Advances in systemic treatment for nasopharyngeal carcinoma
Current systemic treatment for nasopharyngeal carcinoma (NPC)
The optimal treatment of NPC involves a multidisciplinary approach. NPC (especially the endemic subtype) is a radiosensitive tumor, and as its deep-seated anatomic location limits a surgical approach, radiotherapy (RT) has been the mainstay and primary curative treatment modality. Whilst early stage NPC can be treated with RT alone with five year survival rates of more than 90% (1); survival rates decline with increasing tumor and nodal stage to only 50–70% at 5 years with locally advanced disease. Unfortunately, more than half of all patients will present at an advanced stage, with 10% of patients harboring distant metastases at the time of initial diagnosis. The development of concurrent chemoradiation (CRT) strategies has been important in improving treatment outcomes in locally advanced NPC, with no fewer than nine randomized clinical trials demonstrating that addition of concurrent chemotherapy during radiation leads to improved progression-free survival and response, and with overall survival (OS) benefit being demonstrated in the majority of trials (Table 1).
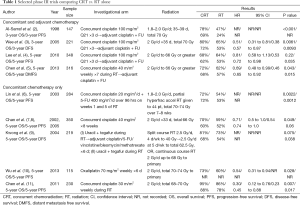
Full table
The landmark phase III Intergroup 0099 (INT-0099) compared CRT with high dose cisplatin followed by adjuvant fluorouracil (5-FU) and cisplatin chemotherapy versus conventional RT alone, and showed a significant survival benefit of 31% increase in 3-year OS with the addition of chemotherapy (2). Following this, many other trials, including Phase III trials published from Singapore and Hong Kong (3-7) also demonstrated similar, albeit smaller degree of benefit from concomitant chemotherapy. Cisplatin was employed in the majority of studies, usually either at a high dose 3-weekly (at 80–100 mg/m2) or a weekly low dose at 30–40 mg/m2 during RT. While no study has compared the efficacy of the weekly vs. 3-weekly cisplatin regimen, several retrospective analyses appear to show similar outcomes (12-14), and the published data supports the conclusion that either of these dosing regimens is acceptable and equivalent in terms of toxicity and efficacy. The cumulative dose of cisplatin delivered during CRT may impact on locoregional control and OS (15,16), and the survival benefit may be larger in patients with T3 and T4 tumors (8,10). Other drugs such as oxaliplatin, tegafur-uracil have been evaluated only in limited studies (9,17) and do not yet form part of standard therapy. In patients with borderline renal function, or who experience significant toxicities associated with high dose cisplatin, carboplatin can be considered as a substitute for cisplatin, based on a phase III randomized non-inferiority trial which demonstrated equivalent survival outcomes with either cisplatin or carboplatin concurrent with RT and followed by adjuvant chemotherapy (18). As expected, patients treated with cisplatin experienced more renal toxicity, leucopenia, anemia, nausea and vomiting, whereas patients with carboplatin had more thrombocytopenia. A higher proportion of patients in the carboplatin arm completed the planned CRT as well as the planned adjuvant therapy.
Three meta-analyses have also supported the benefit of chemotherapy, reporting an 18% reduction in the risk of death and absolute survival benefit of 4% to 6% at 5 years (19-21). The latest update from the MAC-NPC meta-analysis (22) which included 19 trials and 4,806 patients, with a median follow up of 7.7 years, confirmed that the addition of concomitant chemotherapy to RT significantly improves survival in patients with locoregionally advanced NPC (HR for OS 0.79, P<0.0001; absolute benefit at 5 years 6.3%).
