Toxicity of checkpoint inhibitors
Introduction
For several years, metastatic melanoma treatment was based on dacarbazine. Results have been disappointing (1). Despite this, it has been the main choice for control arms, even in recent randomized controlled trials (1-5). Alternatively, immunotherapy has been studied for many years, as a promising therapy. The main reason for investment in research on immunotherapeutic agents is data supporting the idea that the immunologic system may interfere with melanoma outcome. Observations that support this theory are the findings of immune activity at spontaneous regression sites (6-8), melanoma with better outcome in patients who develop autoimmune events (e.g., vitiligo) (9) and worse outcome in patients with immunodeficiency (10-12).
Many immunological agents, such as vaccines and cytokines, have been used in melanoma treatment. To the present, there is no single therapy vaccine considered active for metastatic melanoma or in the adjuvant scenario (13,14). The only positive results observed were in a single trial with gp100, which provided better results when added to high-dose of interleukin-2 (IL-2), but not enough to change practice (15).
Among cytokines, the only two drugs available for melanoma treatment are IL-2, in metastatic disease, and interferon-alfa in the adjuvant scenario. Despite their recognized activity, both therapies have yet to be universally adopted, mainly due to their toxicity profiles (16-18).
High-dose IL-2 has been approved based on data showing a long-term survival rate for approximately 5% of patients treated (16,17). No prospective molecular biomarker has been found to enrich these results, although clinical presentation was correlated with response rate (19). Complex and severe toxicity demanded more in terms of logistics and learning-curve for the treating institutions, in order to improve safety for patients (20). A significant point to consider in the IL-2 therapy was the dose adjustment according to toxicity: the number of doses administered depended on the toxicity, with the recommendation being to reach patients’ tolerance threshold. This strategy, without the proper training of the entire treatment team, leads to higher risk of morbidity and mortality. Although there are defined guidelines, most community oncology centers have not adopted high-dose IL-2 as an alternative (21).
In the pursuit of the long-term benefit provided by immunotherapy, new agents have been developed. The new class of agents, called immunological checkpoint inhibitors, includes monoclonal antibodies without direct immune activity against tumor cells. They act through receptor blockade at specific points in the immune response. The first drug in this class was ipilimumab, an IgG antibody directed at the CTLA4 receptor of the T-Lymphocyte. This molecule is expressed on the T-cell surface after its activation and competes for B7 (a molecule of the antigen-presenting cell surface) interaction, which leads to an inhibitory signal for the T-cell (22,23). Through CTLA4 blockade, ipilimumab promotes a release of this inhibition and in turn, enhances the immune response (24). Its clinical activity has been demonstrated in two randomized clinical trials. The first trial dealt with previously treated melanoma patients, using four cycles of ipilimumabe at a dosage of 3 mg/kg (25). In this trial, the relative risk reduction for death was 34% for the patients that received ipilimumab, in comparison to those in the control arm (gp100). In a second randomized trial, with untreated patients, ipilimumab combined with dacarbazine was compared with dacarbazine alone. In this trial, four cycles of 10 mg/kg of ipilimumab were administered, followed by maintenance infusions every 12 weeks until progression. Again, overall survival benefit was verified, with a 28% risk reduction of mortality (2).
Although registration for ipilimumab in many countries was based in a single randomized trial, its development was rather long, with thousands of patients treated. This led to the important observation of toxicity and allowed the developers to define a comprehensive core of guidelines for patient management, which made the drug safe for community-based usage. To follow, toxicity and its managing guidelines will be reviewed and discussed.
Recently, three new drugs had their initial data presented. Nivolumab (26,27) and lambrolizumab (28) are both PD1 (Programmed Death 1) blockers. MPDL3280A (29) is a PD-L1 inhibitor. PD1, is a T-Lymphocyte surface receptor which promotes inhibition of cell immune response upon interaction with its ligands: PD-L1 and PD-L2. It promotes such reduction by a rapid up regulation at the antigen cell presentation, causing an acute inhibitory signal. Another mechanism of T-cell inactivation is through chronic exposure to antigens and, in particular, PD-L1. This may be observed in tumor infiltrating lymphocytes, which become exhausted (30). The blockade of PD1 interaction with its ligands, promotes improvement of acute T-cell activation, and also restores chronic activity, revert the exhaustion (31). The toxicity profile discussed in the text to follow will be based on the initial data presented on these drugs.
Anti-CTLA4
Two anti-CTLA4 have been developed, but only ipilimumab was registered. The other, tremelimumab, was not registered due to a lack of data showing overall survival benefit (4). The content to follow will be based on ipilimumab data.
