
Surgical resection of early stage hepatocellular carcinoma: balancing tumor biology with the host liver
Introduction
Worldwide, hepatocellular carcinoma (HCC) is the sixth most common cancer and the second leading cause of cancer-related deaths (1,2). In 2017, there were 953,000 incident cases of liver cancer and 819,000 deaths globally (3). Most commonly occurring in the setting of chronic liver disease, HCC represents the leading cause of death in patients diagnosed with cirrhosis (4). Factors determining outcome following HCC diagnosis are complex and heterogenous in nature, dependent on not only tumor burden and biology, but on patient performance status and underlying liver function as well. These complexities require maintaining a tenuous balance between tumor-related and patient-related factors with decisions regarding treatment best made in the context of a multidisciplinary team approach (5).
Surgical resection if one of the mainstays of curative HCC treatment and has been associated with a median overall survival (OS) of >60 months with 5-year OS rates approaching 60% (6-8). Clinical practice guidelines have recommended the use of surgical resection in early stage HCC (9). However, due to heterogeneity of the patient population and underutilization of HCC screening, in the past only 10–37% of patients were eligible for surgical resection at the time of initial HCC diagnosis (10-12). With recent implementation of HCC screening programs resulting in earlier diagnosis, the number of patients that might be candidates for curative surgical resection has increased (13). In this article, we review the indications for curative HCC surgical resection as it pertains to underlying tumor- and patient-related factors.
Indications for surgical resection
Deciding to proceed with surgical resection requires careful consideration of tumor biology, including the number of tumor nodules, tumor size, and presence of vascular involvement, as well as underlying liver dysfunction and overall patient performance status. A multidisciplinary approach with input from surgical oncology, transplant hepatology, medical oncology, transplant surgery, radiation oncology, and both diagnostic and interventional radiology should be utilized. This approach has been associated with improved patient outcomes following HCC diagnosis (5). The existence of a multitude of HCC staging systems exemplifies the complexities present in evaluating a newly diagnosed HCC patient for curative surgical resection. Based on the Barcelona Clinic Liver Cancer (BCLC) system which is endorsed by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), early stage (0/A) disease is recommended for consideration of surgical resection. BCLC stage 0 is defined as patients with a single nodule ≤2 cm in size with preserved liver function, while BCLC stage A represents a solitary nodule or up to 3 nodules ≤3 cm in size with preserved liver function and good patient performance status (14,15). The ultimate goal of hepatic resection for HCC is achieving an appropriate oncological margin while maintaining a functional liver remnant.
Tumor-related factors
Tumor size
Within the initially described BCLC staging system, a solitary HCC tumor greater than 5 cm was considered intermediate stage (BCLC B) and locoregional therapy consisting of intraarterial therapies was recommend (16). Within the updated BCLC staging guidelines, tumor size in a solitary nodule is no longer a criterion. While tumor size by itself is not an independent predictor of HCC recurrence following surgery, increasing tumor size is associated with increasing incidence of microvascular invasion and distant metastases, factors portending increased recurrence and worse OS (17).
Despite the increased preponderance of risk factors associated with the likelihood of worse prognostic features, surgical resection of large HCC tumors (>5 cm in size) is associated with both similar surgical complication rates and long-term oncological outcomes compared to resections done of smaller tumors. With improvements in patient selection and perioperative management, morbidity rates range from 30–40% with post-operative mortality rates ranging from 3% to 5% in patients undergoing surgical resections for tumors >5 cm in size (18,19). Similarly, oncological outcomes following resection of solitary HCC tumors >5 cm in size support the role of resection versus non-curative therapies in this patient cohort, with 5-year OS rates ranging from 27% to 53% (18,20-24).
