). In a phase 2/3 study, the efficacy of neoadjuvant CRT [total radiation therapy (RT) dose: 54 Gy] combined with weekly administration of intravenous gemcitabine (400 mg/m2) within a 6-week period followed by surgical resection was compared to that of upfront surgery and adjuvant CRT according to the same schedule for the treatment of patients with BRPC (43). The neoadjuvant therapy group showed a significantly higher R0 resection rate (51.8% vs. 26.1%, P=0.004) and better OS outcomes than the upfront surgery group, with a hazard ratio (HR) at 2 years of 1.495 (P=0.028) (43). In addition, the phase 3 PREOPANC trial compared the efficacy of neoadjuvant gemcitabine-based CRT followed by surgery and additional postoperative gemcitabine and that of upfront surgery followed by 6 cycles of adjuvant gemcitabine for the treatment of BRPC and resectable PDAC (45). Among the 246 patients included, 133 were diagnosed with BRPC; the study failed to meet its primary endpoint with a median OS HR of 0.78 (P=0.096), although the follow-up data reported at the 2021 ASCO annual meeting showed better outcomes in the neoadjuvant arm than in the upfront surgery arm (HR: 0.72, P=0.025) (45,50). A subgroup analysis of 33 patients with BRPC showed significantly better R0 resection rate (79% vs. 13%, P<0.001) and survival (median OS: 17.6 vs. 13.2 months, P=0.029) in the neoadjuvant arm than in the upfront surgery arm (45).
However, the benefit of adding RT to neoadjuvant chemotherapy in the era of modern chemotherapy is unclear in the treatment of BRPC in terms of resection rate and survival outcomes. A phase 1 study on the efficacy of two cycles of GA followed by CRT with concurrent GA regimen proved the feasibility and safety of this regimen; the resection rate was 63.2% (R0 resection rate: 96%) (44). The feasibility and safety of FOLFIRINOX followed by CRT in patients with BRPC was also demonstrated in a phase 1 trial, although the outcomes were like those reported in the Korean phase 2 trial on neoadjuvant FOLFIRINOX alone as treatment for BRPC (23,51). The recently reported phase 2 trial failed to prove the efficacy of additional RT after FOLFIRINOX as neoadjuvant therapy for BRPC (42). The primary endpoint of the study was an 18-month OS rate of >50% compared to that of the historical cohort, and the patients were either treated with eight cycles of neoadjuvant modified FOLFIRINOX (mFOLFIRINOX) followed by surgery and adjuvant modified 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX) (70 patients) or seven cycles of neoadjuvant mFOLFIRINOX followed by hypofractionated RT or stereotactic body RT (SBRT) and surgical resection with adjuvant mFOLFOX (56 patients) (42). The neoadjuvant mFOLFIRINOX group had an 18-month OS rate of 66.4%, with a resection rate of 49% and preoperative Common Terminology Criteria for Adverse Event (CTCAE) grade of 3–4 in 64% patients, while the neoadjuvant mFOLFIRINOX + RT group had an 18-month OS of 47.3%, with a resection rate of 35% and a CTCAE grade of 3–4 in 57% patients (42). On the contrary, a phase 2 trial including 48 patients with BRPC treated with FOLFIRINOX followed by individualized CRT demonstrated promising outcomes (41). According to the FOLFIRINOX response, patients who showed resolution of vascular involvement received a short-course CRT (25 Gy/five fractions, proton), while those with persistent involvement received conventional CRT. Overall, the resection rate was 65%, with a R0 resection rate of 97% and median OS of 37.7 months (41).
