The management of BRCA1 and BRCA2 carriers in Singapore
The landscape of Singapore healthcare
Singapore is an island nation located in Southeast Asia, measuring 721.5 km2 and 50 km end to end. Singapore is a densely populated country, with a population of 5.7 million. The population is multiethnic and comprises 74% Chinese, 13% Malay, 9% Indian and 3% others (1). In addition to being racially diverse, Singapore is also multireligious with Buddhism/Taoism (43%), Christianity (19%), Islam (14%), Hindu (5%) and unaffiliated (19%) making up the population’s religion (2). Singaporeans are 97.3% literate, with 57.3% of the population having post-secondary qualifications and 32.4% holding a university degree (1).
The average life expectancy of a Singaporean at birth is 83.2 years, with females and males at 85.4 and 81.0 years respectively (1). Cancer is the leading cause of death in Singapore (28.8%), with pneumonia (20.6%) and ischemic heart disease (18.1%) as the second and third most common cause of death (3). Amongst patients with cancer, breast cancer is the most common cancer diagnosed in females and the second most common cancer in the overall Singapore population (4). It affects approximately 1 in 14 Singaporeans in their lifetime. On average, more than 1,900 women in Singapore are diagnosed with breast cancer yearly, and over 400 die from the disease each year (4).
There are nine public tertiary hospitals in the country, with a doctor to population ratio of 1:410 (1). Two formal cancer genetics services are available in Singapore, based in National Cancer Institute Singapore (NCIS) and National Cancer Centre Singapore (NCCS). Both genetics services serve both paediatric and adult patients. NCCS also runs satellite outreach clinics in other public hospitals distributed across Singapore. All genetic counsellors working at NCCS have Master’s level qualification in genetic counselling. Genetic counsellors in Singapore are in the process of establishing national proficiency norms through the establishment of a professional society. Public healthcare in Singapore is subsidized by the government and is provided via a co-payment system (5). There is a nationwide insurance program “Medishield Life” that covers all Singaporeans from birth. Additional coverage can be bought from private insurers at one’s own expense. Government subsidies are tiered and provided based on financial need, with systems such as Community Health Assist Scheme (CHAS) and Medifund to help the economically disadvantaged.
Breast cancer screening
The national screening programme for breast cancer, BreastScreen Singapore (BSS), offers low cost mammographic screening to all Singaporean women above the age of 50, with biennial mammograms (6). Only 40% of Singaporean women are up to date with their mammogram screening in Singapore (7). However, this figure is an underestimation as it excludes patients who had their mammogram done in the private sector as they are not captured by BSS.
Genetics literacy and cultural awareness
The demand and resultant supply of genetic testing has grown rapidly globally (8). Since 2014, there has been significant increases in the number of cases referred for genetic counselling in Singapore as well (9-11). This is likely contributed by the rising genetics literacy and awareness amongst healthcare professionals and the Singaporean population (12), in part due to sustained effort from the genetics services (13).
Genetic counselling involves more than just provision of information from healthcare professional to patient, it addresses the psychosocial aspects of hereditary conditions (14). With the availability of genetic counsellors, time is spent with patients and their families to provide information, clarify doubts, promote informed decision-making, whilst providing the necessary emotional support. This has been shown to improve patient outcomes, including empowerment, behavioural change and decisional satisfaction (15-18). Moreover, there is a cascade effect where patients are better equipped to share the genetics knowledge from the consult with their extended families to help with downstream risk management (19,20).
In multiethnic countries, it is important for genetic counselling services to be culturally sensitive (21). In a multilingual country such as Singapore, English may be the working language, but not necessarily the native language of the patient. All information leaflets on common hereditary conditions are translated into the three most common languages in Singapore (English, Mandarin and Malay) in both cancer genetics services in Singapore.
Cultural sensitivity requires healthcare professionals to understand the strong impact of culture on health beliefs (22). Amongst the ethnic groups, healthcare outcomes amongst the Malay community are known to be poorer (23,24). We explored the cultural beliefs of the Malay community via focus groups to understand their views towards breast cancer screening and genetic testing. The study found that spiritual and religious beliefs act as barriers towards uptake of screening and genetic testing, preference for traditional medicine competes with Western medicine recommendations, family and community influence health-related decisions, complexed by differences in intergenerational beliefs creating contrasting attitudes towards screening and prevention (25). Moving forward, we aim to collaborate with key Malay community leaders in order to address these cultural factors, as well as expand research to other ethnic groups.