Intra-arterial therapy for large HCC tumors including trans-arterial chemoembolization (TACE) or trans-arterial radioembolization (TARE) is the non-curative treatment option recommended by the updated BCLC guidelines (17). A recent propensity score analysis comparing outcomes of patients with solitary HCC tumors >5 cm in size undergoing hepatic resection versus TACE demonstrate improved outcomes with resection. Five-year OS in the resection group was 41.3% vs. 18.5% in the TACE group (P=0.007) (25). Given the safety and efficacy of surgical resection combined with the lack of effective alternative curative treatment options, tumor size of a solitary HCC tumor should not preclude hepatic resection given patient-related factors including liver function, liver volume and performance status are considered acceptable.
Multifocality of tumors
Indications for hepatic resection in the face of multifocal HCC tumors is controversial and generally limited to patients who lack liver transplantation options, as multifocality is an independent risk factor associated with tumor recurrence following resection (26,27). Multiple published studies have demonstrated that surgical resection in patients with multifocal HCC, but still within the Milan criteria (≤3 tumors ≤3 cm in size), have 5-year OS rates ranging from 46% to 69% (19,22,28). Outcome measures in patients undergoing hepatic resection for multifocal HCC tumors outside of the Milan Criteria are not as favorable, with 5-year OS rates ranging from 12% to 24% (24,29). The major limitation of these surgical series are their retrospective nature with multifocality determined on pathology results from surgical resections rather than a priori based on pre-operative imaging studies. Given the lack of conclusive data demonstrating an oncological benefit in multifocal HCC tumors, liver resection should be reserved for circumstances where liver transplantation is not available and only non-curative therapies including TACE, TARE, or systemic therapy are an option.
Presence of macrovascular invasion (MVI)
The presence of MVI, either in the form of portal venous tumor thrombus (PVTT) or hepatic venous tumor thrombus (HVTT), is a poor prognostic factor following HCC diagnosis and is seen as a harbinger of systemic metastatic spread (30). Patients presenting with HCC tumors demonstrating MVI are classified as BCLC C stage (advanced) and are most commonly treated with systemic therapy with median OS times ranging from 6.5 to 13.6 months in the first-line setting (31-33).
Although 5-year OS rates after hepatic resection for HCC in the presence of MVI are dismal, ranging from 10% to 40% in retrospective series, careful patient selection based on extent of MVI and subsequent extent of hepatectomy have slightly improved outcome measures (21,22,34-38). Based on a classification scheme developed by the Liver Cancer Study Group of Japan (39), PVTT can be divided into five grades: Vp0, no tumor thrombus in the portal vein; Vp1, presence of a tumor thrombus in the second-order branches of the portal vein; Vp3, presence of a tumor thrombus in the first-order branches of the portal vein; and Vp4, presence of a tumor thrombus in the main trunk of the portal vein or a portal vein branch contralateral to the primarily involved lobe (or both). A large Japanese retrospective study using propensity score matching demonstrated that liver resection in the presence of PVTT had significantly prolonged median survival times compared to non-liver resection therapy (locoregional or systemic therapy (2.5 vs. 1.6 years, P<0.001). In the liver resection cohort, this survival benefit was only demonstrated in patients with Vp1–3 and not Vp4 PVTT. Ninety-day mortality following liver resection was also significantly higher in the Vp4 vs. Vp1–3 cohort (40). Given the lack of relative efficacious therapy compared to hepatic resection, patients presenting with MVI in the form of PVTT (Vp1–3) can be safely considered for liver resection following a multidisciplinary discussion in high volume centers. There is no current published literature supporting hepatic resection in patients presenting with Vp4 PVTT or HVVT.
Patient-related factors
Although perioperative mortality following hepatic resection for HCC has decreased over the past three decades (41,42), as indications for resection continue to expand, careful preoperative assessment of the degree of functional impairment of the liver is crucial to ensuring that oncological benefit outweigh the risks of post-hepatectomy liver failure (PHLF). PHLF, defined by the International Study Group of Liver Surgery as an increased prothrombin time and concomitant hyperbilirubinemia on or after post-operative day 5, is associated with perioperative mortality rates of more than 50% (43).
