Neurosurgical and radiosurgical decision making in brain metastasis patients in the area of targeted therapies?
Introduction
Brain metastases (BM) represent a major health problem in patients with cancer. It is estimated that approximately 20-40% of patients with malignant neoplasia will develop brain metastasis during their disease (1,2). These lesions, whose incidence is increasing due to the improvement of primary cancers management, represent the most frequent intra-axial brain tumors.
Whole-brain radiation therapy (WBRT) (3-5) has been for a while the standard treatment of BM. However, the advent of modern imaging techniques (CT and MRI), the improvement of surgical techniques and neuroanesthesia (6-9), and the positive impact of stereotactic radiotherapy (SRT) [radiosurgery, hypofractionated stereotactic radiotherapy (HSRT)] (10), led to a reappraisal of local treatment modalities in BM management. Therapeutic decision depends on several factors related to tumor characteristics (number, radiological aspect, size, location…), patient clinical status (neurological deficit, general condition, comorbidities, performance status…) and primary disease status (controlled or uncontrolled, extracranial active metastatic disease) (11).
In this article, we will present an overview of local treatment modalities in BM namely surgical and SRT indication. Respective toxicity of each approach will also be discussed.
Survival impact of surgery in BM
Actual impact, in terms of overall survival (OS), of surgery associated with WBRT in patients with single brain metastasis of solid cancers, in comparison with WBRT alone, has been demonstrated in several studies (Table 1). In 1990, Patchell et al. (8) firstly showed that surgery associated with WBRT led to a significant increase of OS in patient with a unique brain metastasis compared to WBRT alone. In 1993, Vecht et al. (9) confirmed the positive impact on OS of the association of surgery and WBRT, in single brain metastasis. In 1996, Mintz et al. (12) did not find such a positive impact of surgery on OS. However, in this study only 21.4% of patients had a controlled extra-cerebral disease, and none of the patients had brain MRI assessment conversely to the two other studies, this has to interpret with caution these results.
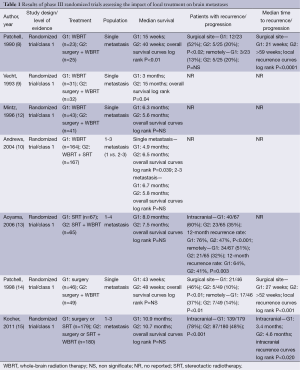
Full table
The impact of surgery associated with WBRT in comparison with surgery alone has then been evaluated (14,15) (Table 1). While adjuvant WBRT led to a significant improvement of cerebral control, no effect was observed in terms of OS or time to functional independence (14,15).
Surgical indications
BM surgery goal is to improve brain tumor control, allow patient’s neurological symptoms relief and provide an accurate tumor molecular characterization. Large tumors responsible for intracranial hypertension and symptomatic tumors located in eloquent area represent a surgical indication. Posterior fossa location with associated obstructive hydrocephalus should also be removed surgically. For cystic or necrotic tumors with cortico-subcortical topography, surgery should also be discussed considering the low efficacy and the potential adverse effects of radiotherapy in these situations.
Surgery may also have a diagnostic role. In case of unknown primary, surgery is warranted to have a histological diagnosis. Also, when a differential tumor diagnosis or pseudo-progression (radionecrosis) is suspected, a histological authentication may be necessary (6).
Finally, in some cases, it may be interesting to document biologically the cerebral metastatic disease. Indeed, molecular or gene expression changes may occur between primary tumor and BM. This could actually impact surgical decision making in patients with BM. Furthermore, for some patients whose initial tumor material is not available, biological metastatic disease documentation could identify patient eligible for a specific targeted therapy. Therefore, surgical resection of BM, in these cases, represents a pivotal step in the treatment strategy decision making process that can lead to an actual change in the therapeutic management.
In summary, surgical excision, when possible, should be performed in the following situations:
- Therapeutic:
- Voluminous lesion >3 cm, symptomatic or not;
- Cystic or necrotic lesion with edema;
- Symptomatic lesion located in eloquent area;
- Lesion located in the posterior fossa with mass effect or associated hydrocephalus.
- Diagnostic:
- No known primary cancer;
- Potential differential diagnosis;
- Suspected radionecrosis in previously irradiated patients.
- Strategic:
- Biological documentation of brain metastatic disease in patients potentially eligible for new targeted therapy.
Finally, surgical resection of brain metastatic lesions also contribute to the constitution of a BM tissue database that could allow for a better understanding of the molecular determinants underlying the brain metastatic disease and for identifying new potential molecular targets and its associated treatments.