Education of healthcare professionals
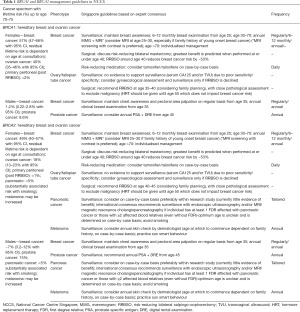
At the professional level, both genetics services play a key role in raising awareness and sharing genetics knowledge in the healthcare community. Genetic counsellors attend multidisciplinary tumour boards and attach checklists in clinics to identify patients appropriate for genetics referral and help busy clinicians pick these patients up (26,27). This is pertinent in light of the expanding indications and continued lowering of threshold for genetic testing. Genetics education is also a key component of cancer genetics work. The clinical geneticist and genetic counsellors are involved in educating a wide spectrum of healthcare professionals, including nurses, medical officers, residents, senior residents and consultants, both locally and in the region. This strategy aims to improve genetics knowledge amongst the next generation of healthcare providers, so that patients with hereditary cancer syndrome such as Hereditary Breast and Ovarian Cancer (HBOC) are appropriately managed. Considerable effort has been put into tailoring referral and risk management guidelines specific to the Singapore context. The Singapore Cancer Action Network (SCAN) guidelines for referral for genetic evaluation of common hereditary cancer syndromes was published in 2015 (28) and is due for revision in 2020. It was a collaboration between Singapore’s tertiary cancer centres to address common hereditary cancer syndrome, including HBOC and Lynch syndrome. SCAN adopted guidelines from international societies, including National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO), and modified them to fit the local Singaporean context. Given the BRCA1/2 Manchester scoring prediction model was included in these guidelines, we have also validated the model for the Singaporean population (29). More recently in 2017, NCCS aimed to standardize care pathways for HBOC and other adult hereditary cancer syndromes. A multidisciplinary team was convened to adapt Singapore-specific risk management guidelines for our hospital. The recommended surveillance for BRCA1/2 pathogenic variant/likely pathogenic variant (PV/LPV) carriers adopted in NCCS are shown in Table 1.
Full table
Access-referral source and costs
Two academic institutions, NCIS and NCCS, see the majority of adult cancer genetics referral in Singapore. Approximately 70% of all cancer cases in Singapore are seen at NCCS, with more than 9000 new cancer cases seen yearly (30). Out of these, approximately 450 patients (5%) may carry a cancer predisposition gene (31). Patients are referred based on personal and/or family history of cancers. Waiting time is in the range of two to three months, however, patients are seen sooner if they require urgent testing for treatment purposes. Long waiting time has been shown to increase non-attendance in clinics with a lost opportunity to identify patients with BRCA1/2 PV/LPV and their at-risk family members (32,33). Referrals originate from other departments within public institutions, general and specialist practitioners from the private sector and also from the surrounding South East Asian region.
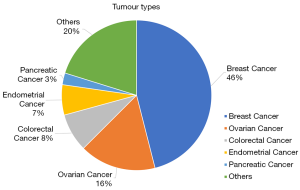
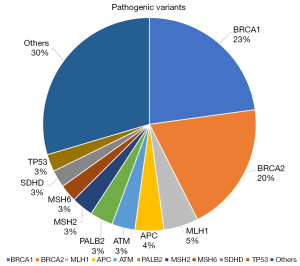
From 2014 to 2019, more than 2600 patients were seen at NCCS Cancer Genetics Service (CGS). Affected patients present with a diverse range of tumour types (Figure 1). Breast and ovarian cancers account for 62% of the observed tumour types. Seventy-one percent of patients seen at CGS will proceed with genetic testing. Amongst patients who did genetic testing, pathogenic variants were found in 16.3% of patients, variant of uncertain significance (VUS) in 34.6% of patients and a combination of pathogenic variants and VUS were seen in 8.2% of patients. 40.9% of patients had a negative test result. Due to the large diversity of cases seen and the routine use of multi-gene panel testing, a large spectrum of germline pathogenic genes are found, of which BRCA1/2 contributes to 43% (Figure 2).