Pre-operative assessment: clinical and blood tests
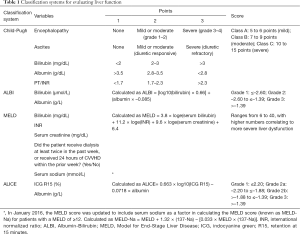
Multiple validated tools exist and are used to stratify patients based on pre-resection liver function to determine both feasibility of resection and the extent of resection tolerability. In the West, the three most utilized prognostic tools are the Child-Pugh (CP) classification, the Model for End-Stage Liver Disease (MELD) score, and the Albumin-Bilirubin (ALBI) score.
In 1964, Child and Turcotte developed a classification based on total bilirubin, serum albumin, and prothrombin time, as well as the presence and grade of hepatic encephalopathy and ascites, to predict short-term mortality following portacaval shunt surgery (44). Although widely used to stratify patients for hepatic resection for HCC, neither the original CP score nor the Pugh modification was designed specifically for this purpose (45). Nevertheless, the CP score remains widely utilized for surgical decision making. In the West, liver resection for HCC is limited to CP A patients, with CP C status universally accepted as a contraindication to resection.
A critique of the CP classification is that it relies on the use of subjective variables (severity of ascites and encephalopathy). In response to these concerns, Johnson et al. proposed a grading system based only on the serum ALBI as an alternative system (46). Similar to CP classification, the ALBI score was not intended to serve as a predictive biomarker for PHLF following liver resection for HCC. However, investigators have reported the ALBI grade more accurately predicts PHLF than CP score (47). Currently, the AASLD/EASL guidelines recommend that liver resection for HCC only be performed in patients with a serum total bilirubin of ≤1 mg/dL. In some Asian centers, a cutoff of ≤2 mg/dL is widely used (48). Given that liver resection for HCC is usually limited to patients with normal bilirubin levels, the usefulness of the ALBI grade in determining surgical candidacy may be based simply on the albumin level alone.
The MELD score, based on serum total bilirubin, international normalized ratio (INR), and creatinine, was first reported to predict early death after elective transjugular intrahepatic portosystemic shunt placement but is now used primarily for allocation of organs for liver transplantation (49). Multiple studies have suggested MELD score might be associated with PHLF following hepatectomy for HCC in cirrhotic patients. Teh et al. demonstrated that cirrhotic patients with cirrhosis and a MELD score of <9 have generally low morbidity of around 8% and negligible mortality from PHLF (50). Patients with a MELD score ≥9 have been shown to have greater risk of post-operative liver failure and peri-operative mortality (51,52). Originally designed as a continuous score in patients with poor underlying liver function, the use of MELD as a discrete variable with an a priori cut-off point to determine PHLF might limit its usefulness.
Pre-operative assessment: portal hypertension
The presence of portal hypertension, defined as a hepatic venous pressure gradient (HVPG) ≥10 mmHg, is defined by both AASLD and EASL guidelines as a contraindication to hepatic resection for HCC (14,17). These recommendations were based on a study by Bruix et al. in 1996 in 29 patients undergoing liver resection for HCC. In their study, elevated HVPG and thrombocytopenia was associated with decompensation following hepatic resection in Child A cirrhotic patients (53). Twenty-three of the 29 study patients underwent at least a major hepatectomy (sectionectomy or greater), calling into question the applicability of their findings in operations involving less liver parenchyma. In contrast, several other studies have demonstrated that resections for HCC can be safely performed in patients with portal hypertension with resultant low perioperative mortality rates and clear oncologic benefits (28,54,55).
As HVPG is an invasive procedure requiring institutional expertise, clinical parameters including splenomegaly >12 cm, clinical signs of collateralization such as a recanalized periumblical vein, and thrombocytopenia <100/nL are surrogate markers for clinically significant portal hypertension. The decision to perform hepatic resection in a patient with clinically significant portal hypertension must be weighed in the context of possible alternative curative therapies including liver transplantation and ablation.
