
The modern management of untreated large (>2 cm) brain metastases: a narrative review
Introduction
Brain metastases are the most common intracranial malignancy, outnumbering primary brain tumors by five-fold (1). Approximately 30% of patients with a solid tumor diagnosis will develop a brain metastasis, with the incidence of central nervous system (CNS) spread increasing over the years due to advancements in treatment and longer survival duration of cancer patients (2). Many individuals with brain metastases will be symptomatic, with nearly half of all patients experiencing headache (3). Other common clinical manifestations include seizure, cognitive decline, and focal neurologic dysfunction, which are typically associated with expanded tumor size and the resulting edema. Metastases are also quantified based on size, with large brain lesions defined as ≥2 cm in maximal diameter or ≥4 cm3 (4).
Given that neurologic symptomatology is often related to intracranial burden, it is no surprise that large brain metastases result in a poor patient prognosis. The outcome for most patients with CNS metastases is already dismal ranging from three months to about three years, and large brain lesions confer an additional risk factor for worse local control and overall survival than counterparts with small CNS lesions alone (5). Surgery plus cavity radiation or definitive radiation therapy (6,7) remain the mainstays of treatment, however large brain metastases present a unique treatment challenge where an adequate radiation dose must be delivered to the metastasis while minimizing exposure to normal brain tissue (2).
Recent randomized clinical trials support the use of stereotactic radiosurgery (SRS) over whole-brain radiotherapy (WBRT) in the definitive or post-op setting for brain metastases to limit neurocognitive toxicity (7-11), especially for tumors that are asymptomatic and low volume. However, it is difficult to safely administer an adequate dose of SRS to large brain tumors. If toxic levels of radiation are delivered to the brain parenchyma, radiation necrosis may occur, which can approach rates of 50% for large treatment volumes (12).
A paucity of data exists suggesting optimal management of large metastases, however improved understanding of radiation dose-fractionation for stereotactic radiation therapy have identified emerging treatment options to improve local control while minimizing radiation toxicity. The objective of this review was to provide an overview of the current management options of large brain metastases, and their associated treatment outcomes and toxicity. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-21-136/rc).
Methods
A literature search was undertaken using the keywords large brain metastases, radiotherapy, radiosurgery, surgery, targeted therapy, and treatment (as per Index Medicus/MESH keywords database) using the ovid-MEDLINE database. Articles which were in English language, published recently (2011–June 2021) and examined human adults were included.
The abstracts of all obtained articles were reviewed independently for relevance, in which all primary research articles that examined the management of large brain metastases with surgery, radiation, targeted therapy, or a combination of treatments were included for review. Large brain metastases were defined according to the RTOG 90-05 definitions of >2 cm in size. Although the definition of large brain metastases may vary between >2–4 cm (where >2 cm may be considered as intermediate sized), for the purpose of this review we have included all trials reporting “large” brain metastases as being greater than >2 cm in size. Some articles reported tumor volume instead of lesion diameter, which corresponds to metastases larger than 4.19 cm3. Articles were excluded if no disease specific outcomes were reported (such as local control or overall survival), small sample size (n<4, such as case studies), and if studies did not separately report patients who previously received WBRT from those receiving initial management alone for their large brain metastases, as previous radiation may affect the primary outcome.
After meeting criteria for review, data from the included studies was extracted for the variables identified in Tables 1,2. A protocol was not registered for this project, however the Narrative Review checklist for performing a review was followed.
