
Systemic therapy of gallbladder cancer: review of first line, maintenance, neoadjuvant and second line therapy specific to gallbladder cancer
Introduction
Gallbladder cancer the sixth most common cancer of the gastrointestinal tract and the most common malignant cancer of the biliary system (1,2). It is mostly associated with late diagnosis, an aggressive disease course and poor prognosis (1).
There is marked international variation in incidence; the top five countries are in South America and East Asia: Bolivia, Chile, Thailand, South Korea and Nepal [age-standardised rate (ASR) per 100,000 of 14.0, 9.3, 7.4, 6.8 and 6.7 respectively] (3). There is also great variation within countries, for example, the indigenous populations of North and South America have an increased risk of gallbladder cancer, as do the people of Northern India. Recent reports calculate an ASR of 7.16 per 100,000 for Northern India’s Gwalior district, however historical data suggests that the ASR could be even higher (21.5 per 100,000) for women in Delhi (1,4,5). The United States of America (US), India and the United Kingdom (UK) have much lower rates of gallbladder cancer (with an ASR of 1.5–2.0 per 100,000) (6,7).
The predominant histological type of gallbladder cancer is adenocarcinoma (approaching 98%) and two thirds of these are moderately or poorly differentiated (8-10). The remaining histological variants are papillary, mucinous, squamous and adenosquamous alongside extra-pulmonary small cell and neuroendocrine tumours (8). Risk factors for gallbladder cancer include age, female sex, ethnicity, gallstones, gallbladder polyps, chronic cholecystitis, chronic infection with Salmonella typhi, an abnormal pancreatobiliary duct junction, obesity and diabetes (11,12). Many of these are distinct from the risk factors for cancers arising elsewhere in the biliary tract (11). Gallbladder cancer development is associated with several molecular aberrations, some of which are distinct to gallbladder cancer, such as EGFR, ERBB3, PTEN, ARID2, MLL2, MLL3, TERT promotor mutations, and others, such as TP53, BRCA1, BRCA2, PIK3CA mutations, which are common to all forms of biliary tract cancer (13,14).
Gallbladder cancer is staged in accordance with the TNM categories of the American Joint Committee on Cancer (AJCC) Union for International Cancer Control (UICC) 8th edition (15,16). The primary tumour might invade the lamina propria (T1a) or the muscular layer (T1b) of the gallbladder wall. The primary tumour might invade further into the perimuscular connective tissue without involvement of the serosa (T2a) or into the perimuscular connective tissue on the hepatic side with no extension into the liver (T2b), further into the liver, into the serosa or an adjacent organ (T3) or into the main portal vein, hepatic artery or several extrahepatic organs or structures (T4). Disease is further categorised by regional lymph node spread: no regional lymph node metastasis (N0), metastases to one or three regional lymph nodes (N1) or metastases to four or more regional lymph nodes (N2), and by distant metastasis: no distant metastatic spread (M0) or distant metastases (M1). If there is no regional or distant spread (N0M0) then gallbladder cancer is staged according to the primary tumour: T1, T2a, T2b, T3 disease is categorised as stage I, IIA, IIB and IIIA respectively. Stage IIIB disease corresponds to T1-3 with N1 M0 disease, stage IVa to T4 N0-1 M0 and stage IVB to T1-4 with N2 or M1 disease.
Surgery can be attempted in patients with up to stage IVa disease, but gallbladder cancer is usually diagnosed at an unresectable locally advanced or metastatic stage (6,9). Gallbladder cancer is usually diagnosed as either an incidental finding in patients with cholelithiasis who are treated with cholecystectomy, in patients investigated for localising symptoms such as jaundice or right upper quadrant pain, or with systemic symptoms such as anorexia or weight loss (6,9). Even if extensive surgical resection is performed, gallbladder cancer frequently recurs and is associated with a poor prognosis of less than 4–5 months without systemic therapy (6,9). Therefore, many gallbladder cancer patients will be candidates for and could benefit from systemic chemotherapy, which is the focus of this article.
Systemic chemotherapy for locally advanced or metastatic gallbladder cancer
Advanced, unresectable and metastatic gallbladder cancer commonly infiltrates into the hepatic duct and therefore the first treatment step is frequently palliative endoscopic or percutaneous stenting or drainage (17-19). Functioning biliary drainage improves symptoms of jaundice, nausea, vomiting and itch, reduces the risk of death by cholangitis or liver failure and is frequently part of best supportive care treatment (17,18). Best supportive care may well be best option for some gallbladder cancer patients with locally advanced, unresectable or metastatic disease, particularly those with multiple co-morbidities, poor end-organ function, and a poor Eastern Cooperative Oncology Group (ECOG) performance score (PS) >2 (6).
As well as systemic chemotherapy, alternatives such as chemoradiation with a concomitant fluoropyrimidine in selected cases of locally advanced, unresectable or metastatic gallbladder cancer and immunotherapy in patients with microsatellite instability (MSI-) high tumours may be considered (20). Chemoradiotherapy and immunotherapy will be considered in separate articles within this edition. This article is focused on the role of systemic chemotherapy in advanced gallbladder cancer, and focuses on response rates (RRs) and survival data specifically reported for gallbladder cancer patients. In the referenced studies, the histological sub-types within cohorts are frequently incompletely reported, but we have aimed to present studies which either select for adenocarcinoma. A brief discussion of the management of gallbladder neuroendocrine carcinomas is also provided.
Best supportive care
To provide a reference point for the survival of patients on the multiple regimens described in the literature, we will describe examples of first-line patients managed with best supportive care. In 2010, Sharma et al. published a single centre randomised study in which 82 patients with unresectable or metastatic adenocarcinoma of the gallbladder were allocated to the systemic chemotherapy or best supportive care arm (21). The aim of the study was to assess the role of chemotherapy in this setting. Twenty-seven patients were randomized into the best supportive care arm, one patient (3.7%) had stable disease as best response (first CT scan was performed at 15 weeks) and the rest showed progressive disease (96.3%); the median progression free survival (PFS) was 2.8 months (95% CI, 1.8–3.8) and median overall survival (OS) was 4.5 months (95% CI, 0.2–8.8) (21). A retrospective case series from Ji et al. in Japan reported OS data for 35 gallbladder cancer patients who had an ECOG PS of 0-2 and were advised to have best supportive care (the reasons for this clinical decision were not reported) (22). The reported median OS was 4.4 months (95% CI, 2.90–5.90), which similar to Sharma et al. (21) Singh et al. described an even poorer survival from 20 patients in an Indian prospective case series; median OS was 3.25 months (95% CI, 2.75–3.75) (23). However, a proportion of the patients who were included in this cohort were unfit for systemic chemotherapy which could explain the poorer prognosis of the whole series (23).