Pre-operative assessment: functional imaging
Quantitative assessment by indocyanine green (ICG) clearance is the most often utilized pre-operative test in the East. ICG is a water-soluble fluorescent cyanine dye that is exclusively excreted by the liver into the bile without metabolism or enterohepatic circulation and its retention, measured as percentage serum retention at 15 minutes (R15), is an indirect assessment of functional hepatic blood flow (56). A surgical decision algorithm based on R15 was first reported by Makuuchi et al. in 1993 and is now widely used in many Eastern centers (57).
A recent multi-center Japanese study developed the ALICE grading system, a system utilizing serum albumin and ICG R15 evaluation (58). Like ALBI, ALICE has predictive power comparable with CP classification, but allows further stratification of CP A patients. These results were validated in a retrospective European cohort (59). However, the use of ICG clearance over other functional tests is still under debate and is seldom utilized in the US (Table 1).
Full table
Assessing future liver remnant (FLR) volume
Knowledge of standard liver volume proportion is necessary to determine the if extent of surgical resection will result in an appreciable decline in remnant functional liver volume. Generally, the right liver (segments V–VIII) contributes two-thirds of the total liver volume and the left liver (segments II–IV) contributes one-third of the liver volume (60). The optimal method for calculating the FLR is heavily debated and relies on formulas involving body surface area and radiographic imaging (61,62). Computed tomography with 3D reconstruction or volumetric MRI traces the hepatic segmental contours and multiples the surface area by slice thickness to calculate the total liver volume (63). To calculate the FLR, the following formula is used: (resected volume-tumor volume)/(total liver volume-tumor volume) (64,65). The minimum FLR considered to be safe following liver resection is based on the function and underlying disease status of the liver. Patients with HCC and cirrhosis necessitate a greater FLR than non-cirrhotic patients, with liver remnants of 40% needed. Small liver remnant volume is associated with higher rates of PHLF and other perioperative complications following hepatic resection (66,67).
Techniques for increasing FLR size
Portal vein embolization (PVE)
In patients with inadequate or borderline standardized FLR (sFLR), selective PVE can successfully increase remnant size. PVE is an image-guided procedure that induces hypertrophy of the FLR by redirecting portal blood flow away from the liver segments to be resected toward the non-tumor-bearing liver. PVE can decrease postoperative morbidity and increase the number of HCC patients eligible for curative intent resection when utilized appropriately. In patients without liver dysfunction, PVE is indicated when sFLR is <20%, which is often the case when extended right hepatectomy is required (68). In cirrhotic patients with HCC, PVE is indicated to when sFLR is <40%. Using ICG criteria, PVE can be considered for FLR ≤40% when ICG R15 is ≤10% and for FLR ≤50% when ICG R15 is between 10–20% (68).
PVE is absolutely contraindicated in cases with extensive ipsilateral tumor thrombus or clinically evident portal hypertension. When extensive ipsilateral tumor thrombus exists, PVE is contraindicated as most of the portal blood flow has already been diverted, and safe delivery of an embolic agent is difficult (69). As clinically evident portal hypertension is a contraindication to hepatectomy, PVE is not indicated in this setting.
The goal of PVE is complete portal occlusion of targeted liver segments. Embolizing the entire portal tree, including distal branches, is critical to prevent portoportal shunts, as well as to maximize hypertrophy of the FLR and prevent hypertrophy of segments planned for resection. Multiple embolic agents have been described for PVE including fibrin glue, n-butyl cyanoacrylate (NBCA), ethanol, ethiodized oil, and microparticles such as trisacryl gelatin or polyvinyl alcohol. No randomized trials have been conducted comparing the various agents, but retrospective studies show similar efficacy (69).
The degree of hypertrophy after PVE depends on the presence/absence and severity of underlying liver disease. Patients with normal livers can be expected to regenerate at 2 weeks post-procedure at rates of 12–21 vs. 9 cm3/day in patients with cirrhosis. In non-cirrhotic patients, sufficient hypertrophy usually occurs within 2–4 weeks, while sufficient hypertrophy in cirrhotic patients can take up to 4 weeks or more (70). Additionally, 10–20% of cirrhotic patients do not achieve adequate contralateral hypertrophy after undergoing PVE due to diminished liver regenerative capacity (71).