Adjuvant and neoadjuvant chemotherapy for non-metastatic advanced NPC
While there is established benefit from concomitant chemotherapy with RT, the role of adjuvant chemotherapy after CRT is uncertain. Initial trials of chemotherapy in locally advanced disease involved administration of chemotherapy concurrently during RT followed by adjuvant chemotherapy (usually comprising of platinum and 5-FU, i.e., PF), based on the observation that a significant proportion of patients with NPC relapse at distant sites despite local control of disease. These studies demonstrated an OS benefit from this strategy. However, compliance to adjuvant chemotherapy was a significant problem with only about 50–75% of patients who were initially planned for adjuvant chemotherapy receiving the three planned cycles. Furthermore, there were significant toxicities associated with administration of adjuvant chemotherapy, with 23–43% of patients experiencing grade 3–4 toxicities (2-4). In addition, other studies which evaluated concurrent chemotherapy without adjuvant chemotherapy (8,10,17) yielded similar outcomes compared to trials comprising concurrent chemotherapy and adjuvant, calling into question the true benefit of adjuvant chemotherapy to disease control in NPC. However, in the MAC-NPC meta-analysis, the subgroup of patients receiving CRT followed by adjuvant chemotherapy appeared to have a bigger survival benefit compared with CRT alone (HR 0.65, 95% CI, 0.56–76 vs. HR 0.80, 95% CI, 0.70–0.93) (22).
A Chinese phase III trial looked at the role of adjuvant chemotherapy following CRT in 508 patients with non-metastatic advanced NPC (23); patients were randomized to adjuvant chemotherapy (three cycles of cisplatin plus 5-FU) or observation, following CRT consisting of weekly cisplatin at 40 mg/m2 for maximum of seven cycles, starting from day one of RT. After a median follow-up of 37.8 months, there was no statistically significant improvement in the 2-year failure-free survival rates (86% vs. 84%, P=0.13). This study had been criticized for its relatively short follow-up period, exclusion of T3–4N0 patients, variability of RT technique and the choice of chemotherapy regimen; furthermore, only 63% of patients could complete planned chemotherapy.
The role of induction chemotherapy followed by RT or CRT is similarly uncertain. In theory, induction or neoadjuvant chemotherapy may control micrometastases earlier and facilitate RT planning by downstaging locally advanced tumors, especially for large T4 lesions, advanced nodal disease, or when delivery of a full course RT is challenging due to close proximity to critical structures (like the optic bundle and brain). However, to date, the phase III studies on neoadjuvant chemotherapy followed by RT alone have not shown any difference in OS compared with RT alone (24-26). The updated MAC-NPC meta-analysis included data from 6 trials on induction chemotherapy and showed a statistically significant improvement in progression free survival but not in OS (22). A randomized phase II trial by Hui and colleagues showed that neoadjuvant docetaxel plus cisplatin followed by concurrent cisplatin-RT was feasible with manageable toxicities compared to CRT alone, with positive survival impact (3-year PFS for neoadjuvant vs. control was 88.2% and 59.5%, P=0.12; 3-year OS was 94.1% vs. 67.7% respectively, P=0.012) (27). However, subsequent randomized phase 3 trials evaluating neoadjuvant triplet chemotherapy prior to CRT versus CRT alone did not show any benefit of neoadjuvant chemotherapy on PFS nor OS (28,29). In the phase 2/3 study reported by Tan et al. (29), a triplet regimen of gemcitabine, carboplatin and paclitaxel was administered for three cycles followed by CRT with weekly cisplatin 40 mg/m2 and compared with the same CRT regimen alone. There was no difference in outcomes in both arms. Conversely, while there was good compliance to neoadjuvant therapy, with no statistically significant difference in grade 3 and 4 radiation toxicities as well as similar global quality life scores between the two arms, the neoadjuvant arm had lower dose intensity of cisplatin compared with the control arm during the CRT period, and higher rates of leukopenia and neutropenia (29). This highlights the main concern of induction/neoadjuvant approaches possibly compromising on effective dose delivery of chemotherapy and/or radiation during the CRT period, or increasing toxicities, thus offsetting any potential benefits of an induction-based approach.