Table 1
| Study | Population | n (patients); N (mets) | Intervention | Dose/fractions (F) | Outcomes (at follow-up) | Median overall survival | Toxicity (RN) |
|---|---|---|---|---|---|---|---|
| Chon et al., 2019 (retrospective review) (13) | Less than 10 brain metastases, with largest size 2.5–3 cm | n=100; N=105 | SRS (N=67) vs. MFSRT (N=38) | SRS 20 Gy/1 F; MFSRT 35 Gy/3 F | 1 yr LC 66.6% (SRS) vs. 92.4% (MFSRT); P=0.028 | 13 months (SRS) vs. 18 months OS (MFSRT); P=0.239 | 29.9% (SRS) vs. 5.3% (MFSRT); P<0.001 |
| Inoue et al., 2014 (single institution prospective study) (14) | Brain metastases ≥10 cm3 in critical areas (median 16.2 cm3) | n=88; N=92 | MFSRT: size 10–19.9 cm3 27–30 Gy/5 F; 20–29.9 cm3 31–35 Gy/5 F; >30 cm3 35–42 Gy/8–10 F | Median SDE 46.5–48.5 Gy in 5 F | LTC 90.2% at median follow-up 7 months (3–41 months) | 9 months OS | 0 patients with RN ≥ Grade 3 |
| Ito et al., 2020 (retrospective review) (15) | Newly diagnosed BMs, largest≥10 cm3 | n=178; N=182 | MFSRT | 13 Gy/2 F | LTC not recorded. Median follow-up 5.4 months | 6.6 months OS | RN risk 4.2% |
| Kim et al., 2019 (randomized dose-escalation study) (16) | Brain metastases >3 cm diameter, <4 lesions | n=46; N=46 | MFSRT: 24 Gy (n=15); 27 Gy (n=17); 30 Gy (n=14) | 24 Gy/3 F; 27 Gy/3 F; 30 Gy/3 F | 1 yr local PFS: 24 Gy 65%; 27 Gy 80%; 30 Gy 75% (median follow-up 9.6 mo) | 75% 6 months OS; 57% 12 months OS (no statistical difference between groups) | Grade 4 RN: 0% 24 Gy; 13% 27 Gy; 37% 30 Gy |
| Koide et al., 2019 (retrospective review) (17) | Brain metastases >2 cm diameter | n=45; N=58 | MFSRT | 35 Gy/5 F | 1 yr LTC: 64.7% (median follow-up 11.3 months) | Median overall survival 14.2 months | 4.4% RN |
| Minniti et al., 2016 (retrospective review) (18) | Brain metastases >2 cm diameter | n=289; N=343 | SRS (n=151) vs. MFSRT (n=138) | 15–18 Gy/1 F; 27 Gy/3 F | 1 yr LTC: 77% SRS, 91% MFSRT (median follow-up 29 months); P=0.01 | Median OS 13.4 months, 1 yr OS 54% | 20% RN SRS; 8% RN MFSRT; P=0.004 |
| Murai et al., 2014 (prospective dose-escalation study) (19) | Brain metastases >2.5 cm | n=54; N=61 | MFSRT | 18–30 Gy/3 F (2.5–4 cm diameter, N=48); 21–35 Gy/5 F (>4 cm diameter, N=13) | LTC 84% at 6 months, 78% at 1 yr (median follow-up NR) | Median OS 6 months, 1 yr OS 31% | 1 patient developed grade 2 or higher RN |
| Navarria et al., 2016 (retrospective review) (20) | Single large brain metastasis ≥2.1cm | n=102; N=102 | MFSRT | 27 Gy/3 F (2.1–3 cm diameter, N=51); 32 Gy/4 F (3.1–5 cm diameter, N=51) | Median LTC 30 months, 1 yr LTC 96% (median follow-up NR) | Median OS 14 months, 1 yr OS 69% | 5.8% RN |
| Park et al., 2019 (retrospective review) (21) | NSCLC and brain metastases ≥10 cm3 | n=66; N=74 | SRS (N=60) vs. MFSRT (N=14) | Median 16 Gy/1 F; median 24 Gy/3 F | LTC 77% at last follow-up (median follow-up 13 months); P=0.10 between groups | Median OS 21.1 months | 8 SRS patients with RN ≥ Grade 3; 0 MFSRT patients with RN ≥ Grade 3 |
SRS, single fraction stereotactic radiosurgery; MFSRT, multi-fraction stereotactic radiation therapy; mets, metastases; yr, year; mo, month; LC, local control; OS, overall survival; SDE, single dose equivalent; LTC, local tumor control; BM, brain metastases; PFS, progression free survival; NR, not recorded; RN, radionecrosis; NSCLC, non-small cell lung cancer.
Table 2
| Study | Population | n (patients); N (mets) | Intervention | Dose/fractions | Cavity Volume | Outcome (at follow-up) | Median overall survival | Toxicity (LMD) |
|---|---|---|---|---|---|---|---|---|
| Choi et al., 2012 (retrospective review) (22) | Pre-operative brain metastases ≥2.1 cm | n=97; N=102 | Cavity MFSRT | Median dose 24 Gy/3 F | Median cavity volume 9.9 cm3 | 1 yr LC 90.7% (median follow-up 10 months, 21 months for surviving patients) | Median OS 15.6 months | 5% RN; LMD not recorded |
| Dohm et al., 2018 (retrospective review) (23) | Large brain metastasis ≥6 cm3 in volume | n=78; N=85 | Cavity SRS (N=40) vs. MFSRT alone (N=45) | Median cavity dose 16.5 Gy/1 F; median MFSRT dose: 15 Gy fraction #1, 13.5 Gy fraction #2 | Median 14.9 cm3 (surgery + SRS), 13.5 cm3 (MFSRT) | 1 yr LC 94% (surgery + SRS) vs. 92% (MFSRT) (median follow-up 23.2 months); P=0.65 | 13.2 months median OS for MFSRT vs. 9.7 months surgery + SRS; P=0.53 | 6% RN surgery + SRS vs. 5% RN MFSRT, P=1.00; LMD 6% surgery + SRS vs. 8% for MFSRT, P=0.63 |