For selected patients with LAPC, sequential CRT may be administered to patients after induction systemic chemotherapy or upfront RT or CRT may be administered to patients who are not suitable for induction chemotherapy (4). However, CRT after old-fashioned chemotherapy regimens failed to show better efficacy outcomes than modern chemotherapy regimens alone in several trials (Table 3). In a phase 2 study of 20 patients with LAPC treated with SBRT and five cycles of gemcitabine, the median OS was only 11.8 months (36). Another phase 2 trial of gemcitabine plus SBRT treatment including 49 patients with LAPC showed similar results, with a median OS of 13.9 months, and conversion surgery was performed in 4 patients (38). The phase 3 LAP07 trial compared the efficacy of additional CRT after induction chemotherapy and that of chemotherapy alone with gemcitabine-based therapy in patients with LAPC (39). A total of 449 patients received induction gemcitabine with or without erlotinib for 4 months, and patients exhibiting disease control and good performances were randomized to receive either CRT with capecitabine or 2 months of additional chemotherapy (39). Among the 269 patients randomized to receive either CRT or chemotherapy; no significant difference was observed between the CRT and chemotherapy in terms of the median OS (15.2 vs. 16.5 months, P=0.83) (39). In the phase 2 SCALOP trial, 74 patients with LAPC were treated with six cycles of neoadjuvant gemcitabine plus capecitabine followed by either gemcitabine plus RT or capecitabine plus RT (37). The median OS was better in patients who received RT with capecitabine than in patients who received gemcitabine (15.2 vs. 13.4 months, P=0.012); moreover, the results of long-term follow-up showed a longer median OS in patients treated with capecitabine-based RT than in those treated with gemcitabine-based RT (17.6 vs. 14.6 months) (37,52).
A recent phase 2 trial showed promising results of additional CRT (50.4 Gy) with capecitabine after eight cycles of neoadjuvant FOLFIRINOX in 49 patients with LAPC along with losartan; the resection rate was 69% (R0 resection rate: 88%), with a median OS of 33 months (40). RT can be administered to patients with LAPC who are not suitable to undergo surgical resection after receiving first-line chemotherapy. An observational study of 119 patients with LAPC treated with induction chemotherapy (mostly FOLFIRINOX) followed by ablative RT (biologically active dose: 98 Gy) with concurrent fluoropyrimidine reported a median OS of 26.8 months, with a 2-year locoregional progression rate of 32.8% (53).
However, existing randomized trials have reported insufficient information on the efficacy of RT in patients with LAPC. An ongoing randomized phase 2 trial (SABER: NCT04986930) comparing the efficacy of modified FOLFIRINOX + SBRT and that of mFOLFIRINOX as treatment for LAPC will provide a high level of evidence to determine whether RT has clinical relevance in the era of modern chemotherapy regimens as treatment for LAPC. The efficacy of SBRT during the earlier clinical course (first four cycles of mFOLFIRINOX) is being evaluated in this trial.
Conversion surgery and adjuvant therapy after neoadjuvant therapy
Current evidence suggests that surgical resection after neoadjuvant therapy in patients with LAPC is strongly associated with better survival outcomes. An observational study compared the survival outcomes in 293 patients with LAPC exhibiting disease control after receiving FOLFIRINOX who underwent and did not undergo surgery; in terms of OS after surgery and a propensity score matched HR of 0.344 (P<0.01) was reported (54). Another observational study also proved the benefit of surgical resection after neoadjuvant therapy in patients with BRPC and LAPC. Among patients who received surgery, there was no significant difference of clinical outcomes between patients with BRPC and LAPC in terms of OS (median OS 15.0 vs. 14.5 months, P=0.7) (54). An observational study of 135 patients with BRPC and LAPC who underwent conversion surgery after neoadjuvant chemotherapy showed promising outcomes, with a median OS of 29.7 and comparable safety in terms of postoperative complications (55).
It is challenging to predict resectability based on imaging studies after neoadjuvant therapy, and many studies have reported that the radiological appearance after neoadjuvant therapy does not reflect the patient’s response to therapy (56,57). An observational study of 188 PDAC patients who underwent surgery, the R0 resection rate was 92% among 40 patients with LAPC or BRPC who received neoadjuvant FOLFIRINOX despite the fact that 28 patients were still BRPC or LAPC by post-FOLFIRINOX imaging (58). In several studies, decrease or normalization of serum carbohydrate antigen (CA) 19-9 levels after neoadjuvant therapy was associated with better clinical outcomes (59-62). Surgical resection should be strongly considered for patients with LAPC without radiological progression after neoadjuvant therapy, especially in those with a good performance status and decreased CA 19-9 level (63). However, there are currently no established predictor of surgical resectability in patients with LAPC who have received neoadjuvant therapy (62). Several prognostic factors associated with better survival outcomes have been proposed, including CA 19-9, small baseline and post-treatment tumor size, and duration of neoadjuvant chemotherapy of longer than six cycles (62,64-67). Recently, a single center retrospective study showed that reduction of the tumor metabolic activity following neoadjuvant chemotherapy estimated by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)-CT was associated with improved survival outcomes for patients with BRPC or LAPC (68).