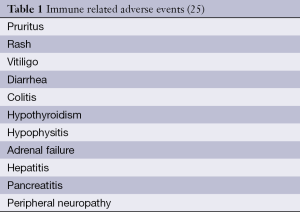
CTLA4 blockade by ipilimumab, provides suppression of the inhibitory signal to the T-cell and, by doing so, increases the chance for activation. Ipilimumab activity has no antigen specificity. Consequently, toxicity occurs due to a reduction in tolerance to antigens previously recognized as “self”, which leads to autoimmune events. Table 1 reports the most commonly observed immune-related adverse events. Aside from immune-related adverse events, other non-specific symptoms have been reported (e.g., fatigue, headache, dyspnea, cough) but their concrete connection might not be evident.
In general, immune-related toxicity due to ipilimumab may be treated according to Table 2 guidelines. This is a summary of the large majority of recommendations. Although not obligatory, grading toxicity based on Common Toxicity Criteria (CTC) by the National Institute of Health and National Cancer Institute is helpful in terms of orientation and is the basis for most guidelines provided.
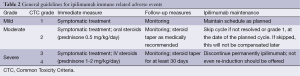
Full table
Dermatological adverse events
Dermatological adverse events may be observed in up to 44% of patients, although less than 2% are considered severe (grade 3 or 4). Still, Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported and must be considered in rapid-onset cases of skin toxicity.
Rash
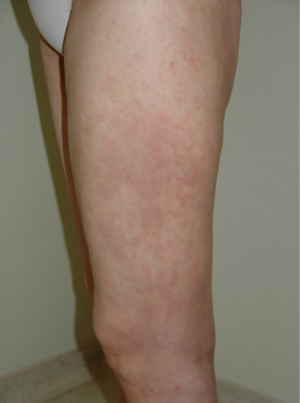
A maculo-papular rash is reported in up to 20% of patients receiving ipilimumab (Figure 1). Appearance usually occurs after the third week of therapy, with its peak at the sixth week. Most of the cases are considered as grade 1, using CTC (less than 10% of body surface area involved) and may be accompanied by symptoms such as pruritus. Restricted lesions might be observed, eventually treated with topical corticosteroids such as betamethasone 0.1% or clobetasol 0.05%. No change in the ipilimumab schedule is necessary. Patients with symptomatic lesions and a body surface area involvement greater than 10% but less the 30% (grade 2), should be treated with topical or oral steroids (prednisone, up to 0.5 mg/kg/day, or equivalent). Rapid improvement is anticipated and the steroid is to be tapered according to medical evaluation. At subsequent ipilimumab infusion, there are of skin involved should be at most a grade 1 in order to proceed with the infusion. Otherwise, infusion must be rescheduled. Rare events of grade 3 rash (greater than 30% of body surface area involved) must be treated with IV steroids (methylprednisolone 1-2 mg/kg/day, or equivalent) and upon improvement, oral steroids may replace IV steroids, with the same tapering plan put into place. The aforementioned skin-area involvement would suggest a permanent discontinuation of ipilimumabe (32).
Pruritus
Pruritus may be observed in up to 25% of patients, with or without visible skin lesions. No debilitating cases have been reported. If accompanied by a skin lesion, pruritus should be treated according to guidelines mentioned above. The pruritus itself may improve with oral anti-histaminic drugs, such as hydroxyzine or diphenhydramine.
Vitiligo
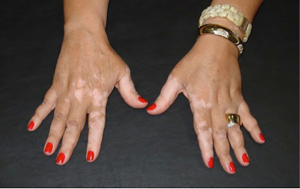
By activating the immune system, response directed to melanocytes may lead to an immune response against melanocytes, causing vitiligo (Figure 2). Although no symptoms or harm, beyond aesthetic, are expected, patients should be warned of the possibility. The occurrence of spontaneous vitiligo has been linked to better melanoma (9) outcome, but it is not known whether the ipilimumabe-induced vitiligo produces the same benefit.
Gastrointestinal adverse events
Diarrhea and colitis
The unspecific immune activation of the immune system might lead to intestinal mucosal infiltration by lymphocytes or neutrophils causing diarrhea (33). This may be accompanied by colitis symptoms. Diarrhea is reported in approximately 30% of the patients receiving ipilimumab at 3 mg/kg (25). Higher dosage trials have documented higher rates of diarrhea (2). The significance given to the issue of diarrhea is a consequence of reports of patients with bowel perforation and deaths related to colitis (25). Proper management has led to an overall improvement in severe diarrhea events and made catastrophic events quite rare.