There are currently no government subsidies for genetic testing. Similarly, most insurance companies do not cover the cost of genetic testing. Over the last decade, testing individual genes with Sanger sequencing has largely been replaced by multigene panel testing with next generation sequencing (34). With this, the cost of testing has fallen significantly to less than SGD$700 per test. However, this lowered cost is still viewed as a barrier to genetic testing in Singapore (10). Public institutions depend on finite short-term philanthropic funds as a plug gap measure for financially needy patients. The provision of philanthropic funding resulted in a significant increase in uptake of genetic testing and improved access for patients in need of genetic testing (10,35). Outcomes from such a model is actively being studied by policy makers. Pharmaceutical companies have also begun to offer genetic testing for patients, in light of expanding drug indications based on germline test results. Unfortunately, the focus is only on groups of patients with druggable mutations in specific cancers (36) and miss out many others who would benefit from genetic testing for risk management indications. While such industry partnerships and support is important, it is vital that the health system ensures access to actionable genetic testing remains equitable, regardless of socioeconomic status as well as disease type and stage.
Genetic counselling—the testing process in Singapore
All patients who attend the genetics clinic undergo pre-test counselling with a genetic counsellor and/or clinical geneticist, the latter if a physical examination is required. It entails taking a medical history, three generation pedigree, risk assessment and a discussion on the pros and cons of germline genetic testing. Patient are then given the opportunity to decide if they are keen to proceed with testing and informed consent taken. All patients who undergo genetic testing are recruited to a prospective registry. The turnaround time ranges from two to eight weeks depending on extent of testing. All laboratories used by public institutions have to be accredited according to national standards to ensure reliability. Laboratories are selected based on quality, methodology, cost, as well as their ability to respond to request for review of clinically suspicious cases. This is especially important in a multiethnic population like Singapore, where there are often limited allelic frequency data available in the public domain for variant classification (37-39). Genetic data is lacking for much of Singapore’s diverse Asian population and this results in a higher rate of VUS (40,41). Due to these limitations, all genetic test reports are discussed at a weekly family review meeting, where the genetics team reviews every report and ensure the correct interpretation of all variants, paying attention to the population source of the reported allelic frequencies. In some cases, we have encountered highly suspicious families with clinically relevant variants where the original test result indicated a VUS. We will then employ several alternative methods to help with variant reclassification (e.g., segregation analysis) and have been successful at resolving a number of such cases.
One key factor contributing to the resolution of these cases is having a centralised registry where information is stored in terms of families and not just individual patients. This allows for the identification of families carrying the same variant. By accumulating families with the same variant, investigations can be pooled together to provide more power for variant reclassification. This is important in understudied populations, such as Singapore and the rest of Asia, where normal variant data is clearly lacking. Singapore has a National Electronic Health Record (NEHR) that stores patient’s health data (42). This makes it accessible to other healthcare providers in Singapore for seamless transfer of care between hospitals and also empowers patients to take charge of their own health.
Follow up—surveillance, predictive testing, patient support group
Patients receive post-test counselling and risk-management recommendations for both individual and at-risk family members in the same setting. When a BRCA1/2 PV/LPV is found in a patient, individualized risk management is offered, and predictive testing of at-risk relatives is facilitated. Letters are written back to referring doctors on surveillance recommendations and therapeutic implications for the individual patient, as well as surveillance guidelines for the extended family. Patients are encouraged to share their genetic results with their relatives to enable cascade predictive testing. Patients continue to be followed up with the cancer genetics service which serves as patient advocates in their downstream care. With patient-centric care as the goal, patients are managed in multi-disciplinary risk management clinics.
