
Prospect of immunotherapy in neoadjuvant/adjuvant treatment for early breast cancer
Introduction
Breast cancer is the most common cancer diagnosed in women and has become one of the main global public health problems. In the recent decade, breast cancer is treated using a multidisciplinary approach that includes surgery, chemotherapy, endocrine therapy, molecular targeted therapy and radiotherapy, which have been beneficial to reduce breast cancer mortality (1). However, numerous unresolved issues surrounding breast cancer still remain. Recently, development of immunotherapies that successfully inhibited immune-check point proteins resulted in a durable effect in some cancers such as malignant melanoma, non-small cell lung cancer, and urothelial cancer. In the other side, breast cancer is generally thought to be a low immunogenic tumor because of the low mutation burden compared with that in the so-called hot immunogenic tumors (2). Nevertheless, immunotherapy is expected to be used as a new treatment modality especially for triple negative breast cancer (TNBC), which has a higher immunogenicity among subtypes of breast cancer. Here, we review the articles and discuss the current position of immunotherapy and future prospects of immunotherapy as neoadjuvant/adjuvant therapy in breast cancer based on our conclusions from the findings in the current literature.
Recent advances in immunotherapy
Revolutionized changes in cancer treatment began with the discovery of immune checkpoint proteins, namely programmed death-1 (PD-1) and cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) as targets (3-5).
Regarding breast cancer, a paradigm shift has occurred in TNBC. The subtype account for around 15% of all breast cancer subtypes, and has been reported to be associated with aggressive characteristics like high grade and poor prognosis compared with characteristics of other breast cancer subtypes (6,7). Chemotherapy still remains the mainstream treatment for TNBC because it biologically lacks estrogen receptors (ER), progesterone receptor expression (PgR) and the human epidermal growth factor receptor 2 (HER2) amplification which are novel targets of breast cancer. In contrast, its aggressive characteristics and higher grade have been revealed to correlate with its immunogenicity; for instance, the number of tumor-infiltrating lymphocytes (TILs) of this type are higher than that of the other subtypes (8). The incidence of lymphocyte predominant breast cancer (LPBC), defined as >50 or 60% lymphocyte infiltration observed in the stroma, is approximately 20% in TNBC, 16% in HER2 expression type and 6% in non-HER2 luminal subtype (8). TILs were also reported to have a positive effect on the clinical outcomes (9). Thus, the immunological feature is one of the reasons why the new targeted approach has been carried out primarily for TNBC.
From the results of the previous studies, triple negative cancer cells are more likely to express the proteins of the programmed death ligand-1 (PD-L1) than that of other breast cancer subtypes (10,11). In breast cancer, focus is placed on the blockade for PD-1/PD-L1 interaction, and not CTLA-4. As seen in other types of cancers, immune checkpoint inhibitors were examined as a monotherapy. Pembrolizumab monotherapy which targets PD-1 in advanced PD-L1+ TNBC was evaluated in a phase Ib study, KEYNOTE-012 as a proof-of-concept study (12). Among 27 PD-L1+ patients who could be evaluated for response to the therapy, the objective response rate (ORR) was 18.5% (95% CI, 6.3–38.1%). In the study, it was 17.9 weeks (range from 7.3 to 32.4 weeks) in terms of the median time to response. The KEYNOTE-086 phase II trial tested pembrolizumab monotherapy in two different cohorts. Cohort A included heavily treated TNBC regardless of PD-L1 status and cohort B included PD-L1+ TNBC treated as first-line therapy (13,14). As looking into cohort A, ORR was 5.3% (95% CI, 2.7–9.9%) in the total cohort and 5.7% (95% CI, 2.4–12.2%) in the PD-L1+ populations. The median progression free survival (PFS) was 2.0 months (95% CI, 1.9–2.0 months), and the 6-month PFS rate was 14.9%. The overall median survival (OS) was 9.0 months (95% CI, 7.6–11.2 months), and the 6-month OS rate was 69.1% (14). In cohort B, 4 patients achieved a complete response and 14 patients achieved a partial response. The ORR was 21.4% (95% CI, 13.9–31.4%). When the data was cut-off, 8 patients (44.4%) were keeping responses among all 18 patients. The response duration was 10.4 months as a median (range, 4.2 to 19.2 months). The median PFS and OS was 2.1 months (95% CI, 2.0–2.2 months) and 18.0 months (95% CI, 12.9–23.0 months), respectively (13). The KEYNOTE-119 trial that compares pembrolizumab with chemotherapy is ongoing (NCT02555657).