The role of adjuvant therapy in patients with LAPC who have undergone conversion surgery after neoadjuvant therapy is not well established (69). A large observational study including 2,016 patients who received neoadjuvant therapy and underwent surgical resection evaluated clinical outcomes according to the status of adjuvant chemotherapy with propensity score matching; adjuvant chemotherapy was associated with better OS than no adjuvant chemotherapy (median OS: 29.4 vs. 24.9 months, P<0.001), irrespective of the pathological nodal status and margin status (70). On the contrary, an observational study of patients who underwent conversion surgery after at least two cycles of FOLFIRINOX showed no difference in OS rates according to the adjuvant chemotherapy status (HR: 0.99, P=0.93), although adjuvant chemotherapy seemed to improve the outcomes of patients with pathological regional lymph node involvement (HR for OS: 0.41, P=0.004) (71). Both studies did not exclusively include patients with LAPC; hence, more prospective studies are needed to determine the benefit of adjuvant therapy and optimal regimen in patients with LAPC patients who have undergone conversion surgery.
Future directions
The application of modern-era chemotherapy regimens including FOLFIRINOX and GA and addition of CRT has improved survival outcomes and the conversion surgery rate in patients with LAPC. Figure 1 provides a summary our suggestion regarding the treatment flow for initially diagnosed patients with LAPC. However, several questions remain unanswered, and more robust randomized clinical trials data are needed. An optimal chemotherapy regimen should be established along with the role of additional CRT. Clinical trials should investigate the appropriate biomarkers to appropriately select high-risk patients who will benefit from additional CRT. In addition, the predictive biomarkers for surgical resectability after neoadjuvant therapy should be determined. Subsequently, additional studies should be conducted to investigate the role of adjuvant therapy after conversion surgery. The ongoing clinical trials registered at clinicaltrials.gov are listed in Table 4. Besides chemoradiation and surgical therapies, several studies also suggest the role of non-pharmacological management including exercise prescriptions and nutritional support, although high-quality evidence showing the efficacy of such interventions still lacking, and further investigations are warranted (72-75).
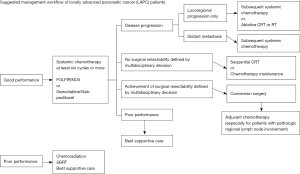
Table 4
| Clinicaltrial.gov | Condition | Intervention | Phase | Completion |
|---|---|---|---|---|
| NCT01821729 | LAPC | FOLFIRINOX + losartan followed by proton beam RT with capecitabine | Phase 2 | September 2021 |
| NCT02128100 | LAPC | FOLFIRINOX followed by SBRT | Phase 2 | May 2025 |
| NCT02578732 | LAPC | FOLFOX + nab-paclitaxel | Phase 2 | December 2021 |
| NCT02635971 | LAPC | GEMOX vs. IA GEMOX | Phase 2 | December 2022 |
| NCT03138720 | BRPC and LAPC | Nab-paclitaxel + GEMCIS + paricalcitol | Phase 2 | March 2022 |
| NCT03158779 | LAPC | FOLFIRINOX or GA followed by SBRT | Phase 2 | March 2022 |
| NCT03523312 | LAPC | Induction chemotherapy (FOLFIRINOX or GA) followed by ablative RT with capecitabine | Phase 2 | April 2023 |
| NCT03815461 | LAPC | Nab-paclitaxel + S-1 | Phase 2 | October 2023 |
| NCT03861702 | LAPC | FOLFOX + nal-irinotecan | Phase 2 | March 2023 |
| NCT03885219 | LAPC | Nab-paclitaxel + S-1 | Phase 2 | April 2022 |
| NCT04089150 | BRPC and LAPC | GA or FOLFIRINOX vs. GA or FOLFIRINOX + SBRT followed by adjuvant GEMCAP or FOLFIRINOX | Phase 2 | August 2023 |
| NCT04481204 | Non-metastatic PDAC | Bayesian platform: Gemcitabine, GA, GemCis or FOLFIRINOX 3–6 months ± RT | Phase 2 | April 2025 |