| Ling et al., 2015 (retrospective review) (24) | Large brain metastases ≥3 cm in diameter | n=99; N=100 | Cavity SRS | Median 22 Gy/3 F | Median volume not recorded | 1 yr LC 72% (median follow-up 12.2 months) | Median OS 12.7 months, 1 yr OS 55%. | 2% grade 3 RN; 6% LMD |
| Marcrom et al., 2020 (retrospective review) (25) | Brain metastases with only one ≥3 cm in diameter | n=125; N=125 | Cavity SRS (n=82) vs. MFSRT alone (n=43) | Median cavity dose 16 Gy/1 F; MFSRT doses: 2 5 Gy or 30 Gy/5 F | Median volume not recorded | 1 yr LC 70% (surgery + SRS), 69% (MFSRT) (median follow-up 7 months); P=0.753 | 6 months OS 86% (surgery + SRS) vs. 63% (MFSRT), not significant after correction for GPA | 1.4% ≥ grade 3 toxicity (surgery + SRS) vs. 6.3% (MFSRT), P=0.248; LMD risk 45% SRS + surgery and 19% MFSRT, P=0.048 |
| Minniti et al., 2013 (retrospective review) (26) | Resected brain metastasis cavity >3 cm | n=101; N=101 | Cavity MFSRT | 27 Gy/3 F | Median cavity volume 17.5 cm3 | 1 yr LC 69%, 2 yr LC 34% (median follow-up 16 months) | Median OS 17 months | 7% RN at 1 year, 16% at 2 years; LMD not recorded |
| Minniti et al., 2019 (retrospective review) (27) | At least one brain metastasis 2–4 cm in size | n=222; N=241 | Cavity MFSRT vs. MFSRT alone | 27 Gy/3 F | Median cavity volume 10.8 cm3 | 1 yr LC 83% (surgery + MFSRT), 92% (MFSRT) (median follow-up 12 months); P=0.15 | Median OS 13.5 months (surgery + SRS), 15.2 months (MFSRT); P=0.2 | 11% symptomatic RN (surgery + MFSRT) vs. 5% (MFSRT) at 1 year, P=0.03; 7% LMD (surgery + MFSRT) vs. 0% LMD (MFSRT), P=0.01 |
| Navarria et al., 2019 (prospective single-arm, phase 2 study) (28) | Oligometastatic disease (maximum 5 lesions) with at least one brain metastasis ≥2.1 cm in maximum diameter | n=101; N=101 | Cavity MFSRT | 30 Gy/5 F | Median cavity volume 31.3 cm3 | 1 yr LC 98.9%, 2 yr LC 85.9% (median follow-up 26 months) | Median OS 22 months, 1yr OS 81.9%, 2yr OS 46.6% | 5.9% Grade 3 RN; 8.9% LMD |
| Pessina et al., 2016 (retrospective study) (29) | Single brain metastases ≥2.1 cm | n=69; N=69 | Cavity MFSRT | 30 Gy/3 F | Median cavity volume 29.0 cm3 | 1 and 2 yr LC 100% (median follow-up 24 months) | Median OS 24 months, 1 yr OS 91.3%, 2yr OS 73.0% | 0% ≥ grade 3 RN, symptomatic RN and LMD not recorded |
| Vogel et al., 2015 (retrospective study) (30) | Brain metastases and post-op cavities ≥2 cm | n=30; N=33 | Cavity MFSRT | Median dose 39 Gy/5 F | Median cavity volume 25.1 cm3 | 1 yr LC 68.5% (median follow-up 9.5 months) | Median OS 10.1 months | 10% RN; 34% LMD |
SRS, single fraction stereotactic radiosurgery; mets, metastases; LMD, leptomeningeal disease; LC, local control; OS, overall survival; MFSRT, multi-fraction stereotactic radiation therapy; RN, radionecrosis; yr, year; GPA, graded prognostic assessment.
Results
Our search strategy yielded 480 articles which were initially evaluated (Figure 1). After review, 51 original articles reporting treatment outcomes for management of large brain metastases were identified (radiation therapy, targeted therapy, surgery, or a combination of treatment modalities). After selecting articles based on the inclusion criteria, 18 primary research articles were included for review which reported treatment outcomes of large brain metastases with either radiation therapy or surgery (Tables 1,2).
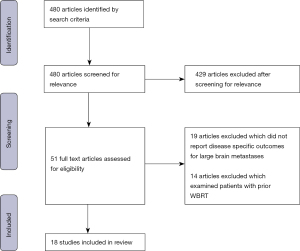
Three review articles were also identified, with two systemic reviews examining the role of SRS or multi-fraction stereotactic radiation therapy (MFSRT) in the management of large brain metastases, which evaluated the literature published until 2018 (31,32), and one narrative review which did not report a systematic search (33). This article differs from these previously reported reviews by broadening the search criteria to include management options for large brain metastases with systemic therapies instead of radiation alone, as well as excluding studies on patients who have received previous WBRT, which may confound our objective of comparing local control and radiation necrosis between modalities.