Clinical data: non-randomised studies of monotherapy
Gemcitabine
First-line gemcitabine monotherapy has shown RRs between 7–36% (24-27). A retrospective phase II study from Tsavaris et al. in 2004 examined the efficacy of single agent gemcitabine (800 mg/m2 of body surface area, weekly) in 14 first line gallbladder cancer patients (24). They showed that 5/14 (35.7%) of patients had a partial response (PR), disease control rate (DCR) was 78.6% (11/14), median time to progression (TTP) was 6.4 months (95% CI, 5.8–7.1) and median OS 17.1 months (95% CI, 15.8–18.5) (24). Gallardo’s phase II study from Chile of single agent gemcitabine at a slightly higher dose but reduced frequency (1,000 mg/m2 weekly for 3 weeks on and 1 week off) showed a PR of 36% (95% CI, 17.1–57.9) (9/25), DCR of 60% (15/25), median duration of response of 5.2 months and median OS of 7.5 months (25). The RRs were lower in two retrospective case series from Japan (26,27). For example, Suzuki et al. reported in 2010, 45 consecutive patients treated in a single hospital achieving an overall RR of 8.8% (4/45) and DCR was 57.8% (26/45) (26).
Non-gemcitabine
Non-gemcitabine regimens may be better suited to patients with reduced biliary drainage. Such alternative regimens include S1 alone, an oral fluoropyrimidine containing three agents to increase circulating fluoropyrimidine levels and reduce bowel toxicity, or other agents such as irinotecan or 5-fluorouracil. The multicentre phase II study from Furuse et al. showed a partial RR of 45.0% (9/20) and a median OS of 8.1 months in first line gallbladder cancer adenocarcinoma patients (28). In an American Phase II study, single agent irinotecan (CPT-11) showed low level activity in 23 GBC patients with a 4% (1/23) complete response rate (CR), 4% (1/23) PR rate, 57% DCR, median PFS of 2.7 months (95% CI, 1.7–3.3) and median OS 7.0 months (95% CI, 5.7–8.4) (29). 5-fluorouracil (5-FU) with folinic acid showed a 5% (1/20) partial RR and 55% DCR in a single centre study from Pakistan (30). Single agent regimens of capecitabine and leucovorin-modulated 5-FU have been reported but included small populations of gallbladder cancer patients and cannot be relied on for drawing conclusions regarding their activity in gallbladder cancer (31-33).
Clinical data: non-randomised studies of combination chemotherapy
Doublet therapy
Gemcitabine-containing doublet therapy
Doublet chemotherapy with gemcitabine and cisplatin in gallbladder cancer has been shown to achieve overall RRs between 22.7–36.7% (34-36). For example, in 2004, Doval et al. indicated early activity with gemcitabine and cisplatin in a gallbladder cancer adenocarcinoma cohort (n=30); the CR rate was 13.3% (4/30), RR was 36.7% (11/30), DCR was 56.7% (18/30), median TTP was 18 weeks (95% CI, 14–24 weeks) and median OS was 20 weeks (95% CI, 14–31 weeks) (36). Lee et al.’s phase II study of combination gemcitabine with cisplatin, published in 2007, described a population of 14 patients diagnosed with gallbladder cancer; the RR was 28.6% (95% CI, 4.9–52.2%) (4/14) and DCR was 42.9% (6/14) (35). In a multicentre phase II study of gemcitabine with cisplatin conducted in Australia and New Zealand, Goldstein et al. reported a partial RR of 22.7% (5/22) for gallbladder patients (34).
Woo et al. examined gallbladder cancer patients who received 2 weekly cycles of gemcitabine (1,000 mg/m2) and oxaliplatin (100 mg/m2) and showed a RR of 36% (12/33) with an impressive disease stabilisation rate of 88%, a median TTP of 5.3 months (95% CI, 3.7–6.9) and median OS of 6.8 months (95% CI, 6.1–7.5) (37). Similar results with gemcitabine oxaliplatin (GEMOX) were shown by Harder et al. in 2006 and led a PR rate of 40%, DCR of 70% and median OS 11.1 months with 10 patients who were all <76 years old and either PS 0 or 1 (38). Sharma et al. studied a 3-weekly regimen of gemcitabine and oxaliplatin in a single centre prospective open label phase II study in India in 48 gallbladder tumour patients and report a complete RR of 6.2%, a lower objective RR of 21.2%, DCR of 56.6%, median PFS of 3 months (95% CI, 2.2–3.8) and median OS of 7.5 months (95% CI, 5.6–8.4) (39). A far lower RR was recorded however in André et al.’s international phase II study of gemcitabine and oxaliplatin; the RR was 4.3% (1/23) and the median PFS was 2.5 months (95% CI, 1.6–4.3 months), despite using the same regimen as the other, more effective studies (40).
Studies of gemcitabine with capecitabine show RRs similar to gemcitabine with cisplatin (41-44). For example, Cho et al. described a phase II combination trial of gemcitabine and high dose capecitabine which gave a 33% (8/24) PR rate, 75% (18/24) DCR, median TTP of 6 months (95% CI, 3.8–8.1) and median OS of 16 months (95% CI, 13.8–18.3) (42). In Riechelmann’s phase II trial of gemcitabine and capecitabine in unresectable gallbladder cancer patients, 1/27 patients had complete responses (4%), 9/27 had PRs (33%), DCR was 64% (15/27), median PFS and median OS were 4.4 months (95% CI, 0.1–9.4 months) and 7.7 months (95% CI, 4.6 months–not reached) (44). Alberts et al.’s phase II study, reported in 2007, combined gemcitabine with pemetrexed which, in 16 gallbladder cancer patients, showed a complete RR of 6.3% and overall RR of 12.5% (45).
Non-gemcitabine doublet chemotherapy
Of the non-gemcitabine containing regimens, a phase II study of doublet therapy with capecitabine and cisplatin in gallbladder cancer adenocarcinoma showed a high partial RR of 53.3% (8/15), along with the same DCR (scans were performed every 6 weeks) and a survival rate at 1 year of 66% (46). The RR of capecitabine and cisplatin was lower in another phase II study; the RR was 32% (6/19) (47). It was also far lower in Woo et al.’s retrospective gallbladder cancer case series of 59 patients; the RR was 14.2%, DCR was 65.2%, median TTP and overall survival of 4.2 and 7.2 months respectively (48).