For female BRCA1/2 PV/LPV carriers in Singapore, women are encouraged to start their annual breast imaging at 25 to 30 years old and to consider risk-reducing bilateral salpingo-oophorectomy when family planning is completed (Table 1). For males with BRCA1/2 PV/LPV, we have an individualized discussion on the benefit of annual prostate-specific antigen (PSA) screening starting from age 40 to 45, especially for BRCA2 carriers (Table 1). For males and females with BRCA2 PV/LPV, pancreatic cancer screening is controversial. We discuss screening with endoscopic ultrasonography and/or MRI/magnetic resonance cholangiopancreatography (MRCP) in our centre if they have high risk features, such as a first-degree relative with pancreatic cancer or two relatives of any degree with pancreatic cancer (Table 1) (43). This is performed within the domains of a study protocol. An annual skin examination for lesions worrisome for melanoma is discussed in selected patients. Sun-smart behaviour is encouraged for all patients with BRCA2 PV/LPV (Table 1). Most Singaporeans with BRCA1/2 PV/LPV adhere to their surveillance protocol despite the need for out-of-pocket testing (44). However, there are economically disadvantaged patients who are not able to afford the cost of surveillance imaging. NCCS has thus partnered with community groups, such as the Singapore Cancer Society, to provide financial support for these patients.
A previous study done in NCCS showed the uptake of predictive testing in our centre is 15%, which lead us to interview our patients to explore factors that influence the sharing of genetic results with their relatives (20). The reasons we identified were similar to our Western counterparts. These include distant family relations, family members not involved in testing process and perception of high likelihood of burden with the knowledge of hereditary cancer. A subsequent study showed that provision of subsidies increased uptake of genetic testing and was cost-effective if the uptake of predictive testing exceeded 36% (10). With this knowledge, we have modified our practice from the first consult, and actively encourage patients to come with a family member for support and to engage the patient’s family on the possible need for predictive testing. Targeted efforts to improve predictive testing rates are currently underway, with campaigns such as “Jeans For Genes” held annually to raise awareness about hereditary cancer syndromes. We are piloting several multi-prong approaches to reach a target of 40% predictive testing uptake over the next few years.
Our patient support group, At-Risk of Cancer (ARC) Support Group, organises quarterly gatherings facilitated by healthcare providers. It is an avenue for patients to seek mutual support and since its inception in 2017, the number of participants has grown significantly. The patients participate in a wide range of activities, such as talks to teach them how to manage the psychological stress of having a hereditary cancer syndrome and also ways to improve self-confidence.
Summary
The field of cancer genetics is constantly evolving and the clinical indications for germline genetic testing will continue to expand. Genetic testing is coming of age in Singapore with increasing genetic literacy and demand from the general population. As such, there is a clear need of dedicated healthcare professionals for pre- and post-test counselling, interpretation of test results and surveillance for patients, as well as predictive testing for at-risk family members. We believe the steps we have taken to develop a comprehensive cancer genetics service will serve as a useful resource for others, particularly in Asia. It is essential for countries in Asia to prioritize the removal of major barriers to the provision of genetics services, including long-term funding sources for genetic testing and surveillance imaging. Improving predictive testing rates is the mainstay of cost-effective delivery of genetic counselling services. With greater uptake of genetic testing, this will likely reduce mortality in patients with hereditary cancer syndromes, lower healthcare cost and advance our understanding of normal genetic variants in the understudied Asian population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Shaheenah Dawood) for the series “Targeting the DNA Damaging Pathway: PARPi and Beyond” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-104). The series “Targeting the DNA Damaging Pathway: PARPi and Beyond” was commissioned by the editorial office without any funding or sponsorship. The authors report research funding from AstraZeneca, and Pfizer, outside the submitted work.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singapore Population [Internet]. Base. [cited 2020 Feb 10]. Available online: http://www.singstat.gov.sg/modules/infographics/population
- General Household Survey 2015 [Internet]. Base. [cited 2020 Feb 10]. Available online: http://www.singstat.gov.sg/publications/ghs/ghs2015content
- MOH | Principal Causes of Death [Internet]. [cited 2020 Feb 10]. Available online: https://www.moh.gov.sg/resources-statistics/singapore-health-facts/principal-causes-of-death
- Cancer Registry - National Registry Of Diseases Office [Internet]. [cited 2020 Feb 10]. Available online: https://www.nrdo.gov.sg/publications/cancer
- Lim J. Sustainable Health Care Financing: The Singapore Experience. Glob Policy 2017;8:103-9.