| NCT04539808 | Non-metastatic PDAC | FOLFIRINOX (switch to GA if progression or toxicity) followed by CRT with capecitabine + losartan | Phase 2 | October 2025 |
| NCT04570943 | LAPC | Sequential GA and FOLFIRINOX followed by SBRT | Phase 2 | October 2026 |
| NCT04986930 | LAPC | FOLFIRINOX vs. FOLFIRINOX + SBRT | Phase 2 | August 2024 |
| NCT02024009 | LAPC | GA vs. GA followed by CCRT (capecitabine) vs. GA followed by CRT (capecitabine) + nelfinavir | Phase 2 | August 2020 |
| NCT01827553 | LAPC | Induction chemotherapy followed by CRT with gemcitabine vs. chemotherapy only (FOLFIRINOX or gemcitabine) | Phase 3 | April 2022 |
| NCT01926197 | LAPC | FOLFIRINOX vs. FOLFIRINOX + SBRT | Phase 3 | September 2022 |
| NCT03257033 | LAPC | Nab-paclitaxel and gemcitabine + RT followed by IA gemcitabine vs. nab-paclitaxel and gemcitabine | Phase 3 | September 2023 |
| NCT03941093 | LAPC | Pamrevlumab + chemotherapy vs. chemotherapy (FOLFIRINOX or gemcitabine + nab-paclitaxel) | Phase 3 | December 2023 |
| NCT03983057 | BRPC and LAPC | FOLFIRINOX vs. FOLFIRINOX + anti-PD1 | Phase 3 | April 2024 |
| NCT04617821 | BRPC and LAPC | Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX | Phase 3 | September 2023 |
| NCT03899636 | LAPC | FOLFIRINOX vs. FOLFIRINOX + IRE with Nanoknife | Phase 3 | December 2023 |
LAPC, locally advanced pancreatic cancer; BRPC, borderline resectable pancreatic cancer; FOLFIRINOX, a combination of 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan; RT, radiation therapy; SBRT, stereotactic body radiation therapy; FOLFOX, a combination of 5-fluorouracil, leucovorin, and oxaliplatin; GEMOX, gemcitabine plus oxaliplatin; GEMCIS, gemcitabine plus cisplatin; GA, gemcitabine plus nab-paclitaxel; GEMCAP, gemcitabine plus capecitabine; CRT, chemoradiation therapy; PDAC, pancreatic ductal adenocarcinoma; IRE, irreversible electroporation; IA, intra-arterial.
Moreover, precision medicine should be introduced to help select the optimal therapeutic modalities for patients with LAPC (7). Studies to investigate molecular and immune biomarkers should be performed to predict the efficacy of preoperative treatment and clinical outcomes. In a previous retrospective study of 49 patients with LAPC who received neoadjuvant therapy followed by surgery, loss of SMAD4 protein was associated with poor survival outcomes (76). In a phase 2 clinical trial of neoadjuvant mFOLFIRINOX and sequential CRT combined with losartan in patients with LAPC, the serum transforming growth factor ß and thrombospondin 1 levels significantly decreased after administration of neoadjuvant therapy compared to those at baseline (40). Systemic inflammatory and immune biomarkers, including low fibrinogen to albumin ratio, lower peripheral monocytes, and higher CD69+ gamma delta T cells levels at baseline, have been shown to be associated with better survival outcomes in patients with BRPC and LAPC (23,77). Recently, a biomarker study of patients with resectable pancreatic cancer showed that a circulating tumor DNA (ctDNA) detected at baseline and postoperative peripheral blood samples were associated with poor survival outcomes, and all patients with postoperative ctDNA positive results had recurrence (78). The serial measurement of ctDNA during treatment along with tumor molecular profiling using next-generation sequencing techniques may help determine the novel biomarkers for LAPC. Although the use of targeted agents and immunotherapies is suggested for the treatment of patients with LAPC, only a few options are available, including pembrolizumab and olaparib, with minimal efficacy for patients with PDAC (79,80).