Selection of patients for surgical resection
The selection of patients who will have surgical resection, should take into account three factors: the clinical and functional status of the patient, the systemic disease status and the characteristics of intra-cranial metastases.
Clinical and functional status of the patient
To have a surgical resection of BM, the patient should be in relatively good general condition and don’t present of major cardiovascular or lung defects, which making incur a significant anesthetic risk. The patient’s functional status must be taken into account. The Karnofsky index is a major element in making local therapeutic decision. Indeed, in the Recursives Partitioning Analysis (RPA) classification of RTOG [age < or >65 years, Karnofsky performance status (KPS) score < or >70, control of systemic disease yes/no], a KPS score <70 is a poor prognostic and should raise the question of the legitimacy of surgical resection (11). However, if the score of KPS is low because of the neurological deficit due to brain metastasis then it is an argument in favor of the excision surgery. The patient’s functional status must challenge a surgical indication only if it is secondary to impaired general condition related to systemic disease or existence of multiple BM, which some symptomatics do not may be subject to resection.
Systemic disease status
The control of systemic disease defined by the activity of the primary site and the existence of extra-cerebral metastases represents an essential factor in choosing the therapeutic strategy. Indeed, in patients with BM, systemic disease status is a major prognostic factor included in RPA classification. Several studies have shown that the control of systemic disease was a confounding factor in detecting of a benefit in OS in patients who underwent surgical resection of BM (6). In the phase III randomized trial of Mintz et al., comparing surgical + WBRT versus WBRT alone, no survival benefit has been demonstrated (12). However, in this study 78.6% of patients had extra-cerebral disease controlled versus 37.5% and 31.7% in studies of Patchell et al. and Vecht et al., respectively (8,9). Analysis of the results of Mintz et al. shows that the majority of deaths were related to the evolution of systemic disease (12). Thus, it does not seem legitimate to propose a surgical resection in patients whose life expectancy is less than 3 months.
Characteristics of intra-cranial metastases
Surgical resection of BM was initially validated for single lesions. The presence of multiple metastases has been longtime an against-indications to the surgical approach. However, the introduction of new technologies and the improvement of surgical techniques have favored the inclusion of surgical resection combined with adjuvant WBRT in therapeutic strategy of multiple metastases. Indeed, several studies have shown the interest of surgical resection in multiples metastases. Bindal et al. have reported a benefit in terms of survival in a series of 56 patients with multiple metastases (2 to 3), when all lesions were resected (16). Another study on a series of 70 patients with BM from breast cancer did not shown survival difference between single and multiple lesions operated (6). More recently, two retrospective studies have shown that in patients with multiple metastases, patients with 2 to 3 lesions should benefit from the resection of dominant lesions associated with an adjuvant WBTR (6). Indeed, these two studies show that the benefit in terms of survival and functional independence was the same as for single metastases.
A similar observation was performed in recurrent metastases. Two retrospective studies have shown that repeated surgical resection of recurrent BM was a benefit in terms of survival and quality of life (17).
New surgical indications in the era of targeted therapies
The interest of the molecular characterization of metastatic disease is to document the existence in the metastatic disease of a potential phenotypic heterogeneity that should assist the clinician in defining its strategy. A recent study has shown that genetic and phenotypic heterogeneity in metastasis of breast cancer explained the resistance to targeted therapies (18). Moreover, it is well established that there can be a molecular phenotypic conversion between primitive and metastatic disease, which is influenced by the time to onset of metastasis and by the metastatic site.
Thus, the possibility to document biologically the cerebral metastatic disease may be justified when the molecular status of primary tumor is insufficiently documented. Indeed, biological documentation of metastatic disease is an approach that can lead to a change of cerebral local treatment, but also to a change in the systemic treatment, and thus to be integrate into the global therapeutic strategy of patients with cancerous lesion, these which emphasizes and enhances the role of the surgeon in this take of care. In 2012, a pioneer randomized phase II study have compared the use of targeted therapies based on the molecular profile of the tumors versus conventional chemotherapy in all types of cancer in treatment failure. This study showed that this approach was well tolerated, feasible, and consistent with routine clinical practice (19). However, if this study has shown that this approach is feasible, it remains to demonstrate that the choice of a target based on the molecular profile of the tumor improves prognosis of patients. Thus, in this perspective, a French multicenter study led by the same group reported the interest of molecular screening by Array-CGH and high-throughput sequencing of metastatic breast cancer. This innovative approach consists to identify the genomic alterations of metastatic disease that could be the subject of potential targeted therapies. However, the results of this study, while promising, were disappointing because to date there is no sufficiently of effective molecular therapies available on the market that targets the identified genomic alterations. Moreover, this approach does not integrate other components of personalized medicine as immunotherapy, modulation of DNA repair and heterogeneity intra-tumoral (20). However, it is clear that the future of cancer treatment is the screening of the primitive and metastatic tumoral disease with the goal of eventually delivering a treatment at card for each patient.