Radiosurgery alone
Role of radiosurgery
Currently, the preferred approach for patients with limited brain metastases remains surgery with post-operative SRS or SRS alone (34). However, no current guideline exists for the subset of patients presenting with brain metastases >2 cm in diameter. Given that large brain metastases may often be symptomatic or cause mass effect, these patients may be considered for upfront surgical resection. Alternatively, patients may be stable on presentation or be ineligible for neurosurgery due to medical comorbidities or eloquent location in the brain and be considered for definitive radiotherapy instead. A number of studies exist reporting the use of SRS to control large brain metastases which are included in our review (Table 1). All studies examined either single-fraction SRS or MFSRT. Given the purpose of our review is to discuss the modern management of large brain metastases, articles which included patient outcomes for those treated with combined SRS and WBRT were excluded, however, this treatment approach is sometimes still employed in clinical practice.
In our review we identified nine articles which explored the outcomes of patients treated with SRS or MFSRT alone for definitive therapy (13-21). Of these, there were three prospective studies (14,16,19), and six retrospective reviews published between the years 2013 to 2020. All reviews either evaluated SRS versus MFSRT or MFSRT alone, with no study reporting outcomes of SRS only on patients. Historically, it is well documented that brain metastasis size is a risk factor for development of radiation necrosis with SRS and treatment for lesions greater than 4 cm have typically avoided single fraction treatment (31). By fractionating SRS regimens, a higher biologically effective dose (BED) may be administered while reducing normal brain tissue toxicity and maintaining local control. Many of the modern studies exploring definitive radiosurgery for management of large brain metastases, including those in our review, have focused on determining the most effective dose and fractionation schedule for local tumor control with minimal rates of radiation necrosis.
Efficacy of fractionated stereotactic radiotherapy
In the studies reviewed, the one-year local control reported for MFSRT alone ranged from 65% (17) to 96% (20). Two of three retrospective studies which directly compared single versus multi-fraction treatment found statistically significant improvements in local control for large brain metastases with MFSRT compared to SRS (13,18,21). In a retrospective review of 289 patients treated with SRS or MFSRT, Minniti et al. (18) identified one-year local control rates of 77% and 91% respectively. These results were then corroborated by Chon et al. (13) in their retrospective review of 100 patients which demonstrated 66.6% one-year local control for patients treated with SRS versus 92.4% with MFSRT. In multivariate analysis, positive predictive factors for local tumor control included hypofractionation, non-gastrointestinal primary cancers, and recent primary cancer diagnosis.
Two dose-escalation trials were identified to determine which dose of MFSRT achieves the highest local control rates while balancing radiation toxicity. In the recently published trial by Kim et al. (16), receiving 30 Gy in three fractions as opposed to 27 or 24 Gy in three fractions was associated with a statistically significant increase in radiation necrosis (the only significant factor on multivariate analysis).
Toxicity associated with radiotherapy
As explained above, the main risk for treatment of large brain metastases with stereotactic radiation therapy is radiation necrosis. This may present one to two years following treatment, either radiographically, or if severe enough, via symptoms such as headache, drowsiness, or seizures and even death (35). It is difficult to ascertain the rates of radiation necrosis from hypofractionated regimens due to the lack of prospective trials, limited follow-up, and variability in BED and time between treatments. Furthermore, institutions vary on their recording system for radiation necrosis, many of which rely on radiographic findings at follow-up.
In the studies which compared SRS with MFSRT, all reported a statistically significant reduction in radiation necrosis with fractionation of treatment (13,18,21). These rates ranged from 13–30% with SRS in comparison to 0–8% of patients who underwent hypofractionated therapy while maintaining adequate local control as described above. It is difficult to delineate what dose fractionation schedule is acceptable for reducing risk of radiation necrosis for large brain metastases due to the incomparability between reporting and definition of radiation necrosis between studies. However, comparability of radiation necrosis in a prospective dose-escalation study by Kim et al. (16) demonstrated that 30 Gy in 3 fractions results in unacceptable radionecrosis (37% of patients treated) in comparison to 27 Gy in three fractions (13% of patients), without improvement in one-year local control (75% and 80% respectively). Similar results using 27 Gy in three fractions were achieved by Minniti et al. (18) [1 year local control (LC) 91%, radionecrosis (RN) 8%], and Navarria et al. (20) (1 year LC 96%, RN 5.8%).
These studies provide convincing results of an optimal dose/fractionation schedule, making MFSRT an attractive treatment option for select patients to reduce risk of radiation toxicity while still providing adequate local control. In a recent meta-analysis combining data from 24 trials treated with SRS or MFSRT for large brain metastases, a relative reduction of radiation necrosis was identified with MFSRT regimens while achieving similar or higher rates of 1-year local control compared to SRS treatment (31). Although the meta-analysis lacked individual patient level data and could not adjust for covariates, these findings are also consistent with the results of our review. Current prospective clinical trials are ongoing to determine MFSRT maximum tolerated dose for both 3 fraction (NCT02054689) and 5 fraction regimens (NCT01705548).