5-FU and cisplatin showed good activity in a Japanese phase II which reported a partial RR of 46.7% (4/15) and DCR of 66.7% (10/15), however the median time to treatment failure was 85 days and median OS 150 days (4.9 months) (49). Ducreux et al. used a similar cisplatin and 5-FU combination, and showed a partial RR of 36% (4/11) in the first line gallbladder cancer adenocarcinoma patients (50). Unfortunately, there is only one study which reported RRs for gallbladder cancer in an S1-containing regimen: Kim et al.’s 2011 single centre phase II doublet regimen of oxaliplatin and S1 showed RRs of 40% PR in 10 first-line patients with gallbladder cancer (51). Oxaliplatin and capecitabine showed good activity in a German prospective multicentre phase II trial reported in 2008 by Nehls et al. (52); they reported a complete response in 1/27 patients (4%), an overall RR of 30% (8/27), DCR of 63% (17/27), median TTP of 4.7 months (95% CI, 4.3–11.7) and overall survival of 8.0 months (95% CI, 4.3–11.7) (52). Low RRs (<10%) are reported in studies of uracil-tegafur combined with intravenous doxorubicin (53,54).
Triple therapies
A recent open-label, single-arm phase II trial of gemcitabine, cisplatin and nab-paclitaxel triple therapy reported by Shroff et al. from two American centres (n=60, intention to treat population) has shown impressive data across BTCs; the RR was 45%, median PFS was 11.8 months (95% CI, 6.0–15.6) and median OS was 19.2 months (95% CI, 13.2 months to not estimable) (55). Gallbladder cancer specific survival data was reported (n=13), but not RRs; the PFS was 4.1 months (95% CI, 2.1–14.9) and median OS was 15.7 months (95% CI, 3.8 months to non-reached) (55). This study is being expanded into a phase III and may change the standard treatment of all or certain biliary tract cancers (intrahepatic cholangiocarcinoma was the histological primary with the best survival data in this study) (55). Sohn et al. reported a trial of triple therapy of 5-FU added to gemcitabine and cisplatin, and showed a RR of 40% (6/15) with 1 (6.7%) complete response, which was slighter higher than reported for gemcitabine cisplatin doublet therapy, however with a high burden of toxicity (for example, 71.4% patients developed Grade 4 neutropenia) (56). Gemcitabine and 5-FU with oxaliplatin has been reported to lead to a 23% (8/35) partial RR, a 69% (24/35) DCR with a median TTP of 5.7 (95% CI, 3.1–8.1) months and median OS of 9.9 months (95% CI, 7.5–12.2) (57). Gemcitabine, leucovorin and 5-FU led to 3/14 (21.4%) PRs, with a median PFS of 5.2 months (95% CI, 1.7–9.1) and median OS of 7.2 months (95% CI, 3.6–11.7) in first line gallbladder cancer patients in the US (58).
Regimens without gemcitabine can lead to reasonable RRs such as 5-FU, high dose levofolinic acid and oral hydroxyurea on a weekly schedule which showed a 9/30 (30%) PR rate, DCR of 57% and median OS of 8 months (59). In addition, less common combination regimens have been studied and some could be effective. For example, triplet chemotherapy with gemcitabine, oxaliplatin and huachansu (a traditional Chinese medicine from the skin of the Bufo toad) showed a partial RR of 34.8%, DCR of 65.2%, median PFS 5.8 months and median OS 10.5 months in a cohort of 23 patients of which 86% were first line (60). Finally, a multicentre phase II from Germany reported by Feisthammel et al. examined triplet chemotherapy with irinotecan, folinic acid and 5-FU and showed a low partial RR of 15% (2/12), DCR of 31% (4/13), an estimated median PFS of 5.3 months (159 days) and OS of 9.1 months (273 days) (61).
Quadruple therapy
A prospective case series was reported by Cereda et al. examining a quartet chemotherapy regimen of gemcitabine, cisplatin, epirubicin and 5-fluorouracil; they report a RR of 33.3% (4/12) gallbladder adenocarcinoma patients and a median OS of 9.6 months (62). Quadruple chemotherapy with 5-FU, cisplatin, doxorubicin and interferon alpha 2b was studied in 2001 with 17 evaluable first line, GBC adenocarcinoma patients who had a complete RR of 5.9%, PR of 35.3%, DCR of 64.7% and median OS of 11.5 months (95% CI, 5.4–17.6), but with notable toxicity such as 41% grade 3/4 neutropenia across the whole study cohort of 41 GBC and cholangiocarcinoma patients (63).
Randomised phase II studies
A summary of the randomised studies is provided inTable 1
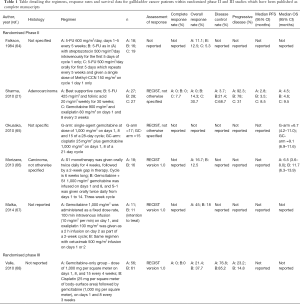
Full table
The first phase II randomised trial exploring the role of the cisplatin and gemcitabine combination in advanced biliary tract cancer was the UK phase II ABC-01 clinical trial. This study was later on converted into a phase III study (ABC-02) and will therefore be discussed later on. Okusaka et al. reported a randomised phase II study (BT22) comparing gemcitabine alone against gemcitabine and cisplatin which suggests a survival advantage for patients treated with gemcitabine and cisplatin over gemcitabine alone of 9.1 months (95% CI, 6.9–11.6) against 6.7 months [(95% CI, 4.2–11.0); P=0.675] (65). The BT22 clinical trial confirmed the previous findings from the ABC-02 study and suggested benefit from combination chemotherapy in an Asian population. However, it did not separately report the GBC response/outcome data (65,69).
The BINGO trial from Malka et al. was conducted across 18 hospitals in Greece and France and reported 11 GBC patients who were treated with GEMOX and 11 gallbladder cancer patients who were treated with GEMOX plus cetuximab (67). The objective RR was 45% (5/11) for the GEMOX cohort and in the intention to treat GEMOX plus cetuximab group of 18% (2/11) (67).