Stereotactic radiotherapy (SRT) indications
Definition and aims indication
SRT is a ‘high precision’ irradiation technique (within 1 mm), using different machines (with invasive contention or frameless, photons X or gamma) delivering high doses (4-25 Gy) in a limited number of fractions (usually 1-5, 10 maximum) with a high dose gradient, to minimize the irradiation of healthy tissues in immediate periphery of volume-target.
SRT can be delivered:
- In one single fraction thus defining the monofractionated stereotactic radiotherapy (MSRT), usually nominated “radiosurgery”, or;
- In several fractions (3-5 most frequently, up to 10) thus defining the HSRT.
The beneficial impact, in terms of OS and local control, of SRT associated with WBRT has been evaluated in management of BM (1 to 3) (Table 1).
In 2004, Andrews et al. (10) have shown a positive impact of SRT, delivered in single fraction, associated with WBRT, on the OS and local control, in comparison with WBRT alone, in patients with single BM. Thus, the local treatment, SRT, was approved for this indication. However, this significant positive impact has not been found in patients with several BM (2 to 3). Two other studies have then compared the impact of the association of SRT + WBRT to the SRT alone in patients with several BM (1 to 4) (13,15). The authors have shown a positive impact on local control, with significant decrease of 12-month rate recurrence, in patients who had the association SRT + WBRT in comparison to patients with SRT alone. However, they did not found this positive impact on OS (13,15).
The choice of type of SRT (dose, number of fractions) will depend on number of BM, size and lesion location. SRT may be proposed: (I) in combination with WBRT with the goal of increasing (modestly) OS of patients with a good performance status, 1 to 3 BM and a controlled extra-cranial disease; (II) for recurrence of 1-3 BM after WBRT; (III) after complete resection of a large and/or symptomatic BM; (IV) after diagnosis of 3 to 5 asymptomatic new or progressing BM during systemic therapy, with the aim of delaying WBRT (avoiding its potential neurotoxicity) and maintaining a high focal control rate.
In summary, main indications of SRT, if life expectancy “expected” of the patient is >3 months, are these following:
- Exclusive SRT, without surgery and without WBRT;
- SRT of tumor bed after macroscopically complete surgery;
- SRT associated with WBRT or SRT after WBRT.
Exclusive SRT, without surgery and without WBRT
This SRT is generally proposed to patients who have an extra-cranial metastatic status controlled, with metastases few or no symptoms, of limited number (4 to 5 at most) and whose size is less than 3 cm. The objective is to favor quality of life and neurocognitive status by pushing maximum the WBRT, without compromising the OS. Monitoring should however be very strict, clinical evaluation and MRI, which must be program systematically every 3 months at least the first year.
SRT of tumor bed after macroscopically complete surgery
This SRT is most often proposed to patients with metastasis whose size >3 cm, cystic or with peri-lesional edema major, highly symptomatic and/or menacing functionally. The extra-cranial status is not the most important factor; the aim is to improve the intra-cranial local control and the quality of survival. Thus, the same standards as those related to the procedures without surgery must be used (21,22). The target volume corresponds then to operative cavity and peripheral contrast enhancement, but does not include the possible edema, or the path of the incision (23).
SRT associated with WBRT or SRT after WBRT
SRT associated with WBRT can be proposed, in particularly for patients with 2 or 3 BM. If the “maximum” option is favored in terms of effectiveness (at the expense of known potential neurocognitive toxicity), the aim may be here a modest gain in OS, compared to WBRT alone. This seems particularly confirm for patients with BM from non-small cell lung cancer and whose the diagnosis-specific graded prognostic assessment (DS-GPA) score is greater than 3 (even with 2 or 3 MC) (24). When SRT is realized after WBRT, doses should consider the doses already delivered to organs at risk (brain stem, optic tract, bone marrow, cochlea) (21). However, the equivalent dose accumulation is difficult to perform because of the lack of clear data to transform MSRT or HSRT doses in “radiobiological equivalent 2 Gy”.