Surgery plus radiation
Role of surgery and cavity SRS
In patients with limited brain metastases, surgery remains a cornerstone of treatment, especially for those with good performance status and controlled primary disease (34). A number of reasons may warrant treatment with surgery versus definitive radiation therapy, such as patients with an unknown primary to confirm the diagnosis of a CNS metastasis. Another indication is among patients who are symptomatic or have mass effect from the lesion, hence making surgery an important component in the management of large brain metastases.
Post-operative cavity radiation is the standard of care following surgical resection of brain metastases, given that surgery alone confers unacceptable control rates of about 50% (6). Randomized trials show that SRS is an effective and less toxic alternative to WBRT for cavity irradiation, however risk of radiation necrosis increases with the volume of cavity irradiated (36). A paucity of data also exists to suggest the optimal dose and fractionation schedule for post-op cavity irradiation with SRS for large brain metastases to balance local control with risk of toxicity. This has been a main focus of modern studies evaluating cavity radiation for large brain lesions.
There is also limited evidence comparing definitive radiosurgery alone versus surgery plus radiosurgery alone in the management of large brain metastases. In our review, we identified nine studies which evaluated tumor control and toxicity in patients treated with surgery followed by cavity SRS or MFSRT for large brain metastases (22-30) (Table 2). All of these were retrospective institutional reviews, apart from one prospective, single-arm phase 2 trial by Navarria et al. (28) to assess the safety and efficacy of MFSRT to the tumor bed following resection of large brain metastases. In total, three reviews compared outcomes between cavity radiation and definitive radiation (23,25,27).
Efficacy of surgery and cavity SRS
The one-year local control rate for large brain metastases treated with surgery plus cavity SRS/MFSRT range from 69–100% (26,29). Six of the nine identified studies treated the tumor bed with MFSRT and only three with SRS, representing a shifting paradigm in the management of surgical resection cavities to account for the larger volume of normal brain tissue radiated following resection of metastases ≥2 cm in size.
In the three institutional reviews comparing outcomes between post-op cavity radiation and definitive radiotherapy alone, none had a statistically significant difference in local control or overall survival (after correcting for patient characteristics) between the two treatment arms. In these studies, most patients who underwent surgical resection compared to MFSRT either had symptomatic lesions warranting surgery, were not surgical candidates, or declined operation. MFSRT alone was also commonly used to treat lesions near critical structures such as the brainstem, in eloquent regions of the brain, or among patients with multiple metastases compared to only a solitary lesion. On analysis of patient characteristics between these groups, the studies by Minniti et al. (27) and Dohm et al. (23) were fairly balanced, apart from more patients receiving treatment for multiple metastases in the MFSRT group than surgery + cavity radiation cohort. The two groups in the study by Marcrom et al. (25), however, had a statistically significant difference in graded prognostic assessment (GPA) of 2.5 (surgery + SRS) versus 1.5 (MFSRT alone). Although local control was similar between groups, six-month OS differed by 86% versus 63% respectively, but the difference was not statistically significant after correcting for difference in GPA. Interestingly, the median overall survival was higher in the definitive MFSRT cohorts in the other two trials in comparison to patients who received cavity radiation (23,27). Another notable difference in clinical outcomes from these studies was identified by Dohm et al. (23). In their study, the rate of distant brain failure was less in the patient cohort with MFSRT alone, at 21% in one year compared to 59% in the surgical group. The authors hypothesized that this difference may be due to delayed initiation of systemic therapy among surgical patients to allow for post-op healing.
Toxicity associated with surgery and cavity SRS
As with the studies evaluating radiation necrosis following MFSRT, it is difficult to compare rates of radiation necrosis among patients who received post-operative cavity radiation due to differences in identification, grading and reporting. However, most studies recorded rates of Grade 3 or higher radiation necrosis (meaning requires neurosurgical intervention), which range from 0% (29) to 5.9% (28). Toxicity is easiest to analyze in studies comparing treatment modalities from the same institution, where post-op cavity radiation was compared to MFSRT. In a study by Minniti et al. (27) comparing 27 Gy/3 fractions as definitive treatment versus the same dose and fractionation following surgical resection, they found the rates of radiation necrosis higher in the cavity + radiation group, with 11% of patients having symptomatic toxicity versus 5% in the MFSRT group alone. On univariate analysis, larger gross tumor volume (GTV) and volume of normal brain radiated with 18 Gy were associated with a statistically increased risk of RN (27).