In conclusion, modern-era chemotherapeutics, including FOLFIRINOX and GA, and additional CRT have markedly improved the clinical outcomes of BRPC and LAPC patients. Particularly, the number of LAPC patients receiving conversion surgery which was provided for only a small proportion of patients is growing along with the application of FOLFIRINOX and GA.
However, there are limitations in the current practice as large randomized trials aimed at identifying the best treatment options for this patient group are ongoing. Hence, future clinical trials will improve the management of patients with LAPC. Molecular and immunologic biomarker studies are also warranted to determine whether precision medicine should be applied.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Yoji Kishi) for the series “Pre- and Post-operative Treatment for Pancreatic Cancer” published in Chinese Clinical Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-21-166/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-21-166/coif). The series “Pre- and Post-operative Treatment for Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet 2020;395:2008-20. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Viale PH. The American Cancer Society's Facts & Figures: 2020 Edition. J Adv Pract Oncol 2020;11:135-6. [PubMed]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014;270:248-60. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Casolino R, Braconi C, Malleo G, et al. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine. Ann Oncol 2021;32:183-96. [Crossref] [PubMed]
- Hank T, Hinz U, Tarantino I, et al. Validation of at least 1 mm as cut-off for resection margins for pancreatic adenocarcinoma of the body and tail. Br J Surg 2018;105:1171-81. [Crossref] [PubMed]
- Tummers WS, Groen JV, Sibinga Mulder BG, et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg 2019;106:1055-65. [Crossref] [PubMed]
- Janssen QP, Buettner S, Suker M, et al. Neoadjuvant FOLFIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J Natl Cancer Inst 2019;111:782-94. [Crossref] [PubMed]
- Oba A, Ho F, Bao QR, et al. Neoadjuvant Treatment in Pancreatic Cancer. Front Oncol 2020;10:245. [Crossref] [PubMed]
- Müller PC, Frey MC, Ruzza CM, et al. Neoadjuvant Chemotherapy in Pancreatic Cancer: An Appraisal of the Current High-Level Evidence. Pharmacology 2021;106:143-53. [Crossref] [PubMed]
- Ychou M, Conroy T, Seitz JF, et al. An open phase I study assessing the feasibility of the triple combination: oxaliplatin plus irinotecan plus leucovorin/ 5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol 2003;14:481-9. [Crossref] [PubMed]
- Conroy T, Paillot B, François E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol 2005;23:1228-36. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Goldstein D, El-Maraghi RH, Hammel P, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 2015;107:dju413. [Crossref] [PubMed]
- Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007;25:2607-15. [Crossref] [PubMed]
- Raufi AG, Manji GA, Chabot JA, et al. Neoadjuvant Treatment for Pancreatic Cancer. Semin Oncol 2019;46:19-27. [Crossref] [PubMed]
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med 2006;5:101-17. [Crossref] [PubMed]
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801-10. [Crossref] [PubMed]
- Yoo C, Lee SS, Song KB, et al. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a Phase 2 study for clinical and biomarker analysis. Br J Cancer 2020;123:362-8. [Crossref] [PubMed]
- Barenboim A, Lahat G, Geva R, et al. Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: An intention to treat analysis. Eur J Surg Oncol 2018;44:1619-23. [Crossref] [PubMed]
- Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013;108:236-41. [Crossref] [PubMed]
- Rombouts SJ, Walma MS, Vogel JA, et al. Systematic Review of Resection Rates and Clinical Outcomes After FOLFIRINOX-Based Treatment in Patients with Locally Advanced Pancreatic Cancer. Ann Surg Oncol 2016;23:4352-60. [Crossref] [PubMed]
- Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18:543-8. [Crossref] [PubMed]
- Hackert T, Sachsenmaier M, Hinz U, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg 2016;264:457-63. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Nitsche U, Wenzel P, Siveke JT, et al. Resectability After First-Line FOLFIRINOX in Initially Unresectable Locally Advanced Pancreatic Cancer: A Single-Center Experience. Ann Surg Oncol 2015;22:S1212-20. [Crossref] [PubMed]
- Kunzmann V, Siveke JT, Algül H, et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:128-38. [Crossref] [PubMed]
- Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol 2020;5:285-94. [Crossref] [PubMed]
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. [Crossref] [PubMed]
- Reni M, Balzano G, Zanon S, et al. Phase 1B trial of Nab-paclitaxel plus gemcitabine, capecitabine, and cisplatin (PAXG regimen) in patients with unresectable or borderline resectable pancreatic adenocarcinoma. Br J Cancer 2016;115:290-6. [Crossref] [PubMed]
- Kondo N, Uemura K, Sudo T, et al. A phase II study of gemcitabine/nab-paclitaxel/S-1 combination neoadjuvant chemotherapy for patients with borderline resectable pancreatic cancer with arterial contact. Eur J Cancer 2021;159:215-23. [Crossref] [PubMed]
- Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011;81:e615-22. [Crossref] [PubMed]
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317-26. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5:1020-7. [Crossref] [PubMed]
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:963-9. [Crossref] [PubMed]
- Katz MHG, Shi Q, Meyers JP, et al. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol 2021;39:abstr 377.