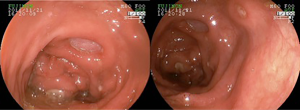
Diarrhea generally occurs after the 5th week of therapy and patients must be advised to report its onset, as prompt intervention and adequate monitoring are the keys to successful management. Grade 1 diarrhea (increase of up to 4 bowel movements over baseline) may be treated with anti-diarrheic medication, such as loperamide (2 mg PO, every 4-6 hours). Restriction of milk and dairy products is also recommended, as this allows for the investigation of other plausible causes, such as Clostridium difficile infection. Monitoring is essential in order to assure patients are properly hydrated and to detect eventual worsening to grade 2 (increase of 4 to 6 bowel movements over baseline), or the occurrence of abdominal pain or the presence of either blood or mucus in stool (colitis, grade 2). In such cases, oral steroids (prednisone 0.5 mg/kg/day or equivalent) should be added to the treatment plan. Although improvement should be seen within a few days of commencement of treatment, the steroid tapering period should be no less than 30 days. Early interruption carries a high risk of relapse. The need for performing a sigmoidoscopy or colonoscopy is not clear. The intrinsic mechanism of ipilimumab-induced diarrhea is inflammatory and perhaps, the only benefit of endoscopic documentation (Figure 3) would be to monitor outcome and justify the use of TNF blockers, as will be discussed later. In severe cases, with 7 or more bowel movements over the baseline, peritoneal signs, dehydration or any reason for hospitalization must be treated with IV steroids (methylprednisolone, 1-2 mg/kg/day IV) until improvement. The lack of a complete response or the inability for the complete taper of steroids should lead to the use of anti-TNF inhibitors (i.e., infliximab). These drugs are approved for colitis and have been useful in treating patients with steroid-refractory ipilimumab-induced diarrhea (34,35).
Endocrine adverse events
Just like any other tissue, endocrine glands are subject to autoimmunity and several known endocrinopathies have these mechanisms. By enhancing immune activity through negative checkpoint inhibition, as ipilimumab does, patients receiving it are at risk of endocrine immune-related adverse events. Knowledge of said risk led to the recommendation of thyroid function monitoring during ipilimumab therapy. Primary hypothyroidism may be detected while patient is still asymptomatic, through detection of an elevated TSH associated or not with free T4 lowering. Hormone replacement therapy with levothyroxine, adjusted according to TSH and free T4 levels, is usually enough to control this. There is no usual recommendation for steroid use, as the odds of recovering thyroid function are low. An elevation of free T4 has been observed, characterizing hyperthyroidism, albeit generally asymptomatic.
Low free T4 levels associated with normal or low TSH levels should raise suspicion for secondary hypothyroidism, or even more frequently, hypophysitis. Hypophysitis is in general insidious and detected by laboratory changes. Symptoms include fatigue, myalgia, orthostatic hypotension, loss of libido and erectile dysfunction. It may be promptly treated with the proper hormone replacement, such as levothyroxine, testosterone or estradiol, or corticosteroid (fludrocortisone or prednisone). High-dose steroids are not usually used to preserve or recover gland function, as they are in general exhausted by the time the disorder is detected. Although, in cases of hypohysis edema with headache and eventual sight changes, they are essential, and should be used with and initial IV steroid course (1-2 mg/kg/day of methylprednisolone or equivalent) and tapered over 30 days, at least, as symptoms improve.
Isolated adrenal dysfunction is possible, although less common than hypohysitis. The diagnosis may be given with the detection of elevated ACTH, which is much more sensitive than baseline cortisol level. In general, a cortrosyn stimulation test is not necessary.
In general, endocrine dysfunction will start after six weeks of therapy, but delayed cases, even after one year of therapy, may occur. As fatigue symptoms may also be due to systemic symptoms of a progressive illness, this should be ruled out as well (32).
Hepatic adverse events
Hepatic parenchyma inflammation may lead to cholestasis, liver enzyme elevation or even hepatitis. Such hepatic changes, have, overall, been reported in less than 5% of patients, receiving 3 mg/kg. The severity is, in general, measured by transaminases and bilirubin elevation, as described in CTC-NCI. Grade 1 liver toxicity [AST (aspartate aminotransferase) and ALT <3× ULN and Bilirubin <1.5× ULN] demands only close monitoring. Further elevation that characterizes grade 2 (AST and ALT >3× and <5× ULN and bilirubin >1.5× and <3.0× ULN) should lead to initiation of oral steroid therapy and the interruption of ipilimumab, until normalization. Grade 3 (AST and ALT >5× and <20× ULN and bilirubin >3× and <10× ULN) or higher demands treatment with IV steroids and permanent discontinuation of ipilimumab.