In light of this, additional phase III clinical trials are underway to confirm the optimal approach. The Hong Kong Nasopharyngeal Cancer Study Group conducted a 6-arm study (NCT00379262) comparing concurrent-adjuvant chemotherapy (using the Intergroup 0099 regimen of concurrent cisplatin-RT and adjuvant PF as the standard arm) with induction-concurrent chemotherapy. It also explored the benefit of replacing 5-FU with capecitabine, and the use of accelerated RT vs. conventional RT fractionation. Preliminary analyses did not meet its study endpoints, as induction PF versus adjuvant PF did not indicate any significant improvement in outcome; other results suggested that oral capecitabine may be a safe substitute for 5-FU, but accelerated RT is not recommended for patients with locally advanced NPC who receive CRT due to higher toxicities (30). As final results of this study are awaited, two other ongoing studies are ongoing in China are evaluating induction TPF followed by CRT compared with CRT alone (NCT01245959), and induction TPF compared with induction PF followed by CRT (NCT01536223). Overall, the role of neoadjuvant chemotherapy in stage III-IVB NPC at present is still investigational and not standard of care.
While current data has not fully defined the role of adjuvant chemotherapy, the current focus of research has shifted somewhat towards two strategies of (I) identification of patient subgroups that may benefit most from adjuvant chemotherapy; and (II) exploration of different chemotherapy regimens apart from cisplatin/5-FU. Based on previous findings that elevated levels of EBV post-treatment correlated with higher risk of recurrent cancer, the NRG-HN001 (NCT02135042) trial will stratify patients based on post-treatment EBV levels. In this study, patients with detectable EBV levels will be randomized to standard cisplatin/5-FU versus gemcitabine/paclitaxel (randomized phase II), while patients with undetectable EBV DNA levels will be randomized between standard adjuvant cisplatin/5-FU versus observation (phase III). Another ongoing study initiated in Hong Kong randomizes patients with residual EBV DNA to either adjuvant gemcitabine/cisplatin chemotherapy or observation (NCT00370890). These studies will enable better understanding of the benefit, if any, of adjuvant and/or induction chemotherapy, help select the optimal patient population who will benefit, and delineate the optimal chemotherapy regimen.
Systemic treatment for stage II NPC
Although the prognoses of patients with stage I and stage II NPC are generally excellent, a few studies have highlighted that particularly in stage II NPC with nodal disease, survival rates may be poorer than stage I disease with more frequent loco-regional as well as distant recurrences and survival rates of only about 73.1% in certain series (31,32). In patients treated with IMRT alone, 5-year distant-metastases-free survival rate was 94% in patients with T2N1 disease compared with 99–100% for T1–2N0 or T2N0 NPC (33). Chen et al. randomized patients with previously untreated stage II NPC (T1–2N1M0 or T2N0M0 disease with parapharyngeal space involvement) to concurrent weekly cisplatin at 30 mg/m2 during RT and RT alone. They reported a significant improvement in OS (5-year OS 95% vs. 86%, HR 0.30, P=0.007) (11). However, there are some caveats from this study, such as routine body computed tomography not being included in the pre-treatment staging, and all patients having undergone 2D RT when IMRT is now treatment of choice. It is also noteworthy that 31 of these patients (13%) were upstaged to stage III when they were restaged according to the 2010 revised TMN staging system. The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology (version 1.2015) (34) recommends CRT for patients with stage II NPC; so does the European Head and Neck Society–European Society for Medical Oncology-European Society of Radiotherapy and Oncology Clinical practice guidelines (35). Given the significant toxicities of concurrent chemoradiotherapy and the generally excellent prognosis of stage II nasopharyngeal cancer with IMRT, the role of administering chemotherapy concurrently with radiation in all stage II patients remains to be clearly defined, although consideration on individual bases should be made based on risk factors such as significant nodal disease, parapharyngeal tumor extension, and plasma EBV level.
Limitations and ongoing investigative approaches in locally advanced NPC
Despite significant improvements in outcomes in NPC with administration of concurrent chemotherapy with radiation, survival in patients with locally advanced NPC is still only 50–70% at 5 years, with a substantial proportion of patients experiencing relapse either loco-regionally, or at distant sites, or both. Given the already significant toxicities of CRT, addition of further chemotherapeutic agents to current treatment regimens is not a feasible approach. Instead, further research is directed towards firstly identifying and defining patients at high risk of relapse prior to, and at the end of CRT, for which further investigative approaches can be focused on. Secondly, use of biological agents concurrently with RT or with chemoradiotherapy is being evaluated.