Combination arterial and portal embolization
Interest has developed in applying TACE sequentially with PVE before performing resection for HCC. In theory, this combination of TACE + PVE offers several benefits. Firstly, the liver necrosis that is typically seen after TACE may lead to increased regeneration rates. Secondly, hepatic arterial flow within the embolized segment increases after PVE, which can lead to increased tumor growth as HCC tumors preferentially derive blood supply from the hepatic artery. TACE therefore might provide local control of tumors in the interval between PVE and hepatectomy. Lastly, the formation of arterioportal shunts has been associated with HCC. These shunts can diminish the efficacy of PVE, which is usually performed upstream of these shunts. TACE targets these shunts and may render PVE more effective.
A French study compared 36 HCC patients with cirrhosis treated between 1998–2004, half (n=18) underwent TACE + PVE while the remaining half underwent PVE alone prior to right hepatectomy. The TACE + PVE treated patients experienced significantly higher mean increases in FLR volume compared to the PVE alone group. Operative blood loss, liver failure, and mortality were comparable between groups. The TACE + PVE group was significantly more likely to have complete tumor necrosis and had higher 5-year disease-free survival (37% vs. 19%) (72). A more recent Korean study published in 2011 of 135 patients undergoing TACE+PVE or PVE alone prior to right hepatectomy found similar results, with patients receiving combination therapy experiencing higher mean increase in sFLR and improved overall and disease-free survival compared with PVE alone (73). While there is a growing experience with TACE + PVE, including a randomized controlled trial currently ongoing in China, this procedure is still considered experimental and is not widely utilized in the US.
Associated liver partition with portal vein ligation for staged hepatectomy (ALPPS)
ALPPS is a novel, alternate method to PVE which has been utilized for FLR hypertrophy in patients with extensive colorectal liver metastasis (CRLM) undergoing extended right hepatectomy. In this technique, portal vein ligation and in situ splitting of the liver along the falciform ligament is performed to induce rapid hypertrophy of the left lateral section. After a short median interval of 9 days, patients undergo completion hepatectomy. Recently, ALPPS has been attempted in HCC patients. A retrospective review of 35 patients in the ALPPS registry from 2010 to 2015 with HCC found rapid and extensive FLR hypertrophy; however, this was significantly lower than for CRLM patients (47% vs. 76%) (74). The degree of hypertrophy was negatively correlated with the severity of liver fibrosis. The 90-day perioperative mortality was high at 31% (compared to 7% for CRLM) and the long-term oncologic outcomes are unknown. This procedure is still experimental and PVE remains the preferred technique to induce FLR hypertrophy prior to hepatectomy in HCC patients. There may be a limited role for ALPPS in younger, healthy non-cirrhotic patients who are not candidates for liver transplant and for whom the operative risks are deemed acceptable.
Stem cell infusion
A growing interest has developed in infusing stem cells into the FLR during PVE to improve hepatic regeneration. Small phase II trials have shown promising initial results, with stem cell-treated patients experiencing significant increases in hepatic growth volume compared with PVE-only patients, as well as improvements in underlying liver function (75,76). Further studies are needed to determine whether stem cell infusion positively impacts surgical outcomes and survival.