Morizane et al. compared the RRs and median OS of S1 monotherapy against doublet therapy of gemcitabine and S1 in 2013 (66). Gallbladder cancer patients treated with S1 alone had a 16.7% (3/18) RR and gemcitabine and S1 was 12.5% (2/16); the median OS was 6.5 months (95% CI, 3.6–8.0) with S1 alone and 11.7 months (95% CI, 6.3–13.9) in the combination arm (66). Due to the low toxicity of the combination regimen a higher dosage was taken forward into the phase III trial, which has been reported in abstract form and is discussed in the next section.
Sharma et al. randomised patients diagnosed with gallbladder cancer to three groups (21). The first arm was described previously in this review and was managed with best supportive care (21). The second arm received gemcitabine with oxaliplatin and showed 7.7% (2/27) complete responses, 23% (6/27) PRs (23%) and 38% (10/27) incidences of stable disease leading to a marked increase of median PFS [8.5 months (95% CI, 5.7–11.3)] and median OS [9.5 months (95% CI, 5–14)] (21). FU and folinic acid (n=28) was the third gallbladder cancer arm and Sharma et al. suggests that is weakly active regimen with negligible effect on median OS (4.5 to 4.6 months), although 4 (14.3%) PRs and 2 (7.1%) incidences of stable disease were recorded (21). Several regimens were used in Falkson’s 1984 study which showed for patients with untreated gallbladder cancer that the objective RR for 5-FU oral alone was 11.1% (2/18), for 5-FU with streptozotocin was 12.5% (2/16) and for 5-FU and Methyl-CCNU it was 5.3% (1/19) (64).
Randomised phase III
The ABC-02 trial reported in 2010 was a multi-centre phase III trial conducted across the UK which compared gemcitabine against gemcitabine with cisplatin in advanced/metastatic biliary tract cancers (68). Gemcitabine alone was administered at a dose of 1,000 mg/m2 on days 1, 8, and 15 every 4 weeks. When the whole population was analysed, the doublet chemotherapy showed a benefit in PFS (median PFS in the gemcitabine group was 5.0 and 8.0 months in the gemcitabine cisplatin group) and in OS (median OS was 8.1 months in the gemcitabine group and 11.7 months in the gemcitabine cisplatin group). In patients with gallbladder tumours, the gemcitabine alone arm showed a partial RR of 21.4% (12/56), DCR of 76.8% (43/56) and progressive disease of 23.2% (13/56) (68). The doublet chemotherapy arm administered cisplatin (25 mg/m2) and gemcitabine (1,000 mg/m2) on days 1 and 8 every 3 weeks and, in the subgroup of GBC, the trial reported a partial RR of 37.7% (23/61), DCR of 85.2% (52/61) and progressive disease in 14.8% (9/61) (68). The median PFS and OS survival for gallbladder was not specifically reported however there was improved median OS in terms of the hazard ratio (HR) =0.61; (95% CI, 0.42–0.89) in favour of the doublet chemotherapy when the subgroup with gallbladder cancer was analysed (68). When these results are compared to the data from other biliary tract cancer in the same clinic trial [partial RR 18.0% for the cisplatin gemcitabine group, rate of progression as best response 21% for the cisplatin gemcitabine group and OS HR 0.57 (95% CI, 0.34–0.94) for intrahepatic cholangiocarcinoma, 0.73 (95% CI, 0.43–1.23) for extrahepatic cholangiocarcinoma, 0.59 (95% CI, 0.32–0.90) for hilar cholangiocarcinoma and 0.62 (95% CI, 0.21–1.82) for ampullary tumours)], there seems to be a towards increased objective RR in the gallbladder cancer group (compared to other biliary tract cancer). However, the impact on differences on OS benefit seemed marginal, with all subgroups presenting a similar HR. Whether this is increased partial RR reflects an easier assessment of the radiological response assessment is unclear (70).
There are two, additional relevant phase III trials which have been presented in abstract form and compare gemcitabine and cisplatin with alternative gemcitabine-containing doublet therapies. JCOG1113/ FUGA-BT, has been conducted in Japan with contained a total of 354 patients with biliary tract cancer who were randomised to be treated with either gemcitabine plus cisplatin or gemcitabine plus S1. A preliminary report was presented at the American Society of Clinical Oncology (ASCO) 2018 meeting which showed non-inferiority in terms of the primary endpoint of median OS; gemcitabine-cisplatin led to a median OS of 13.4 months and gemcitabine with S1 led to a median OS of 15.1 months [HR =0.945; (95% CI, 0.777–1.149), P=0.00459] and comparable, if not slightly better secondary endpoints in median PFS, clinically significant AEs and SAEs, planned dose delivery and convenience (since gemcitabine with S1 does not require the pre-hydration needed to administer cisplatin), although the RR was slightly better with gemcitabine with cisplatin (32.4% vs. 29.8%) (71). However, the gallbladder data has not been published yet for this trial.
The second trial is a single centre phase III randomised study which compared gemcitabine cisplatin (GemCis) against modified gemcitabine and oxaliplatin (mGEMOX) for patients with GBC specifically (72). This trial included 108 patients in each arm and reports similar responses rates of 23.5% and 22.6% in mGEMOX and GemCis arms respectively, statistically similar median PFS times of 6 months (95% CI, 4.72–7.27) in mGEMOX arm and 4.5 months in GemCis arm [(95% CI, 3.44–5.55); P=0.123] and statistically similar median OS in the mGEMOX cohort of 9 months (95% CI, 7.77–10.22) and 8 months in GemCis arm [(95% CI, 7.40–8.59); P=0.152] (72).
Maintenance therapy
Whether to continue chemotherapy beyond a fixed period of 6 months or not remains unclear. Following the rationale of trials such as the PARAMOUNT trial continuation maintenance therapy in non-small cell lung cancer, Ostwal’s 2017 retrospective cohort study reported that gallbladder cancers who achieved at least stable disease with 6–8 cycles of gemcitabine-cisplatin had statistically better median PFS and OS if they received continuation chemotherapy rather than second line chemotherapy on progression (73,74). Although the two groups seemed to be well matched, the data needs confirmation in a prospective trial.
Biomarkers
Clinical and biochemical biomarkers for gallbladder cancer prognosis and chemotherapy response prediction are poorly defined. Across BTC there are numerous biomarkers including recurrent vs. metastatic disease, number and site of metastases, PS, human equilibrative nucleoside transporter (hENT1) and carbohydrate antigen (CA) 19-9 which seem to affect outcome (6,11,27,68,75-79).