In addition to the risk of radionecrosis following cavity SRS/MFSRT, the combined modality approach of surgery + radiation also confers the additional risk of leptomeningeal disease (LMD) due to possible tumor seeding at the time of surgery (37). Even with modern advances in chemotherapy and radiation, the prognosis following LMD diagnosis is poor with a median survival of 3–6 months, and consideration of this risk when determining the best course of treatment is warranted (38). In the included studies, LMD rates ranged from 6% (23) to 45% (25) following resection and cavity radiation. In the three studies comparing definitive radiation to cavity radiation, two reported a statistically significant increase in LMD following surgery + MFSRT/SRS (18,25). Vogel et al. (30) identified significant risk factors for LMD including the simultaneous resection of multiple metastases and greater than 50 days delay before receiving cavity radiosurgery. Marcrom et al. (25) specifically looked at leptomeningeal failure as a primary outcome for their study evaluating management of large brain metastases. The authors corroborated these results, identifying the number of brain metastases as a risk factor for LMD (higher with more brain metastases), as well as performing surgery in general (25). Although the management of large brain metastases with surgery followed by radiation may predispose patients to leptomeningeal carcinomatosis, it is difficult to quantify this risk due to differences in cytologic, radiographic, and clinical reporting of LMD in studies. However, since LMD is often associated with the need for further interventions such as salvage WBRT, it is necessary to consider the best treatment option for improved local control while reducing the risk of LMD.
The above studies affirm the role of MFSRT for large post-operative cavities by demonstrating optimal treatment efficacy with minimal toxicity. These results are supported by a recent retrospective study by Soliman et al. (39), identifying high rates of local control using a five-fraction treatment regimen and median total dose of 30 Gy to the tumor cavity. Although this study did not exclusively include larger tumours , 84% of patients in the study had brain metastases ≥2 cm. Similar one year local control and radiation necrosis rates were reported at 84% and 6% respectively. Notably, preoperative tumor size was not a predictor of local control or toxicity, which supports MFSRT as an optimal treatment approach with improved outcomes for large tumor cavities (39).
A treatment displaying future promise is neoadjuvant SRS/MFSRT prior to surgery. In our review, one prospective trial protocol was identified administering neoadjuvant SRS prior to surgery for large brain metastases, with the primary outcome being radionecrosis and secondary outcomes including one-year local control and LMD (40). This new treatment paradigm is based on findings from a retrospective analysis of lesions with a median size 3 cm, identifying lower rates of LMD (3% vs. 17%) and symptomatic radiation toxicity (1.5% vs. 14.6%) with pre-operative versus post-operative SRS (41). These results have been corroborated by similar, later studies (42), however higher quality data is still needed on patients with large brain metastases. Future clinical trials may elucidate the value of neo-adjuvant radiation therapy, such as the study by Takami et al. (40) and the Phase II neo-adjuvant SRS trial which is evaluating radiation toxicity as the primary outcome (NCT03368625).
Targeted therapy
In the modern management of brain metastases, targeted therapy represents an emerging treatment modality which may be a useful adjuvant to the treatment options mentioned above. Recent advances in some agents have demonstrated improved CNS penetrance and current National Comprehensive Cancer Network (NCCN) guidelines state that a trial of targeted therapies may be considered in patients with metastatic melanoma or anaplastic lymphoma kinase (ALK)-rearrangement positive non-small cell lung cancer (NSCLC) and epidermal growth factor receptor (EGFR)- mutant NSCLC presenting with brain metastases (34). Targeted therapies of particular interest are second and third generation tyrosine kinase inhibitors (TKIs) such as alectinib, which has demonstrated a CNS response rate of 82% and 12-month rate of CNS progression of 9% ALK-positive NSCLC patients (43). Another TKI which has shown promise is osimertinib, with 77% 12-month progression free survival (PFS) in EGFR-mutant NSCLC, demonstrating good blood brain barrier penetration and adequate tumor control (44). For small, asymptomatic brain metastases, it is not unreasonable to delay radiation treatment while intracranial disease is controlled on targeted therapy in these patients. However, no recommendation exists for the subset of individuals presenting with large brain metastases, and few trials have combined radiation with targeted therapies.
Unfortunately, our literature search did not yield any studies evaluating the role of targeted therapy in patients with large brain metastases. However two retrospective reviews were identified which included patients with either symptomatic brain lesions or those ≥1 cm in diameter. Both study populations evaluated NSCLC patients who were ALK-rearranged or EGFR-mutant positive. The first study by Lin et al. (45) reported a response rate of 73.3% and a median CNS duration of response 19.3 months in NSCLC patients on alectinib. All eight patients with symptomatic metastases had clinical improvement with alectinib (five also requiring steroids). Dutta et al. (46) reported similar results with TKIs, demonstrating 94% intracranial response at 3 months and a median PFS of 13.9 months as initial therapy for large or symptomatic brain lesions. Although these studies demonstrated promising results for the management of brain metastases, both reviews had small population sizes, and have inherent limitations given the nature of prospective studies considering both are only from a single institution and data collection is prone to selection bias. Caution must still be used in the management of large brain metastases, given the lack of data available to guide decision making.
Targeted therapies may also increase the risk of radiation toxicity in combination with SRS/MFSRT with some retrospective reviews reporting significantly higher rates of radiation necrosis in patients receiving concurrent immunotherapy (47) and TKIs (48). There may also be neurocognitive symptomatology associated with these drugs, and from a quality of life perspective these side effects must be considered for similar reasons why WBRT has fallen out of favor. Current ongoing trials will provide more clarity to the safety and efficacy of targeted therapies and their effect in combination with SRS. We look forward to the results of the OZM-094 phase II randomized controlled trial (RCT) comparing osimertinib alone or in combination with SRS for patients with EGFR-positive lung cancer (NCT03497767), with progression free survival and neurocognitive changes as treatment endpoints. Studies such as these will not only clarify the role of targeted therapy in the management of brain mets, but also in conjunction with SRS.