Overexpression of epidermal growth factor receptor (EGFR) is present in 80% or more of NPC, and is associated with poorer survival outcome (36,37). In head and neck squamous cell carcinoma, administration of cetuximab, a chimeric monoclonal antibody directed against EGFR, concurrently with RT in locally advanced HNSCC led to significant improvement in OS compared with RT alone (38). In NPC however, evaluation of cetuximab concurrently with RT has not been shown to be more efficacious compared with standard CRT, and was associated with increased mucositis rates (39). The combination of cetuximab, weekly cisplatin and IMRT was evaluated in stage III/IVA and IVB patients (40). This study demonstrated significant mucositis in more than 80% of patients, grade 3 radiation dermatitis in 20% of patients and acneiform rash related to cetuximab in 10% of patients, with 2-year PFS rate of 89%. Nimotuzumab is a humanized monoclonal antibody against EGFR which has significantly lower rates of mucosal and skin toxicities (41). The combination of nimotuzumab concurrently with radiation or CRT has shown efficacy in improving locoregional control and OS in locally advanced HNSCC (42); although its role in NPC remains to be defined (43).
The feasibility of administration of bevacizumab, a monoclonal antibody directed against VEGF, concurrently with chemoradiotherapy in stage IIB–IVB NPC was evaluated in a phase II trial, with grade 4 hemorrhage or grade 5 adverse events as the primary end point. Neither grade 3 or higher hemorrhages nor grade 5 events were recorded, yielding 2-year PFS of 74.7% and 2-year OS of 90.9% (44).
Clearly, a therapeutic plateau in the refinement of therapy in locally advanced NPC has been reached, with combinations of biological agents or molecularly targeted therapies with RT/CRT not showing significant improvements over standard therapy and at the cost of incremental toxicities. A key limitation of these studies that raises concern is about increasing short- and long-term side effects in this patient population of which more than half may be cured of their cancer eventually.
Palliative chemotherapy for metastatic and recurrent NPC
NPC is a chemo-sensitive tumor and palliative chemotherapy plays an important role in disease control and prolonging survival in the metastatic setting. Standard treatment comprises chemotherapy with platinum doublets of drugs such as gemcitabine, paclitaxel, and 5-FU together with cisplatin/carboplatin. For treatment-naive patients who receive platinum-based chemotherapy, response rates as high as 80% and a median survival of 12 to 18 months may be achieved (45). Higher response rates are associated with combination regimens rather than monotherapy, and no particular platinum regimen is regarded as superior or as standard of care. However, regardless of the chosen first line treatment regimen, median time to progression remains relatively constant and static at 7–10 months (46-48); in part due to the development of platinum resistance. A triplet drug regimen of gemcitabine/carboplatin/paclitaxel showed impressive response rates of nearly 80%, however the reported median duration of response of 8 months was similar to historical controls of two-drug regimens (49). In patients progressing after first line platinum therapy, common cytotoxic agents used for second line include 5-FU (including capecitabine), taxanes (paclitaxel, docetaxel), irinotecan, vinorelbine, and gemcitabine, but response rates are generally lower compared to first-line therapy. Phase II trials of second-line monotherapy or combination regimens reported response rates ranging 14% to 48% (50-53) and to date there is similarly no single treatment regimen that is considered as the standard of care. Further phase III trials are needed to establish the optimal palliative chemotherapy regimen. On the other hand, it is increasingly clear that further utility of chemotherapy beyond 2nd or 3rd line therapy may not yield significant and meaningful prolongation of survival in a majority of patients.