MSRT versus RHCS: respective interests and discussion item
Although effective, SRT performed in a single session with high doses (15-25 Gy or more), exposes to a known neurological morbidity risk related to of radionecrosis phenomenon (25,26). This risk is even more to consider than the long axis of BM exceeds 25-30 mm or that BM are located near (less than 3 mm) sensitive organs. The need to decrease the dose level of MSRT, in case of significant volume, exposes to risk of lower local control (27,28). In these situations, the HSRT represents an alternative to reduce the risk of radionecrosis while maintaining a high level of local control (29). To our knowledge, there are no randomized prospective studies comparing the HSRT to the MSRT. Nevertheless, several retrospective series and a recent prospective series have shown a local control of the HSRT comparable to that of the MSRT and a risk of post-radiation lesser toxicity (30-32). During debates among neurosurgeons, expert in radiosurgery, and radiotherapeute oncologists for choice of better schema of HSRT, discussion items are the following: no consensus on the number of fractions (3 to 5 sessions of 7 to 11 Gy, one series shows no toxicity after 10 sessions of 4 Gy), the sprawl (daily session or on alternate days?) and then on the method of dose prescription (33-36). These limits prevent any definitive comparison inter-center, unlike the radiosurgical series, very homogeneous in their methodology.
Thus, in view of literature (37,38), we can consider that the HSRT will find its indications when the maximum diameter of the BM is important (from 25 mm up to 35-40 mm in postoperative situation), the total number of sessions being modulated according to the volume irradiated from 3 to 5 fractions, at doses per session from 7-8 Gy at 11-12 Gy at maximum, the prescription isodose from 70% to 90%. A margin of 1 mm must be added to the gross target volume taking into account the microscopic infiltration and an additional margin (1-2 mm) must be added due to approximate repositioning.
Radionecrosis after SRT
The radionecrosis appears typically 6 to 12 months after SRT. After radiosurgery, about 50% of lesions appear as pure radionecrosis while others are a mixture of tumor cells and necrosis (39). The incidence varies from 2% to 22% for radiological radionecrosis and from 1% to 14% for symptomatic (26,40). The differentiation between evolutive recurrence and radionecrosis is very difficult. On MRI scan, there is an increase of contrast-enhancement in crown on T1-sequence after gadolinium injection and an important edema on FLAIR-sequence, absolutely non-specific. Thus, the clinical examination remains important because of the absence of symptoms which would support radionecrosis. After single fraction, the risk of radionecrosis was evaluated from very large series. The volume which received a dose of 12 Gy (V12 Gy) in a fraction seems predictive to 1 year (39). Few data are available to accurately estimate the risk of radionecrosis 1 year after HSRT. On Tri-fractionating, the risk of radionecrosis to 1 year is estimated for the V21 Gy to 14% if >20.9 cc versus 4% if <20.9 cc (38), and on penta-fractionating: if V28.8 Gy <3 cc, only symptomatic edema is observed, but if V28.8 Gy >7 cc, the risk of necrosis becomes important (41).
With the delivery of increasingly frequent of drug “targeted” such as vemurafenib (in melanomas) or sunitinib (in kidney cancers with clear cell), great attention must be realized to avoid concomitant association with SRT; the current data being quite contradictory: increasing efficiency and/or risk of radiation necrosis (42-45)?
Although there is no ‘curative’ treatment of radionecrosis, the corticosteroids at a dose of 1 mg/kg for a period of at least 1 month, pentoxifylline, low-dose of bevacizumab (5 mg/kg every 3 weeks) (46,47) as well as surgery are the most commonly recommended treatments. Some decisional trees have even been proposed (40).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mehta MP, Khuntia D. Current strategies in whole-brain radiation therapy for brain metastases. Neurosurgery 2005;57:S33-44; discusssion S1-4.