- Wang SC. The Singapore National Breast Screening Programme: principles and implementation. Ann Acad Med Singapore 2003;32:466-76. [PubMed]
- Loy EY, Molinar D, Chow KY, et al. National Breast Cancer Screening Programme, Singapore: Evaluation of participation and performance indicators. J Med Screen 2015;22:194-200. [Crossref] [PubMed]
- Phillips KA, Deverka PA, Hooker GW, et al. Genetic Test Availability And Spending: Where Are We Now? Where Are We Going? Health Aff (Millwood) 2018;37:710-6. [Crossref] [PubMed]
- Chieng WS, Lee SC. Discrepancy Between Initial High Expression of Interest in Clinical Cancer Genetic Testing and Actual Low Uptake in an Asian Population. Genet Test Mol Biomarkers 2012;16:785-93. [Crossref] [PubMed]
- Li ST, Yuen J, Zhou K, et al. Impact of subsidies on cancer genetic testing uptake in Singapore. J Med Genet 2017;54:254-9. [Crossref] [PubMed]
- Lim CW, Samol J, Flook MJJ, et al. Germline BRCA1/2 testing: Trend in Tan Tock Seng Hospital Singapore. Ann Oncol 2019;30:ix127. [Crossref]
- Chin TM, Tan SH, Lim SE, et al. Acceptance, motivators, and barriers in attending breast cancer genetic counseling in Asians. Cancer Detect Prev 2005;29:412-8. [Crossref] [PubMed]
- Abacan M, Alsubaie L, Barlow-Stewart K, et al. The Global State of the Genetic Counseling Profession. Eur J Hum Genet EJHG 2019;27:183-97. [Crossref] [PubMed]
- Austin J, Semaka A, Hadjipavlou G. Conceptualizing genetic counseling as psychotherapy in the era of genomic medicine. J Genet Couns 2014;23:903-9. [Crossref] [PubMed]
- Edwards A, Gray J, Clarke A, et al. Interventions to improve risk communication in clinical genetics: systematic review. Patient Educ Couns 2008;71:4-25. [Crossref] [PubMed]
- Meiser B, Irle J, Lobb E, et al. Assessment of the Content and Process of Genetic Counseling: A Critical Review of Empirical Studies. J Genet Couns 2008;17:434-51. [Crossref] [PubMed]
- Yuen J, Lee SY, Courtney E, et al. Evaluating empowerment in genetic counseling using patient-reported outcomes. Clin Genet 2020;97:246-56. [Crossref] [PubMed]
- Courtney E, Li ST, Shaw T, et al. Predictors of next-generation sequencing panel selection using a shared decision-making approach. NPJ Genom Med 2018;3:11-7. [Crossref] [PubMed]
- Lieberman S, Lahad A, Tomer A, et al. Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med 2018;20:1446-54. [Crossref] [PubMed]
- Li ST, Sun S, Lie D, et al. Factors influencing the decision to share cancer genetic results among family members: An in-depth interview study of women in an Asian setting. Psychooncology 2018;27:998-1004. [Crossref] [PubMed]
- Saha S, Beach MC, Cooper LA. Patient centeredness, cultural competence and healthcare quality. J Natl Med Assoc 2008;100:1275-85. [Crossref] [PubMed]
- Abad PJB, Tan ML, Baluyot MMP, et al. Cultural beliefs on disease causation in the Philippines: challenge and implications in genetic counseling. J Community Genet 2014;5:399-407. [Crossref] [PubMed]
- Lim JN, Potrata B, Simonella L, et al. Barriers to early presentation of self-discovered breast cancer in Singapore and Malaysia: a qualitative multicentre study. BMJ Open 2015;5:e009863. [Crossref] [PubMed]
- Bhoo-Pathy N, Hartman M, Yip CH, et al. Ethnic differences in survival after breast cancer in South East Asia. PLoS One 2012;7:e30995. [Crossref] [PubMed]
- Shaw T, Ishak D, Lie D, et al. The influence of Malay cultural beliefs on breast cancer screening and genetic testing: A focus group study. Psychooncology 2018;27:2855-61. [Crossref] [PubMed]
