
Is it time for a specific score for venous thromboembolism risk assessment for non-solid tumors?
Cancer patients have a high incidence of venous thromboembolism (VTE), VTE recurrence, bleeding and reduced quality of life (1). Thrombosis is the second most common cause of death in cancer patients (2). VTE prophylaxis is not routinely recommended for all outpatients with cancer undergoing chemotherapy.
Lymphoma patients are at a higher risk of VTE, compared to different cancer types, particularly with solid tumors (3). The evaluation of thrombosis risk to guide VTE prophylaxis strategies is recommended for patients with newly diagnosed neoplastic diseases. Khorana et al. developed a prospectively validated risk assessment tool for predicting chemotherapy-associated VTE. However, in this cohort study, only a minority of patients (12.6%) had lymphomas (4).
Most of the available risk assessment tools are not specific for hematological tumors. Recently the Khorana score was used as an inclusion criterion (≥2) for two prospective, randomized controlled trials in VTE prevention in out-of-patient cancer patients receiving chemotherapy. The Cassini trial (5) compared rivaroxaban 10 mg once-daily versus placebo and the AVERT trial (6) compared apixaban 2.5 mg twice-daily to placebo as well. Both studies showed reductions around 60% of thrombotic events, with a slight increase of bleeding on the apixaban study. The Cassini trial included around 7% of lymphoma patients where the AVERT trial included only solid tumors. Subgroup analysis of these 2 trials are not possible comparing solid versus hematological tumors, and these are currently the largest studies in this setting. Table 1 describes the currently available VTE risk tools and its features.
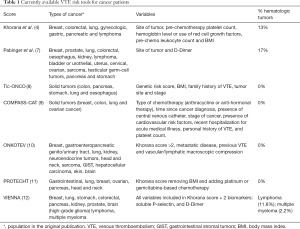
Full table
VTE prophylaxis and treatment in patients with hematologic malignancies is challenging, particularly because severe thrombocytopenia can complicate the course of treatments or may exist since early diagnosis, increasing the chances of bleeding complications (13). The identification of patients with lymphomas who are at high risk of VTE are warranted to identify who might benefit from prophylactic anticoagulation strategies and those who will benefit from avoiding anticoagulation, preventing bleeding (3). We believe that a specific score to evaluate VTE risk assessment in hematological cancers should be developed and prospectively validated. Such score would include on top of the Khorana risk score, previous history of VTE, disease stage, potential pro-thrombotic associated treatments and biomarkers.
Recently, Borchmann et al. (14) evaluated thrombotic events in more than 5,000 patients from the GHSG HD13–15 trials in patients with Hodgkin lymphoma (HL). They have found an overall incidence of thrombosis of 3.3%. The authors reported an incidence of less than 1% events in early-favorable, 1.3% in early-unfavorable and 7.3% in advanced patients, the latter incidence being significantly higher (P<0.001). The majority of the events were deep-venous thrombosis (DVT), and 7.8% arterial thrombosis. Interestingly, the majority of events occurred in the upper extremity (46.3%), mainly catheter associated thrombosis. In 24.6% of the patients, VTE occurred in the lower extremities. In advanced HL, the incidence of VTE events was increased upon more intensive treatment with BEACOPP-14. Applying the Khorana score, only age and smoking correlated with the development of VTE.
The authors concluded that the incidence of VTE in advanced stage HL is comparable to other high-risk cancer patients, with a higher incidence in patients that received dose-dense regimens. This study adds important data regarding incidence of thrombotic events and risk factors for VTE in patients with lymphoma. Previous studies in patients with hematologic malignancies have shown similar results (15). Patients with aggressive non-Hodgkin lymphoma (NHL) and advanced stage disease (III/IV), are also at a high risk to develop VTE. A recent retrospective single center study carried out by Hohaus and collaborators evaluated the occurrence of VTE and identified proposed lymphoma-specific risk factors. It was identified 3 specifics clinical risk factors: central nervous system (CNS) lesions, tumor bulk greater than 10 cm and reduced performance ECOG status. This group proposed that VTE risk factors in patients with lymphoma are not the same VTE risk factors described for patients with solid tumors (3).
As for study design, Borchmann et al. excluded patients over 60 years old and follow-up was limited to 1 year. We believe that future prospective trials involving patients with individual types of cancer, elderly and with longer follow-up periods would provide the definitive evidence about the clinical benefit associated with prophylaxis in out-of-hospital patients, particularly in patients suffering from non-solid tumors.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Donnellan E, Khorana AA. Cancer and Venous Thromboembolic Disease: A Review. Oncologist 2017;22:199-207. [Crossref] [PubMed]
- Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-50. [Crossref] [PubMed]
- Hohaus S, Tisi MC, Bartolomei F, et al. Risk factors for venous thromboembolism in patients with lymphoma requiring hospitalization. Blood Cancer J 2018;8:54. [Crossref] [PubMed]
- Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902-7. [Crossref] [PubMed]
- Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med 2019;380:720-8. [Crossref] [PubMed]
- Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med 2019;380:711-9. [Crossref] [PubMed]
- Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 2018;5:e289-98. [Crossref] [PubMed]
- Munoz Martin AJ, Ortega I, Font C, et al. Multivariable clinical-genetic risk model for predicting venous thromboembolic events in patients with cancer. Br J Cancer 2018;118:1056-61. [Crossref] [PubMed]
- Gerotziafas GT, Taher A, Abdel-Razeq H, et al. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS-Cancer-Associated Thrombosis Study. Oncologist 2017;22:1222-31. [Crossref] [PubMed]
- Cella CA, Di Minno G, Carlomagno C, et al. Preventing Venous Thromboembolism in Ambulatory Cancer Patients: The ONKOTEV Study. Oncologist 2017;22:601-8. [Crossref] [PubMed]
- Verso M, Agnelli G, Barni S, et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med 2012;7:291-2. [Crossref] [PubMed]
- Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377-82. [Crossref] [PubMed]
- Annibali O, Napolitano M, Avvisati G, et al. Incidence of venous thromboembolism and use of anticoagulation in hematological malignancies: Critical review of the literature. Crit Rev Oncol Hematol 2018;124:41-50. [Crossref] [PubMed]
- Borchmann S, Müller H, Hude I, et al. Thrombosis as a treatment complication in Hodgkin lymphoma patients: a comprehensive analysis of three prospective randomized German Hodgkin Study Group (GHSG) trials. Ann Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Caruso V, Di Castelnuovo A, Meschengieser S, et al. Thrombotic complications in adult patients with lymphoma: a meta-analysis of 29 independent cohorts including 18 018 patients and 1149 events. Blood 2010;115:5322-8. [Crossref] [PubMed]