Some studies suggest differential RRs and survival in gallbladder cancer compared to other forms of biliary tract cancer, and future trials may look for robust statistically significant differences (55,67,68). Current opinion is that these differences are likely to be genetically driven. Gallbladder cancer has a greater prevalence of amplifications in human epidermal growth factor receptor 2 (HER2), activatory mutations in KRAS, and their downstream effectors. In contrast, gallbladder cancer is less likely to have isocitrate dehydrogenase (IDH-1) driver mutations and fibroblast growth receptor (FGFR)-2 translocation events in comparison to cholangiocarcinoma (11). However, current pre-clinical data has not addressed how these differences in oncogenes or cell metabolism translate to the aggressiveness of the tumour or susceptibility to standard chemotherapy options. Whether genetic differences lead to differences in prognosis or if these biomarkers are truly predictive remains unclear (11,80).
Neoadjuvant treatment
Most studies show a marked survival benefit for those who are ultimately treated with definitive surgical resection compared to those who could not be treated with surgical resection or received incomplete surgical resection (81-85). For example, Creasy et al. reported in a retrospective case series that 10 of 74 patients (14%) with locally advanced or lymph node positive gallbladder cancer who were treated with neoadjuvant, gemcitabine-containing chemotherapy underwent definitive resection; these 10 patients had a median OS of 51 months (95% CI, 11.7–55.3) compared to an 11 month median OS (95% CI, 4.1–23.6) for those who received chemotherapy and underwent surgery but this was not definitive (n=12/74) (P=0.003) (86). Furthermore, a notable proportion of patients included in these studies do proceed to surgery. For example, Gangopadhyay et al. report, in a retrospective case series, for locally advanced gallbladder cancer patients having a 3 weekly cisplatin gemcitabine regimen, an overall resection rate of 48.7% (59/121) with 43.0% (52/121) having R0 resections (84). Of 106 gallbladder cancer patients who were treated with single agent gemcitabine in a retrospective case series from Kato et al. reported in 2013, 7 received neoadjuvant chemotherapy and all 4 patients with disease control underwent curative surgery (83). Despite cases of increased survival, the overall impact of starting neoadjuvant chemotherapy is unclear and is not robust enough to make recommendation, namely because not all patients can receive curative surgery and there are no studies comparing neoadjuvant chemotherapy with surgery and then adjuvant chemotherapy or palliative surgery and palliative chemotherapy. The difficulty of making comparisons is compounded by the lack of standardised definitions of what is resectable and what is not resectable (85).
Second line
There are few studies which examine biliary tract cancers chemotherapy in the second line setting and none which report a best supportive care cohort to compare against (87). Kang et al. showed that the median OS for gallbladder patients (n=14) who received various forms of gemcitabine based doublet chemotherapy was 4.4 months (95% CI, 3.2–5.7) in the second-line setting (88). This could be improved by several regimens including folinic acid, 5-FU and oxaliplatin (FOLFOX) which showed encouraging responses as a second line agent in the 2015 prospective case series reported by Dodagoudar et al. (89) They reported an impressive RR of 24.2% (16/66) and a DCR of 59.1% (39/66), although the method of cross-sectional imaging response evaluation is not reported (89). The median TTP was 3.9 (95% CI, 3.1–4.7) and median OS 7.6 (95% CI, 6.8–8.2) (89). In Suzuki et al.’s 2013 multicentre phase II study 14 2nd line gallbladder cancer patients received S1 after progression on gemcitabine (90). The RR was 7.1% (1/14) with a single PR, DCR was 42.9% (6/14), median PFS was 1.4 months and median OS was 4.7 months (90). The RR was higher in a previous phase II study reported by Sasaki in 2010; it was 50% (3/6), but no other survival or response data was reported for these patients (91). There are several reported alternative regimens such as irinotecan and capecitabine (XELIRI) or irinotecan monotherapy but many are unsuitable for analysis due to too few gallbladder cancer patients, and poor reporting of gallbladder cancer responses and regimens (78,79,92-100).
Neuroendocrine carcinomas
High grade small cell and large cell neuroendocrine carcinomas of the gallbladder comprise <2% of all gallbladder cancer cases (101). Patients tend to present with advanced disease, and are frequently treated with similar chemotherapy schedules to those used in small cell lung cancer (102). Standard chemotherapy for advanced or metastatic disease is based on a combination of platinum (carboplatin or cisplatin) and etoposide (or infrequently gemcitabine or paclitaxel); there is no standard second line therapy (102-108). First-line systemic therapy recommendations have not been modified for around 30 years, due to the lack of prospective studies (104,107,108).
Conclusions
A variety of systemic chemotherapy regimens have been reported in observational studies and phase II trials suggesting potential effectiveness in gallbladder cancer. Active agents include gemcitabine, platinum compounds and S1. However, reporting bias, inconsistency in the regimens used and variation in patient selection and characteristics, make direct comparisons difficult. The only large phase III trial which has been published and contains accessible gallbladder cancer data is ABC-02 and has established gemcitabine and cisplatin as a standard regimen for both first line biliary tract cancer and gallbladder cancer. However, two new phase III trials have been reported in abstract form and may support the use of gemcitabine combined with oxaliplatin or S1 as the second agent in some first line patients, and a recent non-randomised phase II suggests that the triple therapy of gemcitabine, cisplatin and nab-paclitaxel may prove to extend median overall survival in both biliary tract cancer and gallbladder cancer. No robust data exists comparing the response to chemotherapy between gallbladder and other biliary tract cancer. The ABC-02 clinical trial showed a trend towards increased partial RR in the gallbladder cancer group (compared to other biliary tract cancer), whether this is reflection of an easier radiological response assessment or an actual increased activity of the cisplatin and gemcitabine is unclear, especially in view of similar benefit in terms of OS.
Only three studies report useful data for the second line treatment of gallbladder cancer. Unfortunately, the lack of comparative studies in the second line setting makes it difficult to recommend one of those regimens above others as a salvage therapy after progression to first-line treatment. In addition, whether to treat with neo-adjuvant treatment followed by potential surgery or surgery followed by adjuvant therapy has not been addressed, and the methods and definitions required to examine that question are still lacking.
The prognosis for unresectable and metastatic gallbladder cancer remains poor, and biomarkers for stratifying patients to particular first line therapies are not defined. This could be improved by gallbladder cancer specific analysis and reporting in biliary tract cancer trials or making data available publicly for further analysis. Currently any gallbladder cancer specific signals are being lost within all biliary tract cancers, even though biliary tract cancers are known to have distinct biological and genetic behaviour (13,14). This heterogeneity and data on underlying genetic aberrations also provides an opportunity for combination therapy with targeted or immune-modulatory agents in the first line and on progression which may in carefully designed umbrella trials help us to personalise systemic gallbladder cancer treatment.