- Cohen PA, Nichols CB, Schofield L, et al. Impact of Clinical Genetics Attendance at a Gynecologic Oncology Tumor Board on Referrals for Genetic Counseling and BRCA Mutation Testing. Int J Gynecol Cancer 2016;26:892-7. [Crossref] [PubMed]
- Chan SH, Chew W, Ishak NDB, et al. Clinical relevance of screening checklists for detecting cancer predisposition syndromes in Asian childhood tumours. NPJ Genom Med 2018;3:30-8. [Crossref] [PubMed]
- Singapore Cancer Network (SCAN) Cancer Genetics Workgroup. Singapore Cancer Network (SCAN) Guidelines for Referral for Genetic Evaluation of Common Hereditary Cancer Syndromes. Ann Acad Med Singapore 2015;44:492-510. [PubMed]
- Chew W, Moorakonda RB, Courtney E, et al. Evaluation of the relative effectiveness of the 2017 updated Manchester scoring system for predicting BRCA1/2 mutations in a Southeast Asian country. J Med Genet 2018;55:344-50. [Crossref] [PubMed]
- Teo M, Soo KC. National Cancer Center Singapore: the way forward. Future Oncol 2016;12:433-7. [Crossref] [PubMed]
- Huang KL, Mashl RJ, Wu Y, et al. Pathogenic Germline Variants in 10,389 Adult Cancers. Cell 2018;173:355-70.e14. [Crossref] [PubMed]
- Shaw T, Metras J, Ting ZAL, et al. Impact of Appointment Waiting Time on Attendance Rates at a Clinical Cancer Genetics Service. J Genet Couns 2018;27:1473-81. [Crossref] [PubMed]
- Tan RYC, Met-Domestici M, Zhou K, et al. Using Quality Improvement Methods and Time-Driven Activity-Based Costing to Improve Value-Based Cancer Care Delivery at a Cancer Genetics Clinic. J Oncol Pract 2016;12:e320-31. [Crossref] [PubMed]
- Lynce F, Isaacs C. How Far Do We Go With Genetic Evaluation? Gene, Panel, and Tumor Testing. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Meet 2016;35:e72-8.
- Courtney E, Chok AKL, Ang ZLT, et al. Impact of free cancer predisposition cascade genetic testing on uptake in Singapore. NPJ Genom Med 2019;4:22-7. [Crossref] [PubMed]
- Lim D, Ngeow J. Evaluation of the methods to identify patients who may benefit from PARP inhibitor use. Endocr Relat Cancer 2016;23:R267-85. [Crossref] [PubMed]
- Eggington JM, Bowles KR, Moyes K, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet 2014;86:229-37. [Crossref] [PubMed]
- Wong ESY, Shekar S, Met-Domestici M, et al. Inherited breast cancer predisposition in Asians: multigene panel testing outcomes from Singapore. NPJ Genom Med 2016;1:15003. [Crossref] [PubMed]
- Bentley AR, Callier S, Rotimi CN. Diversity and inclusion in genomic research: why the uneven progress? J Community Genet 2017;8:255-66. [PubMed]
- Ow SGW, Ong PY, Lee SC. Discoveries beyond BRCA1/2: Multigene testing in an Asian multi-ethnic cohort suspected of hereditary breast cancer syndrome in the real world. PLoS One 2019;14:e0213746. [Crossref] [PubMed]
- Ndugga-Kabuye MK, Issaka RB. Inequities in multi-gene hereditary cancer testing: lower diagnostic yield and higher VUS rate in individuals who identify as Hispanic, African or Asian and Pacific Islander as compared to European. Fam Cancer 2019;18:465-9. [Crossref] [PubMed]
- Stephanie L. Singapore’s NEHR: Challenges on the path to connected health. In: 2017 IEEE International Conference on Industrial Engineering and Engineering Management (IEEM) 2017:1128-32.
- Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7-17. [Crossref] [PubMed]
- Courtney E, Chin XW, Yuen J, et al. Risk management adherence following genetic testing for hereditary cancer syndromes: a Singaporean experience. Fam Cancer 2018;17:621-6. [Crossref] [PubMed]