Conclusions
In conclusion, the management of large brain metastases requires adequate local control with preservation of healthy brain tissue and minimization of treatment-related toxicity. The mainstay of treatment remains surgery followed by cavity radiation, or definitive SRS/MFSRT. Targeted therapies are an upcoming treatment modality with certain drugs such as osimertinib and alectinib showing promising results, however these drugs are reserved for a very select subgroup of patients. Current evidence suggests that MFSRT is a reasonable alternative to standard SRS in the definitive treatment setting for asymptomatic patients with large brain metastases to achieve adequate local control while balancing the risk of radiation necrosis. After discussion in the multidisciplinary setting, if surgery is indicated then MFSRT should also be considered for large post-op cavity volumes to reduce risk of radiation necrosis. Future prospective studies are required to provide a more definitive dose and fractionation regimen.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Simon S. Lo, Balamurugan Vellayappan, Kevin Shiue, and Jonathan P. S. Knisely) for the series “The Modern Approaches to the Management of Brain Metastases” published in Chinese Clinical Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-21-136/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-21-136/coif). The series “The Modern Approaches to the Management of Brain Metastases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fox BD, Cheung VJ, Patel AJ, et al. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am 2011;22:1-6. v. [Crossref] [PubMed]
- Suh JH, Kotecha R, Chao ST, et al. Current approaches to the management of brain metastases. Nat Rev Clin Oncol 2020;17:279-99. [Crossref] [PubMed]
- Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin 2003;21:1-23. vii. [Crossref] [PubMed]
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000;47:291-8. [Crossref] [PubMed]
- Sperduto PW, Mesko S, Li J, et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J Clin Oncol 2020;38:3773-84. [Crossref] [PubMed]
- Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1040-8. [Crossref] [PubMed]
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1049-60. [Crossref] [PubMed]
- Kayama T, Sato S, Sakurada K, et al. Effects of Surgery With Salvage Stereotactic Radiosurgery Versus Surgery With Whole-Brain Radiation Therapy in Patients With One to Four Brain Metastases (JCOG0504): A Phase III, Noninferiority, Randomized Controlled Trial. J Clin Oncol 2018; Epub ahead of print. [Crossref] [PubMed]
- Li J, Ludmir EB, Wang Y, et al. Stereotactic Radiosurgery versus Whole-brain Radiation Therapy for Patients with 4-15 Brain Metastases: A Phase III Randomized Controlled Trial. Int J Radiat Oncol 2020;108:S21-2. [Crossref]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011;6:48. [Crossref] [PubMed]
- Chon H, Yoon K, Lee D, et al. Single-fraction versus hypofractionated stereotactic radiosurgery for medium-sized brain metastases of 2.5 to 3 cm. J Neurooncol 2019;145:49-56. [Crossref] [PubMed]
- Inoue HK, Sato H, Suzuki Y, et al. Optimal hypofractionated conformal radiotherapy for large brain metastases in patients with high risk factors: a single-institutional prospective study. Radiat Oncol 2014;9:231. [Crossref] [PubMed]
- Ito D, Aoyagi K, Nagano O, et al. Comparison of two-stage Gamma Knife radiosurgery outcomes for large brain metastases among primary cancers. J Neurooncol 2020;147:237-46. [Crossref] [PubMed]
- Kim KH, Kong DS, Cho KR, et al. Outcome evaluation of patients treated with fractionated Gamma Knife radiosurgery for large (> 3 cm) brain metastases: a dose-escalation study. J Neurosurg 2019; Epub ahead of print. [Crossref] [PubMed]
- Koide Y, Tomita N, Adachi S, et al. Retrospective analysis of hypofractionated stereotactic radiotherapy for tumors larger than 2 cm. Nagoya J Med Sci 2019;81:397-406. [PubMed]
- Minniti G, Scaringi C, Paolini S, et al. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J Radiat Oncol Biol Phys 2016;95:1142-8. [Crossref] [PubMed]
- Murai T, Ogino H, Manabe Y, et al. Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol (R Coll Radiol) 2014;26:151-8. [Crossref] [PubMed]
- Navarria P, Pessina F, Cozzi L, et al. Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol 2016;11:76. [Crossref] [PubMed]
- Park K, Kim JW, Chung HT, et al. Single-Session versus Multisession Gamma Knife Radiosurgery for Large Brain Metastases from Non-Small Cell Lung Cancer: A Retrospective Analysis. Stereotact Funct Neurosurg 2019;97:94-100. [Crossref] [PubMed]
- Choi CY, Chang SD, Gibbs IC, et al. What is the optimal treatment of large brain metastases? An argument for a multidisciplinary approach. Int J Radiat Oncol Biol Phys 2012;84:688-93. [Crossref] [PubMed]
- Dohm AE, Hughes R, Wheless W, et al. Surgical resection and postoperative radiosurgery versus staged radiosurgery for large brain metastases. J Neurooncol 2018;140:749-56. [Crossref] [PubMed]
- Ling DC, Vargo JA, Wegner RE, et al. Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases: clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neurosurgery 2015;76:150-6; discussion 156-7; quiz 157.