Novel therapies: molecular-targeted agents, immunotherapy and vaccines
The development of novel therapies in NPC has been somewhat slow, with little advances beyond standard cytotoxic approaches in the past 10 years and only exploratory phase II studies. Molecular agents that inhibit EGFR-mediated signaling pathways leading to cell growth suppression and cell apoptosis in NPC include monoclonal antibodies such as cetuximab (37), and tyrosine kinase inhibitors such as gefitinib (54). A multicenter phase II study by Chan and colleagues (55) evaluated the combination of cetuximab and carboplatin in platinum-resistant recurrent NPC, and demonstrated clinical activity with an overall response rate of 11% and acceptable safety profile. However, the response rates and PFS do not appear superior to chemotherapy alone. Gefitinib monotherapy had poor response rates in a phase II single-center study in recurrent and metastatic NPC pretreated with platinum-based chemotherapy (56).
Multi-kinase inhibitors target various receptor tyrosine kinases such as platelet derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR) and stem cell factor receptor (c-KIT). These are involved in the initiation of various cascades of intracellular signaling events that lead to cell proliferation and/or influence processes critical to cell survival and tumor progression, such as angiogenesis, inhibition of apoptosis and metastasis, on the basis that simultaneous inhibition of these targets may reduce tumor vascularization and trigger cancer cell apoptosis and lead to tumor control. Drugs such as sorafenib (VEGFR, PDGFR, Raf kinases), pazopanib (VEGFR, PDGFR, FGFR, c-KIT), and sunitinib (PDGFR, VEGFR, C-KIT), have been evaluated in NPC (57-60). Pazopanib was evaluated in 33 patients at a dose of 800mg daily, this demonstrated a modest response rate of 6.1%, disease stabilization in more than 50% patients and notably 21% of patients had partial response/stable disease lasting at least 6 months. However, two grade 5 events of tumor hemorrhage occurred. Other toxic effects were fatigue, hand-foot syndrome, anorexia and gastro-intestinal side effects (57). The use of sorafenib monotherapy at a dose of 400 mg twice daily showed a response rate of 3.7%, stabilization of disease in 37%, but only modest survival with time to progression of 1.8 months and median OS 4.2 months, albeit with good tolerability in a phase II trial (58). While the combination of sorafenib with cisplatin and 5-FU showed good response rates of more than 70% and median PFS of 7.2 months and median OS of 11.8 months amongst chemotherapy-naïve patients, this did not appear significantly better compared with historical controls of chemotherapy alone (59). In addition, major side effects of hand-foot skin reaction, myelosuppression, gastrointestinal reaction and 22.2% incidence of hemorrhage and one grade 5 toxicity related to tumor hemorrhage were noted. A phase II study evaluating sunitinib recruited fourteen patients and was stopped prematurely after two grade 5 hemorrhagic events occurring in patients with local tumor invasion into the carotid sheath within the first cycle, and other hemorrhagic events such as epistaxis, hemoptysis and hematemesis being reported in 64% patients (60). In view of the modest response rates, as well as the significant risk of hemorrhagic events and other toxicities associated with the use of these multi-kinase inhibitors, their use currently remains experimental and limited to clinical trial settings.
In cancers such as lung adenocarcinoma, the advent of molecular profiling approaches, better understanding of the tumor genomics and differentiation into various tumor subtypes based on histology has allowed identification of numerous genomic alterations and potential therapeutic targets. This in turn has led to a new era and treatment paradigm with molecularly targeted agents in NSCLC, and significant improvements in disease outcomes have been achieved in select patient subgroups (61). On the other hand, NPC has a relatively low mutational burden comparatively. Whilst various mutated genes have been identified as possible driver mutations in NPC—including TP53 and PIK3CA, genes involved in chromatin transcription (BAP1, MLL2, TSHZ3), as well as those involved in cell proliferation (ERBB3, ERBB2, KRAS, NRAS), prevalence rates of these mutations are nonetheless low (62). Apart from the low prevalence of mutations, further development of targeted therapies for NPC has been slow, also contributed by the difficulty in establishing preclinical NPC cell models to test proof-of-principle of therapeutic approaches and study drugs against actionable genetic aberrations in culture, such as the ERBB-PIK3CA signaling pathway.