Acknowledgments
Dr. Alexander A. Azizi would like to thank The Christie Experimental Cancer Medicine and the Hepato-pancreato-biliary (HPB) teams for their support during his time at The Christie. Dr Angela Lamarca has received funding from The Christie Charity.
Footnote
Conflicts of Interest: A Lamarca received travel and educational support from Ipsen, Pfizer, Bayer, AAA, SirtEx, Novartis, Mylan and Delcath, speaker honoraria from Merck, Pfizer, Ipsen and Incyte and advisory honoraria from EISAI and Nutricia; she is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. JW Valle received travel grants from Celgene, Ipsen, Novartis, NuCana for more than 5 years, received honoraria for participation in Speakers’ Bureau for Abbott, Celgene, Ipsen, Novartis, Pfizer and Sir-tex and provided consulting or advisory role for for Abbott, Agios, AstraZeneca, Baxalta, Bioven, Celgene, Delcath, Genoscience Pharma, Incyte, Ipsen, Keocyt, Lilly, Merck, MidaTech, Mundipharma, Novartis, NuCana, PCI Biotech, Pfizer, Pieris Pharmaceuticals and QED Pharmaceuticals. AA Azizi has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Nemunaitis JM, Brown-Glabeman U, Soares H, et al. Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer 2018;18:665. [Crossref] [PubMed]
- Lai CH, Lau WY. Gallbladder cancer--a comprehensive review. Surgeon 2008;6:101-10. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Barbhuiya MA, Singh TD, Poojary SS, et al. Gallbladder cancer incidence in Gwalior district of India: Five-year trend based on the registry of a regional cancer center. Indian J Cancer 2015;52:430-7. [Crossref] [PubMed]
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591-602. [Crossref] [PubMed]
- Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v28-37. [Crossref] [PubMed]
- Cancer WIAfRo. Global Cancer Observatory: Cancer Today. 2018. Available online: http://gco.iarc.fr/. Accessed 26th June 2019.
- Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology 2009;55:218-29. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Valle JW. Advances in the treatment of metastatic or unresectable biliary tract cancer. Ann Oncol 2010;21 Suppl 7:vii345-8. [Crossref] [PubMed]
- Valle JW, Lamarca A, Goyal L, et al. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov 2017;7:943-62. [Crossref] [PubMed]
- Sharma A, Sharma KL, Gupta A, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol 2017;23:3978-98. [Crossref] [PubMed]
- Goldstein D, Lemech C, Valle J. New molecular and immunotherapeutic approaches in biliary cancer. ESMO Open 2017;2:e000152. [Crossref] [PubMed]
- Sicklick JK, Fanta PT, Shimabukuro K, et al. Genomics of gallbladder cancer: the case for biomarker-driven clinical trial design. Cancer Metastasis Rev 2016;35:263-75. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th edition. Chicago, Illinios: Springer, 2017.
- Lee AJ, Chiang YJ, Lee JE, et al. Validation of American Joint Committee on Cancer eighth staging system for gallbladder cancer and its lymphadenectomy guidelines. J Surg Res 2018;230:148-54. [Crossref] [PubMed]
- Saluja SS, Gulati M, Garg PK, et al. Endoscopic or percutaneous biliary drainage for gallbladder cancer: a randomized trial and quality of life assessment. Clin Gastroenterol Hepatol 2008;6:944-50.e3. [Crossref] [PubMed]
- Kumaran V, Gulati S, Paul B, et al. The role of dual-phase helical CT in assessing resectability of carcinoma of the gallbladder. Eur Radiol 2002;12:1993-9. [Crossref] [PubMed]
- Lamarca A, Rigby C, McNamara MG, et al. Impact of biliary stent-related events in patients diagnosed with advanced pancreatobiliary tumours receiving palliative chemotherapy. World J Gastroenterol 2016;22:6065-75. [Crossref] [PubMed]
- (NCCN) NCCN. NCCN Clinical Practice Guidelines in Oncology. 2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 19th May 2019.
- Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581-6. [Crossref] [PubMed]
- Ji JH, Song HN, Kim RB, et al. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn J Clin Oncol 2015;45:256-60. [Crossref] [PubMed]
- Singh SK, Talwar R, Kannan N, et al. Patterns of Presentation, Treatment, and Survival Rates of Gallbladder Cancer: a Prospective Study at a Tertiary Care Centre. J Gastrointest Cancer 2018;49:268-74. [Crossref] [PubMed]
- Tsavaris N, Kosmas C, Gouveris P, et al. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs 2004;22:193-8. [Crossref] [PubMed]
- Gallardo JO, Rubio B, Fodor M, et al. A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol 2001;12:1403-6. [Crossref] [PubMed]
- Suzuki E, Furuse J, Ikeda M, et al. Treatment efficacy/safety and prognostic factors in patients with advanced biliary tract cancer receiving gemcitabine monotherapy: an analysis of 100 cases. Oncology 2010;79:39-45. [Crossref] [PubMed]
- Murata A, Amano R, Yamada N, et al. Prognostic predictive values of gemcitabine sensitivity-related gene products for unresectable or recurrent biliary tract cancer treated with gemcitabine alone. World J Surg Oncol 2013;11:117. [Crossref] [PubMed]
- Furuse J, Okusaka T, Boku N, et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 2008;62:849-55. [Crossref] [PubMed]
- Alberts SR, Fishkin PA, Burgart LJ, et al. CPT-11 for bile-duct and gallbladder carcinoma: a phase II North Central Cancer Treatment Group (NCCTG) study. Int J Gastrointest Cancer 2002;32:107-14. [Crossref] [PubMed]
- Qayyum A, Mujtaba I. Effects of chemotherapy on patients with unresectable or metastatic adenocarcinoma of gallbladder. J Pak Med Assoc 2007;57:71-4. [PubMed]
- Chen JS, Jan YY, Lin YC, et al. Weekly 24 h infusion of high-dose 5-fluorouracil and leucovorin in patients with biliary tract carcinomas. Anticancer Drugs 1998;9:393-7. [Crossref] [PubMed]