- Marcrom SR, Foreman PM, Colvin TB, et al. Focal Management of Large Brain Metastases and Risk of Leptomeningeal Disease. Adv Radiat Oncol 2019;5:34-42. [Crossref] [PubMed]
- Minniti G, Esposito V, Clarke E, et al. Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys 2013;86:623-9. [Crossref] [PubMed]
- Minniti G, Scaringi C, Lanzetta G, et al. Comparative effectiveness of multi-fraction stereotactic radiosurgery for surgically resected or intact large brain metastases from non-small-cell lung cancer (NSCLC). Lung Cancer 2019;132:119-25. [Crossref] [PubMed]
- Navarria P, Pessina F, Clerici E, et al. Surgery Followed by Hypofractionated Radiosurgery on the Tumor Bed in Oligometastatic Patients With Large Brain Metastases. Results of a Phase 2 Study. Int J Radiat Oncol Biol Phys 2019;105:1095-105. [Crossref] [PubMed]
- Pessina F, Navarria P, Cozzi L, et al. Outcome Evaluation of Oligometastatic Patients Treated with Surgical Resection Followed by Hypofractionated Stereotactic Radiosurgery (HSRS) on the Tumor Bed, for Single, Large Brain Metastases. PLoS One 2016;11:e0157869. [Crossref] [PubMed]
- Vogel J, Ojerholm E, Hollander A, et al. Intracranial control after Cyberknife radiosurgery to the resection bed for large brain metastases. Radiat Oncol 2015;10:221. [Crossref] [PubMed]
- Lehrer EJ, Peterson JL, Zaorsky NG, et al. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J Radiat Oncol Biol Phys 2019;103:618-30. [Crossref] [PubMed]
- Lee EJ, Choi KS, Park ES, et al. Single- and hypofractionated stereotactic radiosurgery for large (> 2 cm) brain metastases: a systematic review. J Neurooncol 2021;154:25-34. [Crossref] [PubMed]
- Gutschenritter T, Venur VA, Combs SE, et al. The Judicious Use of Stereotactic Radiosurgery and Hypofractionated Stereotactic Radiotherapy in the Management of Large Brain Metastases. Cancers (Basel) 2020;13:70. [Crossref] [PubMed]
- Nabors LB, Portnow J, Joachim B. NCCN Central Nervous System Cancer Guidelines. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- Buboltz JB, Tadi P. Hyperbaric Treatment Of Brain Radiation Necrosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; September 29, 2021.
- Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996-1001. [Crossref] [PubMed]
- Nguyen TK, Nguyen EK, Soliman H. An overview of leptomeningeal disease. Ann Palliat Med 2021;10:909-22. [Crossref] [PubMed]
- Nguyen TK, Sahgal A, Detsky J, et al. Predictors of leptomeningeal disease following hypofractionated stereotactic radiotherapy for intact and resected brain metastases. Neuro Oncol 2020;22:84-93. [Crossref] [PubMed]
- Soliman H, Myrehaug S, Tseng CL, et al. Image-Guided, Linac-Based, Surgical Cavity-Hypofractionated Stereotactic Radiotherapy in 5 Daily Fractions for Brain Metastases. Neurosurgery 2019;85:E860-9. [Crossref] [PubMed]
- Takami H, Nassiri F, Moraes FY, et al. A Phase II Study of Neoadjuvant Stereotactic Radiosurgery for Large Brain Metastases: Clinical Trial Protocol. Neurosurgery 2020;87:403-7. [Crossref] [PubMed]
- Asher AL, Burri SH, Wiggins WF, et al. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys 2014;88:899-906. [Crossref] [PubMed]
- Patel KR, Burri SH, Asher AL, et al. Comparing Preoperative With Postoperative Stereotactic Radiosurgery for Resectable Brain Metastases: A Multi-institutional Analysis. Neurosurgery 2016;79:279-85. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; Epub ahead of print. [Crossref] [PubMed]
- Lin JJ, Jiang GY, Joshipura N, et al. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J Thorac Oncol 2019;14:683-90. [Crossref] [PubMed]
- Dutta SW, Mack ML, Aliotta E, et al. Intracranial disease control for EGFR-mutant and ALK-rearranged lung cancer with large volume or symptomatic brain metastases. J Neurooncol 2020;149:357-66. [Crossref] [PubMed]
- Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016;125:17-23. [Crossref] [PubMed]
- Kim JM, Miller JA, Kotecha R, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol 2017;133:357-68. [Crossref] [PubMed]