Conclusions
Most HCC patients are not candidates for resection at diagnosis, often due to underlying liver dysfunction, inadequate FLR, or tumor characteristics. The perioperative risks of hepatic resection in cirrhotic patients with HCC must be balanced against the oncological benefits. Tumor characteristics including large tumor size, vascular invasion, and multifocality are not absolute contraindications to hepatic resection in well-compensated patients with a lack of alternative curative options. Underlying liver dysfunction must be careful assessed, accounting for the degree of dysfunction, extent of planned liver resection, and alternative curative therapies including liver transplantation and ablation. Adjuncts such as PVE or ALPPS may allow an increased role for hepatic resection in HCC patients with small FLR.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mehmet Akce and Shishir K. Maithel) for the series “Hepatocellular Carcinoma” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-130). The series “Hepatocellular Carcinoma” was commissioned by the editorial office without any funding or sponsorship. ACY serves as an unpaid editorial board member of Chinese Clinical Oncology from May 2019 to April 2021. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Ioannou GN, Weiss NS, Kowdley KV. Relationship between transferrin-iron saturation, alcohol consumption, and the incidence of cirrhosis and liver cancer. Clin Gastroenterol Hepatol 2007;5:624-9. [Crossref] [PubMed]
- Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014;21:1287-95. [Crossref] [PubMed]
- Pang TC, Lam VW. Surgical management of hepatocellular carcinoma. World J Hepatol 2015;7:245-52. [Crossref] [PubMed]
- Maluccio MA, Zang Y, Pi W, et al. Survival in patients with hepatocellular carcinoma (HCC): A report of 1444 patients treated within a multidisciplinary program. J Clin Oncol 2017;35:e15652. [Crossref]
- Liu W, Wang K, Bao Q, et al. Hepatic resection provided long-term survival for patients with intermediate and advanced-stage resectable hepatocellular carcinoma. World J Surg Oncol 2016;14:62. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999;229:790-9; discussion 799-800. [Crossref] [PubMed]
- Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-30. [Crossref] [PubMed]
- Colella G, Bottelli R, De Carlis L, et al. Hepatocellular carcinoma: comparison between liver transplantation, resective surgery, ethanol injection, and chemoembolization. Transpl Int 1998;11 Suppl 1:S193-6. [Crossref] [PubMed]
- Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol 2020;72:250-61. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Pandey D, Lee KH, Wai CT, et al. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol 2007;14:2817-23. [Crossref] [PubMed]
- Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg 2002;194:592-602. [Crossref] [PubMed]
- Cho YB, Lee KU, Lee HW, et al. Outcomes of hepatic resection for a single large hepatocellular carcinoma. World J Surg 2007;31:795-801. [Crossref] [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg 2005;140:450-7; discussion 457-8. [Crossref] [PubMed]
- Ruzzenente A, Capra F, Pachera S, et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg 2009;13:1313-20. [Crossref] [PubMed]
- Yamashita Y, Taketomi A, Shirabe K, et al. Outcomes of hepatic resection for huge hepatocellular carcinoma (>/= 10 cm in diameter). J Surg Oncol 2011;104:292-8. [Crossref] [PubMed]
- Zhong JH, Xiang BD, Gong WF, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One 2013;8:e68193. [Crossref] [PubMed]
- Zhu SL, Ke Y, Peng YC, et al. Comparison of long-term survival of patients with solitary large hepatocellular carcinoma of BCLC stage A after liver resection or transarterial chemoembolization: a propensity score analysis. PLoS One 2014;9:e115834. [Crossref] [PubMed]
- Chang WT, Kao WY, Chau GY, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery 2012;152:809-20. [Crossref] [PubMed]
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007;141:330-9. [Crossref] [PubMed]
- Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908-16. [Crossref] [PubMed]
- Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013;257:929-37. [Crossref] [PubMed]
- Mokdad AA, Singal AG, Marrero JA, et al. Vascular invasion and metastasis is predictive of outcome in barcelona clinic liver cancer stage C hepatocellular carcinoma. J Natl Compr Canc Netw 2017;15:197-204. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Ban D, Shimada K, Yamamoto Y, et al. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg 2009;13:1921-8. [Crossref] [PubMed]
- Le Treut YP, Hardwigsen J, Ananian P, et al. Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature. A European case-control series. J Gastrointest Surg 2006;10:855-62. [Crossref] [PubMed]