In general, the onset of hepatitis or liver enzyme changes occurs after six weeks of therapy. The treating physician must be vigilant to possible progressive illness causing hepatic changes. This possibility must be ruled out prior to investigating hepatic toxicity associated with ipilimumab (32).
Pancreatitis
The onset of abdominal pain and vomiting, without any evident cause, should prompt and investigation into pancreatitis. The diagnosis of acute pancreatitis is readily given through testing for amylase and lipase changes. The occurrence of exocrine or endocrine dysfunction is rare. Again, based on CTC grading, which uses amylase and lipase levels, oral steroids (at the same dosages) are recommended for grade 2 toxicity and IV steroids for grade 3 or 4 (32).
Neurologic adverse events
Autoimmune activity to neuronal tissue may be diagnosed anywhere from mild peripheral paresthesia up to a severe Guillain-Barré syndrome. This, in general, does not occur prior to the second infusion of ipilimumab. Fortunately these events are rare and make up less than 1% of patients complaints, but one should be watchful for these symptoms from their onset, as their worsening may follow a predictable pattern (25). Moderate symptoms should be treated with oral steroids, and severe symptoms, with IV steroids as well as assignment of neurologist to follow the patient and advice on therapy options (32).
General guidelines for ipilimumab toxicity management
Table 2 summarizes the recommendation for steroid use, in accordance with the CTC grading system. It is very important, though, that the treating physician rate the toxicity, for the purposes of therapy planning and outcome monitoring. Although the CTC system is not compulsory, it has been extensively used for ipilimumab toxicity management and proven itself practically and effective in daily practice.
Patients must be instructed to report their symptoms and be aware that delay in this may result in more severe toxicity and raise the chances of therapy discontinuation. The immunosuppressive action of steroids should not be reason for non-use during adverse events. Although, there is data suggesting that toxicity is directly related to its efficacy, this has not been widely accepted (36,37).
PD1 blockers
Early activity data has been reported on a new class of immunotherapeutic agents: PD1 blockers. The available data is, up to this moment, based solely on phase 1 and phase 2 trials. Consequently, there are no specific guidelines for toxicity management. Although, with an immune mechanism and in part, with tolerance inhibition, it is expected that toxicity will be less than but similar to the ipilimumab profile. Table 3 shows G3 and G4 adverse events reported during the use of nivolumab and lambrolizumab, in their reported trials (26-28).
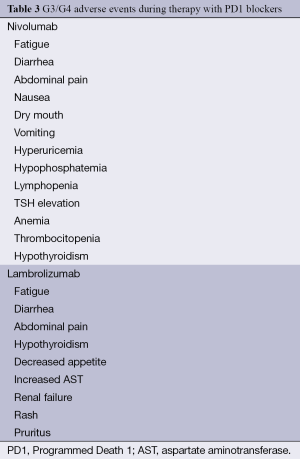
Full table
Diarrhea, endocrine adverse events, skin toxicity, myalgia, arthralgia, pyrexia and fatigue were reported and their treatment should include symptom management for mild events and the use of steroids for severe cases (26-28).
A unique toxicity observed has been lung toxicity. In the lambrolizumab phase 2 trial, cough was reported in 8% of participants, dyspnea in 4% and pneumonitis in 4%, but no grade 3 or 4 events were observed. In the nivolumab phase 1 trials, 4% of patients reported cough (none G3 or G4). The basis of its management should be similar to the general management guidelines for ipilimumab toxicity, but with special attention paid to respiratory infectious agents that my resemble pneumonitis (28).
PD-L1 blockers
Following development of PD1 blockers, come two PD-L1 blockers. Although the blockade of the interaction of PD1-PDL1 occurs with either antibody, PD-L1 blockade does not limit the interaction of PD1 with PD-L2, which preserves homeostasis and is a theoretical mechanism for PD1 blocker-induced pneumonitis. Very few G3 and G4 adverse events were reported and most common were hyperglycemia, AST and ALT elevation, back pain and adrenal failure (29).
With more patients treated and with more involved follow ups, this data is expected to become clearer and guidelines for toxicity management, more defined.
Combination of checkpoint inhibitors
Even without a definite role for PD1 blockers in the treatment of melanoma, and unknown ipilimumab effects on melanoma therapy with these new drugs, the strategy of combining anti-CTLA4 and anti-PD1, is already in development. An extended phase 1 trial reported provocative efficacy data (38).