Avelumab, which blocks PD-L1 was evaluated in 168 patient cohort that included all breast cancer subtypes in the JAVELIN trial (15). ORR was relatively low and reached 3.0% and 5.2% in all patients and 58 patients with TNBC, respectively. However, PD-L1+ patients could tend to response with higher ORR rate (16.7% in all patient cohort, 22.2% in TNBC patient cohort) compared with PD-L1- patients (1.6% in all patient cohort, 2.6% in TNBC patient cohort).
Another PD-L1 inhibitor, atezolizumab was clinically evaluated as a monotherapy for several types of cancers including 115 TNBC patients in phase Ia study (16). The OS was 17.6 months as a median in patients who were treated as a first line. PD-L1 was defined as positive if its expression was observed at 1% or more tumor-infiltrating immune cells (ICs). PD-L1+ patients had higher ORR (12%) and longer OS (10.1 months), compared with PD-L1- patients (0% and 6.0 months, respectively). The previous monotherapy data of PD-1/PD-L1 blockade consistently led to a durable response in 10–20% of TNBC patients. However, expected development in breast cancer has not been performed like in other types of cancers. Regarding the degree of immunogenicity, a successful approach for breast cancer is thought to involve a combination with chemotherapy that can lead to an increase in neoantigens.
Atezolizumab has been evaluated with weekly nab-paclitaxel in a phase Ib study for TNBC patients regardless of their PD-L1 status (17). ORR was 39.4% and the disease control rate was 51.5%. The response duration was 9.1 months as a median (95% CI, 2.0–20.9 months). The median PFS was 5.5 months and the median OS was 14.7 months. A phase III IMpassion 130 trial was conducted and the results on the addition of atezolizumab to nab-paclitaxel for advanced TNBC were reported (18). The median PFS was 7.2 months in patients receiving atezolizumab plus nab-paclitaxel, and was statistically higher than that of patients with nab-paclitaxel alone with 5.5 months (HR: 0.80; 95% CI, 0.69–0.92; P=0.002). In PD-L1+ patient cohort, the median PFS was 7.5 months in atezolizumab experimental arm and 5.0 months in placebo control arm (HR: 0.62; 95% CI, 0.49–0.78; P<0.001). The median OS was 25.0 months in atezolizumab experimental arm compared with 15.5 months in placebo control arm (HR: 0.62; 95% CI, 0.45–0.86). Based on the results, atezolizumab has been approved for clinical use globally. Pembrolizumab has been evaluated with eribulin for 95 advanced TNBC patients regardless of their PD-L1 status (19). In 17 patients treated as first line therapy, ORR was 41.2%. In those treated with second- or third-line therapies, ORR was 27.3%. The clinical benefit rate (CBR) was 41%. There was no correlation between PD-L1 status and response. ORR and CBR were 29.4% and 35.3%, respectively, for the PD-L1+ patients and 33.3% and 44.4%, respectively, for the PD-L1- patients. The KEYNOTE-355 phase III trial is proceeding to examine the effect of first line pembrolizumab combined with chemotherapy compared to chemotherapy alone for advanced TNBC (NCT02819518).