- Inoue Y, Hasegawa K, Ishizawa T, et al. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery 2009;145:9-19. [Crossref] [PubMed]
- Wang Y, Yuan L, Ge RL, et al. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol 2013;20:914-22. [Crossref] [PubMed]
- Chok KS, Cheung TT, Chan SC, et al. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg 2014;38:490-6. [Crossref] [PubMed]
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339-64. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. [Crossref] [PubMed]
- Dokmak S, Fteriche FS, Borscheid R, et al. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford) 2013;15:908-15. [Crossref] [PubMed]
- Kenjo A, Miyata H, Gotoh M, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg 2014;218:412-22. [Crossref] [PubMed]
- Balzan S, Belghiti J, Farges O, et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824-8, discussion 828-9. [Crossref] [PubMed]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1-85. [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725-34. [Crossref] [PubMed]
- Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-206; discussion 1206. [Crossref] [PubMed]
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864-71. [PubMed]
- Teh SH, Christein J, Donohue J, et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg 2005;9:1207-15; discussion 1215. [PubMed]
- Cucchetti A, Ercolani G, Vivarelli M, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl 2006;12:966-71. [Crossref] [PubMed]
- Delis SG, Bakoyiannis A, Dervenis C, et al. Perioperative risk assessment for hepatocellular carcinoma by using the MELD score. J Gastrointest Surg 2009;13:2268-75. [Crossref] [PubMed]
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018-22. [Crossref] [PubMed]
- Cucchetti A, Ercolani G, Vivarelli M, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg 2009;250:922-8. [Crossref] [PubMed]
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40. [Crossref] [PubMed]
- Teranaka M, Schenk WG. A Comparison of the indocyanine green and electromagnetic techniques in normal and abnormal flow states in the dog. Ann Surg 1977;185:58-63. [Crossref] [PubMed]
- Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol 1993;9:298-304. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Amikura K, et al. Assessment of preoperative liver function in patients with hepatocellular carcinoma - the Albumin-Indocyanine Green Evaluation (ALICE) Grade. PLoS One 2016;11:e0159530. [Crossref] [PubMed]
- Russolillo N, Forchino F, Conci S, et al. Validation of the albumin-indocyanine green evaluation model in patients with resected hepatocellular carcinoma and comparison with the albumin-bilirubin score. J Hepatobiliary Pancreat Sci 2019;26:51-7. [PubMed]
- Abdalla EK, Denys A, Chevalier P, et al. Total and segmental liver volume variations: implications for liver surgery. Surgery 2004;135:404-10. [Crossref] [PubMed]
- Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol 2008;25:104-9. [Crossref] [PubMed]
- Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995;21:1317-21. [Crossref] [PubMed]
- Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 2005;16:215-25. [Crossref] [PubMed]
- Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176-81. [PubMed]
- Ogasawara K, Une Y, Nakajima Y, et al. The significance of measuring liver volume using computed tomographic images before and after hepatectomy. Surg Today 1995;25:43-8. [Crossref] [PubMed]
- Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210-5. [Crossref] [PubMed]
- Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512-9. [Crossref] [PubMed]
- Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540-8. [PubMed]
- Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol 2005;16:779-90. [Crossref] [PubMed]
- Madoff DC, Hicks ME, Vauthey JN, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics 2002;22:1063-76. [Crossref] [PubMed]
- Kianmanesh R, Regimbeau JM, Belghiti J. Selective approach to major hepatic resection for hepatocellular carcinoma in chronic liver disease. Surg Oncol Clin N Am 2003;12:51-63. [Crossref] [PubMed]
- Ogata S, Belghiti J, Farges O, et al. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg 2006;93:1091-8. [Crossref] [PubMed]
- Yoo H, Kim JH, Ko GY, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol 2011;18:1251-7. [Crossref] [PubMed]
- D'Haese JG, Neumann J, Weniger M, et al. Should ALPPS be Used for Liver Resection in Intermediate-Stage HCC? Ann Surg Oncol 2016;23:1335-43. [Crossref] [PubMed]
- am Esch JS, Schmelzle M, Fürst G, et al. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances functional hepatic reserves after extended right hepatectomy: a retrospective single-center study. Ann Surg 2012;255:79-85.
- Han HS, Ahn KS, Cho JY, et al. Autologous stem cell transplantation for expansion of remnant liver volume with extensive hepatectomy. Hepatogastroenterology 2014;61:156-61. [PubMed]