The widespread existence of type II latent EBV infection in nonkeratinizing NPC has triggered interest in EBV-directed therapies, especially since activated CD8+ T cells have been shown to attack tumor cells and cause tumor regression in NPC models (63). EBV viral antigens expressed by NPC tumor cells [EBNA1, latent membrane protein 1 (LMP1), and LMP2] form good targets for the immune T-cell system. On this basis, adoptive immunotherapy strategies which involve the use of autologous EBV-specific cytotoxic T cells (CTL) to induce LMP2 specific immune response, as well as active immunotherapy using EBV vaccination with dendritic cells (professional antigen-presenting cells) and viral vectors-introduced peptides to activate cytotoxic T-cells (64,65), are being evaluated. These have shown clinical efficacy in heavily treated patients with NPC, and phase II and III trials are presently in progress (NCT00834093, NCT00953420, NCT01094405 and NCT02578641).
Immune checkpoint blockade with novel drugs which target programmed death-ligand 1 (PD-L1) or its receptor, programmed death-1 (PD-1), modulate the immune system by blocking ligand activation of the PD-1 receptor on activated T cells, and have been shown to be an effective therapeutic strategy in a variety of tumors including melanoma, renal cell carcinoma and lung cancer (66-69). Some of these studies report a correlation between PD-L1 positivity and response (69). The activity of pembrolizumab, a selective humanized monoclonal antibody against PD-1, was evaluated in a variety of solid tumors in the KEYNOTE-028 phase 1b trial. Amongst 27 NPC patients enrolled, the overall response rate was 22.2%, stable disease rate was 55.6%, and median response duration of 10.8 months. Grade 3–5 treatment-related adverse effects were hepatitis, pneumonitis, anemia, facial pain, increase blood creatinine phosphokinase, proteinuria and sepsis, with one treatment related death due to sepsis (70). In view of the promising activity of immune checkpoint inhibitors, various phase II trials are underway in recurrent/metastatic NPC, such as the KEYNOTE-122 randomized phase II study evaluating pembrolizumab versus standard treatment (capecitabine, gemcitabine or docetaxel) in platinum pre-treated NPC (NCT02611960); and another multicenter phase II single-arm study evaluating nivolumab (anti-PD1) in patients with recurrent and/or metastatic NPC (NCT02339558). Based on the significant benefit that these drugs have shown in other tumor types, we eagerly await the results of these studies.
Conclusions and future directions
Systemic chemotherapy is an integral part of the multidisciplinary management of NPC, both in the curative and palliative setting. Despite the inherent chemoradiosensitivity of NPC, relapse at both distant and/or local sites is not uncommon particularly amongst patients with locally advanced disease. Ongoing strategies are focused towards identifying patients at high risk of relapse and optimizing CRT as well as adjuvant chemotherapeutic regimens particularly for these patients. In the metastatic setting, despite the relative chemosensitivity of NPC, resistance to chemotherapy inevitably develops with median OS generally less than 24 months. Novel strategies evaluating EBV directed immunotherapy as well as immune checkpoint blockade may offer new hope in palliative treatment of NPC.