Immunotherapy in neoadjuvant/adjuvant treatment for early breast cancer
When investigating new drugs, a neoadjuvant setting is preferred because drug efficacy could be evaluated earlier using pathological complete response (pCR) as an alternative endpoint for survival (20). The strategy is based on the observed results that pCR after neoadjuvant therapy significantly correlates with PFS and OS (21,22). As a part of the I-SPY2 trial, neoadjuvant treatment including pembrolizumab was examined for HER2 negative breast cancer (Table 1) (23). Sixty-nine from a total of 249 patients enrolled received pembrolizumab combined with paclitaxel, and 180 received paclitaxel alone followed by neoadjuvant doxorubicin and cyclophosphamide (AC) in all the patients. The results showed that adding pembrolizumab increased pCR rate by approximately threefold in patients with TNBC from 20% to 60% and in luminal-like patients from 13% to 34%. Pembrolizumab has graduated from the I-SPY 2 trial after Bayesian predictive probability of success was confirmatory estimated based on the results of phase I and II trial. Pembrolizumab was also tested as a neoadjuvant therapy for locally advanced TNBC in the phase Ib KEYNOTE-173 study (24). Ten patients received a single-dose pembrolizumab followed by pembrolizumab + nab-paclitaxel followed by pembrolizumab + AC. Another 10 patients received an addition of carboplatin. Findings show that yT0/Tis ypN0 pCR rate was 60% (90% CI, 30–85%) in the pembrolizumab group and 90% (90% CI, 61–100%) in the pembrolizumab + carboplatin group. Preliminary results from the phase III NeoTRIPaPDL1 Michelangelo study (NCT002620280) were reported (25). In this open-label study, 280 patients with early high-risk and locally advanced or inflammatory TNBC were randomized into the combination of atezolizumab with chemotherapy group or the chemotherapy-alone group in the neoadjuvant setting. Findings showed that the pCR rate with atezolizumab was 43.5% (95% CI, 35.1–52.2%) compared with 40.8% (95% CI, 32.7–49.4%) in the control group, which led to an odds ratio of 1.11 (95% CI, 0.69–1.79; P=0.066). Another PD-L1 inhibitor, durvalumab, was evaluated in two clinical trials but the efficacy was not reported (26,27).
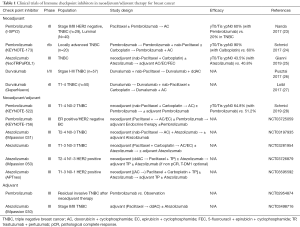
Full table
Whether adjuvant checkpoint inhibitors could improve survival is an unresolved clinical question. In the KEYNOTE 522 trial, the checkpoint inhibitor, pembrolizumab was consistently administered in both neoadjuvant and adjuvant therapies (Table 1) (28). The addition of pembrolizumab to chemotherapy boosted pCR rates compared with chemotherapy alone. An interim analysis showed that at a median follow-up of 15.5 months, pCR rates were 64.8% for pembrolizumab plus chemotherapy versus 51.2% for chemotherapy alone (P=0.00055). A more important issue in this trial was to evaluate the efficacy of adding pembrolizumab in an adjuvant setting. Another pembrolizumab neoadjuvant/adjuvant trial (KEYNOTE-756) recruited not only TNBC but also ER positive HER2 negative breast cancer. In the experimental arm, patients received pembrolizumab + chemotherapy in the neoadjuvant setting, followed by definite surgery and adjuvant pembrolizumab + endocrine therapy. Even though checkpoint inhibitors were discussed primarily for TNBC, KEYNOTE-756 may demonstrate the brand-new findings.
IMpassion 031 trial is a global phase III placebo-controlled study to evaluate the efficacy and safety of neoadjuvant treatment with atezolizumab combined with nab-paclitaxel followed by AC for operable TNBC. In the experimental arm, a total of approximately 12 months of atezolizumab therapy is required after surgery. Neoadjuvant/adjuvant atezolizumab for TNBC is tested in another trial (NCT03281954). It differs with Impassion 031 in that carboplatin is administered in both arms.
For HER2-positive breast cancer, IMpassion050 will evaluate the efficacy and safety of atezolizumab compared with placebo when it is combined with dose-dense AC (ddAC), followed by paclitaxel + trastuzumab + pertuzumab as a neoadjuvant treatment. After definitive surgery, participants will continue to receive the following study treatments to complete up to 1 year of HER2-target therapy inclusive of therapy given both in the neoadjuvant and adjuvant setting (atezolizumab, trastuzumab and pertuzumab). For participants who do not achieve pCR, blinded atezolizumab + trastuzumab, emtansine after surgery is optional. Different types of chemotherapy including omission of anthracycline from IMpassion050 are tested in APT neo trial.