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- Takahashi H, Akita H, Ioka T, et al. Phase I Trial Evaluating the Safety of Preoperative Gemcitabine/nab-Paclitaxel With Concurrent Radiation Therapy for Borderline Resectable Pancreatic Cancer. Pancreas 2018;47:1135-41. [Crossref] [PubMed]
- Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol 2020;38:1763-73. [Crossref] [PubMed]
- Hamada C, Okusaka T, Ikari T, et al. Efficacy and safety of gemcitabine plus S-1 in pancreatic cancer: a pooled analysis of individual patient data. Br J Cancer 2017;116:1544-50. [Crossref] [PubMed]
- Arima S, Kawahira M, Shimokawa M, et al. Gemcitabine Plus Nab-Paclitaxel Versus FOLFIRINOX in Locally Advanced, Unresectable Pancreatic Cancer: A Multicenter Observational Study (NAPOLEON Study). Pancreas 2021;50:957-64. [Crossref] [PubMed]
- Macedo FI, Ryon E, Maithel SK, et al. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg 2019;270:400-13. [Crossref] [PubMed]
- Williet N, Petrillo A, Roth G, et al. Gemcitabine/Nab-Paclitaxel versus FOLFIRINOX in Locally Advanced Pancreatic Cancer: A European Multicenter Study. Cancers (Basel) 2021;13:2797. [Crossref] [PubMed]
- Eijck CHJV, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: Long-term results of the multicenter randomized phase III PREOPANC trial. J Clin Oncol 2021;39:abstr 4016.
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151:e161137. [Crossref] [PubMed]
- Hurt CN, Falk S, Crosby T, et al. Long-term results and recurrence patterns from SCALOP: a phase II randomised trial of gemcitabine- or capecitabine-based chemoradiation for locally advanced pancreatic cancer. Br J Cancer 2017;116:1264-70. [Crossref] [PubMed]
- Reyngold M, O'Reilly EM, Varghese AM, et al. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol 2021;7:735-8. [Crossref] [PubMed]
- Brada LJH, Daamen LA, Magermans LG, et al. Survival Benefit Associated With Resection of Locally Advanced Pancreatic Cancer After Upfront FOLFIRINOX Versus FOLFIRINOX Only: Multicenter Propensity Score-matched Analysis. Ann Surg 2021;274:729-35. [Crossref] [PubMed]
- Yoo C, Shin SH, Kim KP, et al. Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis. Cancers (Basel) 2019;11:278. [Crossref] [PubMed]
- Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [Crossref] [PubMed]
- Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol 2013;2:413-25. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Truty MJ, Kendrick ML, Nagorney DM, et al. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann Surg 2021;273:341-9. [Crossref] [PubMed]
- Satoi S, Yamamoto T, Yamaki S, et al. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg 2019;4:6-13. [Crossref] [PubMed]
- Tsai S, George B, Wittmann D, et al. Importance of Normalization of CA19-9 Levels Following Neoadjuvant Therapy in Patients With Localized Pancreatic Cancer. Ann Surg 2020;271:740-7. [Crossref] [PubMed]
- Michelakos T, Pergolini I, Castillo CF, et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann Surg 2019;269:733-40. [Crossref] [PubMed]
- Fong ZV, Ferrone CR. Surgery After Response to Chemotherapy for Locally Advanced Pancreatic Ductal Adenocarcinoma: A Guide for Management. J Natl Compr Canc Netw 2021;19:459-67. [Crossref] [PubMed]