Further prospective randomized clinical trials are needed to gain insight into how best we can combine sequence and utilize the different treatment modalities (RT, chemotherapy, and novel therapeutics) based on individualized assessment of each patient’s disease and clinical condition. Other than improving survival outcomes for the patients, an imperative will be to look into strategies to reduce acute and long-term treatment-related toxicities, so as to improve the overall quality of life and survivorship for these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2005;61:1107-16. [Crossref] [PubMed]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [PubMed]
- Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730-8. [Crossref] [PubMed]
- Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy Plus concurrent–Adjuvant chemotherapy vs radiotherapy Alone for regionally Advanced Nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188-98. [Crossref] [PubMed]
- Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer 2013;119:2230-8. [Crossref] [PubMed]
- Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003;21:631-7. [Crossref] [PubMed]
- Chan AT, Teo P, Ngan R, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002;20:2038-44. [Crossref] [PubMed]
- Chan AT, Leung S, Ngan RK, et al. Overall Survival After Concurrent Cisplatin-Radiotherapy Compared With Radiotherapy Compared With Radiotherapy Alone in Locoregionally Advanced Nasopharyngeal Carcinoma. J Natl Cancer Inst 2005;97:536-9. [Crossref] [PubMed]
- Kwong DL, Sham JS, Au GK, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol 2004;22:2643-53. [Crossref] [PubMed]
- Wu X, Huang P, Peng P, et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol 2013;24:2131-6. [Crossref] [PubMed]
- Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 2011;103:1761-70. [Crossref] [PubMed]
- Jagdis A, Laskin J, Hao D, et al. Dose delivery analysis of weekly versus 3-weekly cisplatin concurrent with radiation therapy for locally advanced nasopharyngeal carcinoma (NPC). Am J Clin Oncol 2014;37:63-9. [Crossref] [PubMed]
- Tao CJ, Lin L, Zhou GQ, et al. Comparison of Long-Term Survival and Toxicity of Cisplatin Delivered Weekly versus Every Three Weeks Concurrently with Intensity-Modulated Radiotherapy in Nasopharyngeal Carcinoma. PLoS One 2014;9:e110765. [Crossref] [PubMed]
- Isobe K, Uno T, Aruga T, et al. Weekly cisplatin administration concurrent with radiation therapy for locoregionally advanced nasopharyngeal carcinoma. Int J Clin Oncol 2005;10:201-3. [Crossref] [PubMed]
- Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent–adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: Combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 2011;47:656-66. [Crossref] [PubMed]
- Loong HH, Ma BB, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol 2012;104:300-4. [Crossref] [PubMed]
- Zhang L, Zhao C, Peng PJ, et al. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol 2005;23:8461-8. [Crossref] [PubMed]
- Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer 2007;43:1399-406. [Crossref] [PubMed]
- Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 2006;64:47-56. [Crossref] [PubMed]
- Huncharek M, Kupelnick B. Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. Am J Clin Oncol 2002;25:219-23. [Crossref] [PubMed]
- Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 2004;22:4604-12. [Crossref] [PubMed]
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. [Crossref] [PubMed]
- Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163-71. [Crossref] [PubMed]
- International Nasopharynx Cancer Study Group1; VUMCA I Trial. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV (> or= N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. Int J Radiat Oncol Biol Phys 1996;35:463-9. [Crossref] [PubMed]
- Chua DT, Sham JS, Choy D, et al. Preliminary report of the asian-oceanian clinical oncology association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 1998;83:2270-83. [Crossref] [PubMed]
- Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 2001;19:1350-7. [PubMed]
- Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009;27:242-9. [Crossref] [PubMed]
- Fountzilas G, Ciuleanu E, Bobos M, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol 2012;23:427-35. [Crossref] [PubMed]
- Tan T, Lim WT, Fong KW, et al. Concurrent Chemo-Radiation With or Without Induction Gemcitabine, Carboplatin, and Paclitaxel: A Randomized, Phase 2/3 Trial in Locally Advanced Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2015;91:952-60. [Crossref] [PubMed]
- Lee AW, Ngan RK, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 2015;121:1328-38. [Crossref] [PubMed]
- Xiao WW, Han F, Lu TX, et al. Treatment outcomes after radiotherapy alone for patients with early-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2009;74:1070-6. [Crossref] [PubMed]