- Mehta MP, Tsao MN, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2005;63:37-46. [PubMed]
- Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:17-32. [PubMed]
- Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:33-43. [PubMed]
- Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:45-68. [PubMed]
- Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol 2009;92:275-82. [PubMed]
- Lang FF, Sawaya R. Surgical management of cerebral metastases. Neurosurg Clin N Am 1996;7:459-84. [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [PubMed]
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [PubMed]
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78:1470-6. [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [PubMed]
- Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg 1993;79:210-6. [PubMed]
- Bindal RK, Sawaya R, Leavens ME, et al. Reoperation for recurrent metastatic brain tumors. J Neurosurg 1995;83:600-4. [PubMed]
- Almendro V, Kim HJ, Cheng YK, et al. Genetic and phenotypic diversity in breast tumor metastases. Cancer Res 2014;74:1338-48. [PubMed]
- Le Tourneau C, Kamal M, Trédan O, et al. Designs and challenges for personalized medicine studies in oncology: focus on the SHIVA trial. Target Oncol 2012;7:253-65. [PubMed]
- André F, Bachelot T, Commo F, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol 2014;15:267-74. [PubMed]
- Tsao MN, Lloyd N, Wong R, et al. Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database Syst Rev 2006;CD003869. [PubMed]
- Minniti G, Esposito V, Clarke E, et al. Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys 2013;86:623-9. [PubMed]
- Prabhu R, Shu HK, Hadjipanayis C, et al. Current dosing paradigm for stereotactic radiosurgery alone after surgical resection of brain metastases needs to be optimized for improved local control. Int J Radiat Oncol Biol Phys 2012;83:e61-6. [PubMed]
- Sperduto PW, Shanley R, Luo X, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 2014;90:526-31. [PubMed]
- Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996-1001. [PubMed]
- Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011;6:48. [PubMed]
- Yang HC, Kano H, Lunsford LD, et al. What factors predict the response of larger brain metastases to radiosurgery? Neurosurgery 2011;68:682-90; discussion 690. [PubMed]
- Han JH, Kim DG, Chung HT, et al. Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 2012;83:113-20. [PubMed]
- Aoyama H, Shirato H, Onimaru R, et al. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys 2003;56:793-800. [PubMed]
- Bernier-Chastagner V, Baffert S, Thillays F, et al. Évaluation de la radiothérapie fractionnée en conditions stéréotaxiques des métastases cérébrales de l’adulte: résultats cliniques et médicoéconomiques à 2 ans. Cancer Radiothérapie 2008;12:701.
- Kim YJ, Cho KH, Kim JY, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys 2011;81:483-9. [PubMed]
- Fokas E, Henzel M, Surber G, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol 2012;109:91-8. [PubMed]
- Ernst-Stecken A, Ganslandt O, Lambrecht U, et al. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol 2006;81:18-24. [PubMed]
- Fahrig A, Ganslandt O, Lambrecht U, et al. Hypofractionated stereotactic radiotherapy for brain metastases--results from three different dose concepts. Strahlenther Onkol 2007;183:625-30. [PubMed]
- Wiggenraad R, Verbeek-de Kanter A, Kal HB, et al. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol 2011;98:292-7. [PubMed]
- Ohtakara K, Hayashi S, Hoshi H. Characterisation of dose distribution in linear accelerator-based intracranial stereotactic radiosurgery with the dynamic conformal arc technique: consideration of the optimal method for dose prescription and evaluation. Br J Radiol 2012;85:69-76. [PubMed]
- Schlienger M, Nataf F, Huguet F, et al. Hypofractionated stereotactic radiotherapy for brain metastases. Cancer Radiother 2010;14:119-27. [PubMed]
- Minniti G, D'Angelillo RM, Scaringi C, et al. Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol 2014;117:295-301. [PubMed]
- Telera S, Fabi A, Pace A, et al. Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol 2013;113:313-25. [PubMed]
- Stockham AL, Ahluwalia M, Reddy CA, et al. Results of a questionnaire regarding practice patterns for the diagnosis and treatment of intracranial radiation necrosis after SRS. J Neurooncol 2013;115:469-75. [PubMed]
- Inoue HK, Seto K, Nozaki A, et al. Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J Radiat Res 2013;54:727-35. [PubMed]
- Liebner DA, Walston SA, Cavaliere R, et al. Radiation necrosis mimicking rapid intracranial progression of melanoma metastasis in two patients treated with vemurafenib. Melanoma Res 2014;24:172-6. [PubMed]
- Baroudjian B, Boussemart L, Routier E, et al. Dramatic response to radiotherapy combined with vemurafenib. Is vemurafenib a radiosensitizer? Eur J Dermatol 2014;24:265-7. [PubMed]
- Staehler M, Haseke N, Nuhn P, et al. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int 2011;108:673-8. [PubMed]
- Barney BM, Markovic SN, Laack NN, et al. Increased bowel toxicity in patients treated with a vascular endothelial growth factor inhibitor (VEGFI) after stereotactic body radiation therapy (SBRT). Int J Radiat Oncol Biol Phys 2013;87:73-80. [PubMed]
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011;79:1487-95. [PubMed]
- Lubelski D, Abdullah KG, Weil RJ, et al. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neurooncol 2013;115:317-22. [PubMed]