- Klaiber U, Schnaidt ES, Hinz U, et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann Surg 2021;273:154-62. [Crossref] [PubMed]
- Wijetunga AR, Chua TC, Nahm CB, et al. Survival in borderline resectable and locally advanced pancreatic cancer is determined by the duration and response of neoadjuvant therapy. Eur J Surg Oncol 2021;47:2543-50. [Crossref] [PubMed]
- Choi YH, Lee SH, You MS, et al. Prognostic Factors for Patients with Borderline Resectable or Locally Advanced Pancreatic Cancer Receiving Neoadjuvant FOLFIRINOX. Gut Liver 2021;15:315-23. [Crossref] [PubMed]
- Brada LJH, Walma MS, Daamen LA, et al. Predicting overall survival and resection in patients with locally advanced pancreatic cancer treated with FOLFIRINOX: Development and internal validation of two nomograms. J Surg Oncol 2021;124:589-97. [Crossref] [PubMed]
- Lee W, Oh M, Kim JS, et al. Metabolic activity by FDG-PET/CT after neoadjuvant chemotherapy in borderline resectable and locally advanced pancreatic cancer and association with survival. Br J Surg 2021;109:61-70. [Crossref] [PubMed]
- Turpin A, El Amrani M, Bachet JB, et al. Adjuvant Pancreatic Cancer Management: Towards New Perspectives in 2021. Cancers (Basel) 2020;12:3866. [Crossref] [PubMed]
- Kamarajah SK, White SA, Naffouje SA, et al. Adjuvant Chemotherapy Associated with Survival Benefit Following Neoadjuvant Chemotherapy and Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Population-Based Cohort Study. Ann Surg Oncol 2021;28:6790-802. [Crossref] [PubMed]
- van Roessel S, van Veldhuisen E, Klompmaker S, et al. Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment. JAMA Oncol 2020;6:1733-40. [Crossref] [PubMed]
- O'Connor D, Brown M, Eatock M, et al. Exercise efficacy and prescription during treatment for pancreatic ductal adenocarcinoma: a systematic review. BMC Cancer 2021;21:43. [Crossref] [PubMed]
- Luo H, Galvão DA, Newton RU, et al. Feasibility and efficacy of a multicomponent exercise medicine programme in patients with pancreatic cancer undergoing neoadjuvant therapy (the EXPAN trial): study protocol of a dual-centre, two-armed phase I randomised controlled trial. BMJ Open Gastroenterol 2021;8:e000642. [Crossref] [PubMed]
- Akita H, Takahashi H, Asukai K, et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin Nutr ESPEN 2019;33:148-53. [Crossref] [PubMed]
- Trestini I, Carbognin L, Bonaiuto C, et al. Prognostic impact of nutritional support in patients affected by locally advanced or metastatic pancreatic cancer (PC) undergone chemotherapy. Ann Oncol 2017;28:vi47. [Crossref]
- Kadera BE, Sunjaya DB, Isacoff WH, et al. Locally advanced pancreatic cancer: association between prolonged preoperative treatment and lymph-node negativity and overall survival. JAMA Surg 2014;149:145-53. [Crossref] [PubMed]
- Fang L, Yan FH, Liu C, et al. Systemic Inflammatory Biomarkers, Especially Fibrinogen to Albumin Ratio, Predict Prognosis in Patients with Pancreatic Cancer. Cancer Res Treat 2021;53:131-9. [Crossref] [PubMed]
- Lee B, Lipton L, Cohen J, et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann Oncol 2019;30:1472-8. [Crossref] [PubMed]
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353-65. [Crossref] [PubMed]
- Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019;381:317-27. [Crossref] [PubMed]