- Choi CW, Choi IK, Seo JH, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol 2000;23:425-8. [Crossref] [PubMed]
- Patt YZ, Hassan MM, Aguayo A, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 2004;101:578-86. [Crossref] [PubMed]
- Goldstein D, Gainford MC, Brown C, et al. Fixed-dose-rate gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol 2011;67:519-25. [Crossref] [PubMed]
- Lee J, Kim TY, Lee MA, et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol 2008;61:47-52. [Crossref] [PubMed]
- Doval DC, Sekhon JS, Gupta SK, et al. A Phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer 2004;90:1516-20. [Crossref] [PubMed]
- Woo SM, Lee SH, Yoo JW, et al. A Multicenter Phase II Trial of Gemcitabine Plus Oxaliplatin in Unresectable Gallbladder Cancer. Gut Liver 2013;7:594-8. [Crossref] [PubMed]
- Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 2006;95:848-52. [Crossref] [PubMed]
- Sharma A, Mohanti B, Raina V, et al. A phase II study of gemcitabine and oxaliplatin (Oxigem) in unresectable gall bladder cancer. Cancer Chemother Pharmacol 2010;65:497-502. [Crossref] [PubMed]
- André T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 2008;99:862-7. [Crossref] [PubMed]
- Santini D, Virzi V, Vasile E, et al. A phase II trial of fixed-dose rate gemcitabine plus capecitabine in metastatic/advanced biliary tract cancer patients. Oncology 2012;82:75-82. [Crossref] [PubMed]
- Cho JY, Nam JS, Park MS, et al. A Phase II Study of Capecitabine Combined with Gemcitabine in Patients with Advanced Gallbladder Carcinoma. Yonsei Med J 2005;46:526-31. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332-8. [Crossref] [PubMed]
- Riechelmann RP, Townsley CA, Chin SN, et al. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 2007;110:1307-12. [Crossref] [PubMed]
- Alberts SR, Sande JR, Foster NR, et al. Pemetrexed and gemcitabine for biliary tract and gallbladder carcinomas: a North Central Cancer Treatment Group (NCCTG) phase I and II Trial, N9943. J Gastrointest Cancer 2007;38:87-94. [Crossref] [PubMed]
- Hong YS, Lee J, Lee SC, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol 2007;60:321-8. [Crossref] [PubMed]
- Kim TW, Chang HM, Kang HJ, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol 2003;14:1115-20. [Crossref] [PubMed]
- Woo SM, Lee WJ, Han SS, et al. Capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer: a retrospective single-center study. Chemotherapy 2012;58:225-32. [Crossref] [PubMed]
- Kobayashi K, Tsuji A, Morita S, et al. A phase II study of LFP therapy (5-FU (5-fluorourasil) continuous infusion (CVI) and Low-dose consecutive (Cisplatin) CDDP) in advanced biliary tract carcinoma. BMC Cancer 2006;6:121. [Crossref] [PubMed]
- Ducreux M, Rougier P, Fandi A, et al. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol 1998;9:653-6. [Crossref] [PubMed]
- Kim K, Jang G, Hong YS, et al. Phase II study of S-1 combined with oxaliplatin as therapy for patients with metastatic biliary tract cancer: influence of the CYP2A6 polymorphism on pharmacokinetics and clinical activity. Br J Cancer 2011;104:605-12. [Crossref] [PubMed]
- Nehls O, Oettle H, Hartmann JT, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer 2008;98:309-15. [Crossref] [PubMed]
- Furuse J, Okusaka T, Ohkawa S, et al. A phase II study of uracil-tegafur plus doxorubicin and prognostic factors in patients with unresectable biliary tract cancer. Cancer Chemother Pharmacol 2009;65:113-20. [Crossref] [PubMed]
- Furuse J, Okusaka T, Funakoshi A, et al. Early phase II study of uracil-tegafur plus doxorubicin in patients with unresectable advanced biliary tract cancer. Jpn J Clin Oncol 2006;36:552-6. [Crossref] [PubMed]
- Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Sohn BS, Yuh YJ, Kim KH, et al. Phase II trial of combination chemotherapy with gemcitabine, 5-fluorouracil and cisplatin for advanced cancers of the bile duct, gallbladder, and ampulla of Vater. Tumori 2013;99:139-44. [Crossref] [PubMed]
- Wagner AD, Buechner-Steudel P, Moehler M, et al. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: two parallel, multicentre phase-II trials. Br J Cancer 2009;101:1846-52. [Crossref] [PubMed]
- Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer 2005;103:111-8. [Crossref] [PubMed]
- Gebbia V, Majello E, Testa A, et al. Treatment of advanced adenocarcinomas of the exocrine pancreas and the gallbladder with 5-fluorouracil, high dose levofolinic acid and oral hydroxyurea on a weekly schedule. Results of a multicenter study of the Southern Italy Oncology Group (G.O.I.M.). Cancer 1996;78:1300-7. [Crossref] [PubMed]
- Qin TJ, Zhao XH, Yun J, et al. Efficacy and safety of gemcitabine-oxaliplatin combined with huachansu in patients with advanced gallbladder carcinoma. World J Gastroenterol 2008;14:5210-6. [Crossref] [PubMed]
- Feisthammel J, Schoppmeyer K, Mossner J, et al. Irinotecan with 5-FU/FA in advanced biliary tract adenocarcinomas: a multicenter phase II trial. Am J Clin Oncol 2007;30:319-24. [Crossref] [PubMed]
- Cereda S, Passoni P, Reni M, et al. The cisplatin, epirubicin, 5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary tract adenocarcinoma. Cancer 2010;116:2208-14. [PubMed]
- Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of cisplatin, interferon alpha-2b, doxorubicin, and 5-fluorouracil for biliary tract cancer. Clin Cancer Res 2001;7:3375-80. [PubMed]
- Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 1984;54:965-9. [Crossref] [PubMed]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74. [Crossref] [PubMed]
- Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci 2013;104:1211-6. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8. [Crossref] [PubMed]
- Lamarca A, Frizziero M, Mcnamara M, et al. Clinical and translational research challenges in biliary tract cancers. Curr Med Chem 2018. In press.
- Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase III study of gemcitabine plus S-1 combination therapy versus gemcitabine plus cisplatin combination therapy in advanced biliary tract cancer: A Japan Clinical Oncology Group study (JCOG1113, FUGA-BT). J Clin Oncol 2018;36:205. [Crossref]
- Sharma A, Shukla NK, Chaudhary SP, et al. Final results of a phase III randomized controlled trial comparing modified gemcitabine + oxaliplatin (mGEMOX) to gemcitabine+ cisplatin in management of unresectable gall bladder cancer (GBC). J Clin Oncol 2017;34:4077. [Crossref]
- Ostwal V, Pinninti R, Ramaswamy A, et al. Treatment of advanced Gall bladder cancer in the real world-can continuation chemotherapy improve outcomes? J Gastrointest Oncol 2017;8:368-76. [Crossref] [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [Crossref] [PubMed]
- Park HS, Park JS, Chun YJ, et al. Prognostic Factors and Scoring Model for Survival in Metastatic Biliary Tract Cancer. Cancer Res Treat 2017;49:1127-39. [Crossref] [PubMed]
- Grunnet M, Christensen IJ, Lassen U, et al. Decline in CA19-9 during chemotherapy predicts survival in four independent cohorts of patients with inoperable bile duct cancer. Eur J Cancer 2015;51:1381-8. [Crossref] [PubMed]
- Deng T, Pan H, Han R, et al. Gemcitabine sensitivity factors, hENT1 and RRM1 as potential prognostic biomarker for advanced biliary tract cancer. Int J Clin Exp Med 2014;7:5041-9. [PubMed]
- Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer 2015;121:3290-7. [Crossref] [PubMed]
- Fornaro L, Vivaldi C, Cereda S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res 2015;34:156. [Crossref] [PubMed]
- Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 2015;8:58. [Crossref] [PubMed]
- Selvakumar VP, Zaidi S, Pande P, et al. Resection after neoadjuvant chemotherapy in advanced carcinoma of the gallbladder: a retrospective study. Indian J Surg Oncol 2015;6:16-9. [Crossref] [PubMed]
- Agrawal S, Mohan L, Mourya C, et al. Radiological Downstaging with Neoadjuvant Therapy in Unresectable Gall Bladder Cancer Cases. Asian Pac J Cancer Prev 2016;17:2137-40. [Crossref] [PubMed]
- Kato A, Shimizu H, Ohtsuka M, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol 2013;20:318-24. [Crossref] [PubMed]
- Gangopadhyay A, Nath P, Biswas J. Reduced Dose Intensity of Chemotherapy may not Lead to Inferior Palliation in Locally Advanced Carcinoma of the Gall Bladder: An Experience from a Regional Cancer Centre in Eastern India. J Gastrointest Cancer 2015;46:297-300. [Crossref] [PubMed]
- Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - A systematic review. Eur J Surg Oncol 2019;45:83-91. [Crossref] [PubMed]
- Creasy JM, Goldman DA, Dudeja V, et al. Systemic Chemotherapy Combined with Resection for Locally Advanced Gallbladder Carcinoma: Surgical and Survival Outcomes. J Am Coll Surg 2017;224:906-16. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref] [PubMed]
- Kang EJ, Choi YJ, Kim JS, et al. Prognostic Factors for the Selection of Patients Eligible for Second-Line Chemotherapy in Advanced Biliary Tract Cancer. Chemotherapy 2014;60:91-8. [Crossref] [PubMed]
- Dodagoudar C, Doval DC, Mahanta A, et al. FOLFOX-4 as second-line therapy after failure of gemcitabine and platinum combination in advanced gall bladder cancer patients. Jpn J Clin Oncol 2016;46:57-62. [Crossref] [PubMed]
- Suzuki E, Ikeda M, Okusaka T, et al. A multicenter phase II study of S-1 for gemcitabine-refractory biliary tract cancer. Cancer Chemother Pharmacol 2013;71:1141-6. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 2012;30:708-13. [Crossref] [PubMed]
- Zheng Y, Tu X, Zhao P, et al. A randomised phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br J Cancer 2018;119:291-5. [Crossref] [PubMed]
- Lim KH, Kim TY, Lee KH, et al. Efficacy of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) in the treatment of patients with gemcitabine-pretreated pancreatic cancer and analysis of prognostic factors in a salvage setting. Cancer Chemother Pharmacol 2011;68:1017-26. [Crossref] [PubMed]
- Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013;49:329-35. [Crossref] [PubMed]
- Oh SY, Jeong CY, Hong SC, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 2011;29:1066-72. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs 2011;29:1488-93. [Crossref] [PubMed]
- Lim KH, Han SW, Oh DY, et al. Outcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancer. Oncology 2012;83:57-66. [Crossref] [PubMed]
- Kobayashi S, Ueno M, Ohkawa S, et al. A retrospective study of S-1 monotherapy as second-line treatment for patients with advanced biliary tract cancer. Jpn J Clin Oncol 2012;42:800-6. [Crossref] [PubMed]
- Takahara N, Nakai Y, Isayama H, et al. Second-line chemotherapy in patients with advanced biliary tract cancer. J Clin Oncol 2019;37:381. [Crossref]
- Sasaki T, Isayama H, Nakai Y, et al. A retrospective study of gemcitabine and cisplatin combination therapy as second-line treatment for advanced biliary tract cancer. Chemotherapy 2013;59:106-11. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Lamarca A, Frizziero M, Barriuso J, et al. Urgent need for consensus: international survey of clinical practice exploring use of platinum-etoposide chemotherapy for advanced extra-pulmonary high grade neuroendocrine carcinoma (EP-G3-NEC). Clin Transl Oncol 2019;21:950-3. [Crossref] [PubMed]
- Kamboj M, Gandhi JS, Gupta G, et al. Neuroendocrine Carcinoma of Gall Bladder: A Series of 19 Cases with Review of Literature. J Gastrointest Cancer 2015;46:356-64. [Crossref] [PubMed]
- Iype S, Mirza TA, Propper DJ, et al. Neuroendocrine tumours of the gallbladder: three cases and a review of the literature. Postgrad Med J 2009;85:213-8. [Crossref] [PubMed]
- Zarog MA, Lyons EM, O’Leary DP, et al. Incidental small cell carcinoma of the gallbladder—an unexpected finding at elective cholecystectomy. J Surg Case Rep 2018;2018:rjy166. [Crossref] [PubMed]
- Buscemi S, Orlando E, Damiano G, et al. "Pure" large cell neuroendocrine carcinoma of the gallbladder. Report of a case and review of the literature. Int J Surg 2016;28 Suppl 1:S128-32. [Crossref] [PubMed]
- Lee JM, Hwang S, Lee SG, et al. Neuroendocrine tumors of the gallbladder: twelve cases in a single institution. Hepatogastroenterology 2010;57:1064-8. [PubMed]
- Chen H, Shen YY, Ni XZ. Two cases of neuroendocrine carcinoma of the gallbladder. World J Gastroenterol 2014;20:11916-20. [Crossref] [PubMed]