To the best of our knowledge, there are two trials in which checkpoint inhibitors are evaluated in only an adjuvant setting. Pembrolizumab trial has recruited TNBC patients who have invasive residual tumor after neoadjuvant chemotherapy. Patients in the experimental part will receive pembrolizumab for a year in the absence of disease progression or unacceptable toxicity (NCT02954874). IMpassion 030 trial will evaluate the efficacy, safety, and pharmacokinetics of adjuvant atezolizumab when it is combined with paclitaxel, followed by atezolizumab, dose-dense AC or epirubicin and cyclophosphamide (EC), compared with paclitaxel followed by dose-dense AC or EC alone in patients with Stage II-III TNBC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yutaka Yamamoto and Takayuki Ueno) for the series “Neoadjuvant/Adjuvant Treatment for Early Breast Cancer” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2020.04.01). This series “Neoadjuvant/Adjuvant Treatment for Early Breast Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012;344:e2718. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw 2017;15:433-51. [Crossref] [PubMed]
- Iwamoto T, Fukui N, Kinoshita T, et al. Comprehensive prognostic report of the Japanese Breast Cancer Society registry in 2006. Breast Cancer 2016;23:62-72. [Crossref] [PubMed]
- Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol 2016;2:1354-60. [Crossref] [PubMed]
- Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 2016;13:228-41. [Crossref] [PubMed]
- Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 2016;47:52-63. [Crossref] [PubMed]
- Li X, Li M, Lian Z, et al. Prognostic Role of Programmed Death Ligand-1 Expression in Breast Cancer: A Systematic Review and Meta-Analysis. Target Oncol 2016;11:753-61. [Crossref] [PubMed]
- Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460-7. [Crossref] [PubMed]
- Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:405-11. [Crossref] [PubMed]
- Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:397-404. [Crossref] [PubMed]
- Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018;167:671-86. [Crossref] [PubMed]
- Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol 2019;5:74-82. [Crossref] [PubMed]
- Adams S, Diamond JR, Hamilton E, et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol 2019;5:334-42. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Tolaney S, Savulsky C, Aktan G, et al. Phase 1b/2 study to evaluated eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Eur J Cancer 2017;72:S16. [Crossref]
- DeMichele A, Yee D, Berry DA, et al. The Neoadjuvant Model Is Still the Future for Drug Development in Breast Cancer. Clin Cancer Res 2015;21:2911-5. [Crossref] [PubMed]
- Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014;32:3883-91. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY2. J Clin Oncol 2017;35:506. [Crossref]
- Schmid P, Park YH, Muñoz-Couselo E, et al. Pembrolizumab 1 chemotherapy as neoadjuvant treatment for triple negative breast cancer (TNBC): preliminary results from KEYNOTE-173. J Clin Oncol 2017;35:556. [Crossref]
- Gianni L, Huang CS, Egle D, et al. Pathological complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomised study. Cancer Res 2020. [Crossref]
- Pusztai L, Silber A, Wysong Hofstatter E, et al. Safety of MEDI4736 (anti-PD-L1 antibody) administered concomitant with weekly nab-paclitaxel and dose dense doxorubicin/cyclophosphamide (ddAC) as neoadjuvant chemotherapy for stage I-III triple negative breast cancer (TNBC): a phase I/II trial. J Clin Oncol 2017;35:572. [Crossref]
- Loibl S, Untch M, Burchardi N, et al. A randomized phase II neoadjuvant study (GeparNuevo) to investigate the addition of durvalumab, a PD-L1 antibody, to a taxane-anthracycline containing chemotherapy in triple negative breast cancer (TNBC). J Clin Oncol 2017;35:3062. [Crossref]
- Schmid P, Cortés J, Dent R, et al. KEYNOTE-522: Phase 3 study of pembrolizumab (pembro)+ chemotherapy (chemo) vs placebo (pbo) + chemo as neoadjuvant treatment, followed by pembro vs pbo as adjuvant treatment for early triple-negative breast cancer (TNBC). Ann Oncol 2019;30:v851-v934. [Crossref]