- Leung TW, Tung SY, Sze WK, et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head Neck 2005;27:555-65. [Crossref] [PubMed]
- Su SF, Han F, Zhao C, et al. Long-term outcomes of early-stage nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy alone. Int J Radiat Oncol Biol Phys 2012;82:327-33. [Crossref] [PubMed]
- Pfister DG, Spencer S, Brizel DM, et al. Head and neck cancers, Version 1.2015. J Natl Compr Canc Netw 2015;13:847-55. [PubMed]
- Chan AT, Grégoire V, Lefebvre JL, et al. Nasopharyngeal cancer: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii83-5. [Crossref] [PubMed]
- Ma BB, Poon TC, To K, et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma—a prospective study. Head Neck 2003;25:864-72. [Crossref] [PubMed]
- Sung FL, Poon TC, Hui EP, et al. Antitumor effect and enhancement of cytotoxic drug activity by cetuximab in nasopharyngeal carcinoma cells. In Vivo 2005;19:237-45. [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21-8. [Crossref] [PubMed]
- Xu T, Liu Y, Dou S, et al. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: Results of a randomized phase II study. Oral Oncol 2015;51:875-9. [Crossref] [PubMed]
- Ma BB, Kam M, Leung S, et al. A phase II study of concurrent cetuximab–cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol 2012;23:1287-92. [Crossref] [PubMed]
- Crombet T, Osorio M, Cruz T, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 2004;22:1646-54. [Crossref] [PubMed]
- Reddy BK, Lokesh V, Vidyasagar M, et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: A randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol 2014;50:498-505. [Crossref] [PubMed]
- Zhai RP, Ying HM, Kong FF, et al. Experience with combination of nimotuzumab and intensity-modulated radiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Onco Targets Ther 2015;8:3383-90. [PubMed]
- Lee NY, Zhang Q, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol 2012;13:172-80. [Crossref] [PubMed]
- Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci 2008;99:1311-8. [Crossref] [PubMed]
- Ngan RK, Yiu H, Lau W, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol 2002;13:1252-8. [Crossref] [PubMed]
- Tan E-H, Khoo K, Wee J, et al. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol 1999;10:235-7. [Crossref] [PubMed]
- Chua DT, Yiu HH, Seetalarom K, et al. Phase II trial of capecitabine plus cisplatin as first-line therapy in patients with metastatic nasopharyngeal cancer. Head Neck 2012;34:1225-30. [Crossref] [PubMed]
- Leong SS, Wee J, Tay MH, et al. Paclitaxel, carboplatin, and gemcitabine in metastatic nasopharyngeal carcinoma. Cancer 2005;103:569-75. [Crossref] [PubMed]
- Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncol 2003;39:361-6. [Crossref] [PubMed]
- Poon D, Chowbay B, Cheung YB, et al. Phase II study of irinotecan (CPT-11) as salvage therapy for advanced nasopharyngeal carcinoma. Cancer 2005;103:576-81. [Crossref] [PubMed]
- Wang CC, Chang JY, Liu TW, et al. Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck 2006;28:74-80. [Crossref] [PubMed]
- Foo KF, Tan EH, Leong SS, et al. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol 2002;13:150-6. [Crossref] [PubMed]
- Hsu CH, Gao M, Chen CL, et al. Inhibitors of epidermoid growth factor receptor suppress cell growth and enhance chemosensitivity of nasopharyngeal cancer cells in vitro. Oncology 2005;68:538-47. [Crossref] [PubMed]
- Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol 2005;23:3568-76. [Crossref] [PubMed]
- Chua DT, Wei WI, Wong MP, et al. Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head Neck 2008;30:863-7. [Crossref] [PubMed]
- Lim WT, Ng QS, Ivy P, et al. A Phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res 2011;17:5481-9. [Crossref] [PubMed]
- Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol 2007;25:3766-73. [Crossref] [PubMed]
- Xue C, Huang Y, Huang P, et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol 2013;24:1055-61. [Crossref] [PubMed]
- Hui EP, Ma B, King A, et al. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann Oncol 2011;22:1280-7. [Crossref] [PubMed]
- Thomas A, Liu SV, Subramaniam DS, et al. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol 2015;12:511-26. [Crossref] [PubMed]
- Lin DC, Meng X, Hazawa M, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genetics 2014;46:866-71. [Crossref] [PubMed]
- Khanna R, Moss D, Gandhi M. Technology insight: applications of emerging immunotherapeutic strategies for Epstein–Barr virus-associated malignancies. Nat Clin Pract Oncol 2005;2:138-49. [Crossref] [PubMed]
- Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother 2010;33:983-90. [Crossref] [PubMed]
- Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 2014;22:132-9. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Hsu C, Lee SH, Ejadi S, et al. Antitumor activity and safety of pembrolizumab in patients with PD-L1 positive nasopharyngeal carcinoma: Interim results from a phase 1b study. Ann Oncol 2015;26:ix94. [Crossref]