The combination of both inhibitors is yet to be tested in terms of efficacy, in a randomized controlled trial, but clearly, its toxicity is higher than either drug alone, as seen in preliminary data. Depending on the cohort, 50% reported diarrhea, 65% reported rash and pruritus and more than 15% of patients presented grade 3 or 4 transaminases elevation (38). The benefits of this strategy are already being tested in a randomized controlled trial, comparing ipilimumab, nivolumab and their combination. The impact on efficacy and the feasibility of this regimen will be defined in the near future.
Conclusions
Immunotherapy has been pursued for many years, as an alternative to chemotherapy. Despite from many ineffective attempts, high-dose IL-2 was used with limited benefit and presenting toxicity profile which demanded more in depth professional training and improvement in facility logistics. More recently, T-cell infiltrating lymphocyte infusion followed by IL-2 has apparently improved the efficacy, but neither reduced toxicity nor complexity, which makes it even more restrictive (39).
With checkpoint inhibitors, outpatient immunotherapy may be used for the large majority of patients. The toxicity profile of these drugs is predictable and patients’ safety may be ensured with strict adherence to guidelines developed by the researchers.
For many years, metastatic melanoma therapy produced disappointing results, due to the lack of efficacy of chemotherapy or complexity and restrictiveness in terms of IL-2. In addition to the possibility of BRAF and MEK inhibitors, immune checkpoint inhibitors have become an important alternative. With clear guidelines to follow, toxicity should not be an issue in terms of preventing patient access.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer 2011;47:1476-83. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616-22. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- Bulkley GB, Cohen MH, Banks PM, et al. Long-term spontaneous regression of malignant melanoma with visceral metastases. Report of a case with immunologic profile. Cancer 1975;36:485-94. [PubMed]
- Lowes MA, Bishop GA, Crotty K, et al. T helper 1 cytokine mRNA is increased in spontaneously regressing primary melanomas. J Invest Dermatol 1997;108:914-9. [PubMed]
- Wenzel J, Bekisch B, Uerlich M, et al. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol 2005;124:37-48. [PubMed]
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol 2010;21:409-14. [PubMed]
- Vajdic CM, van Leeuwen MT, Webster AC, et al. Cutaneous melanoma is related to immune suppression in kidney transplant recipients. Cancer Epidemiol Biomarkers Prev 2009;18:2297-303. [PubMed]
- Frankenthaler A, Sullivan RJ, Wang W, et al. Impact of concomitant immunosuppression on the presentation and prognosis of patients with melanoma. Melanoma Res 2010;20:496-500. [PubMed]
- Dillon P, Thomas N, Sharpless N, et al. Regression of advanced melanoma upon withdrawal of immunosuppression: case series and literature review. Med Oncol 2010;27:1127-32. [PubMed]
- Morton DL, Mozzillo N, Thompson JF, et al. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol 2007;25:8508.
- Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006;17:563-70. [PubMed]
- Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364:2119-27. [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 1998;228:307-19. [PubMed]
- Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17. [PubMed]
- Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 2001;19:3477-82. [PubMed]
- Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer 1998;83:797-805. [PubMed]
- Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother 2001;24:287-93. [PubMed]
- Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565-94. [PubMed]
- Melero I, Hervas-Stubbs S, Glennie M, et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 2007;7:95-106. [PubMed]
- Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003;100:8372-7. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol 2013;31:abtr 9010.
- Freeman GJ. Structures of PD-1 with its ligands: sideways and dancing cheek to cheek. Proc Natl Acad Sci U S A 2008;105:10275-6. [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [PubMed]
- Baurain JF, Smylie M, Ascierto PA, et al. Outcomes of ipilimumab treatment-related adverse events in patients with metastatic melanoma (MM) who received systemic corticosteroids in a phase III trial. J Clin Oncol 2012;30:abtr 8539.
- Peggs KS, Quezada SA, Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol 2006;18:206-13. [PubMed]
- Johnston RL, Lutzky J, Chodhry A, et al. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci 2009;54:2538-40. [PubMed]
- O'Day S, Weber JS, Wolchok JD, et al. Effectiveness of treatment guidance on diarrhea and colitis across ipilimumab studies. J Clin Oncol 2011;29:abstr 8554.
- Lutzky J, Wolchok J, Hamid O, et al. Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol 2009;27:abstr 9034.
- Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003;100:8372-7. [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [PubMed]
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550-7. [PubMed]