Mining the ACCENT database: a review and update
Introduction
Clinical trials are becoming more sophisticated in design and comprehensive in the information learned from patients, yet the knowledge to be gleaned from even the largest, most data-enriched trial remains limited. Single studies have inherent limitations, such as restricted sample size and geographic region, narrow focus of research questions, and so on. In reality, multiple clinical trials assessing similar treatments for a given disease are often conducted simultaneously, collectively including patients from slightly differing populations or from several different countries. With organized and dedicated efforts such as those initiated and sustained by the Adjuvant Colon Cancer End Points (ACCENT) Group, the information yielded from individual studies may be combined to answer important disease questions that no single trial can address.
Colorectal cancer is the fourth most common cancer worldwide, with a worldwide incidence on the order of 1 million cases per year resulting in on the order of 500,000 deaths. The landmark trial of Moertel et al. (1) first established an effective regimen for the post-surgical treatment of resected stage II/III disease. In the decades since, multiple trials have been conducted worldwide in attempts to refine and improve the original adjuvant therapy of 5-FU with levamisole. The ACCENT database is the amalgamation of individual patient data from many of these trials, selected for their quality and importance. Today, the ACCENT database contains patient-level data from over 33,000 individuals enrolled onto 25 adjuvant colon cancer trials conducted between 1977 and 2008. This project has notably served as a prototype for the construction of databases in other disease settings, and contains selected baseline and treatment data, and critically lengthy and validated recurrence and survival follow-up for all patients.
We highlight some of the most notable research outcomes made possible by the ACCENT database, from the earliest pre-ACCENT pooled analyses of adjuvant trials, to the most recent ACCENT group publications. Additionally, we provide an overview of the collaborative origins, growth, and operational aspects of the ACCENT database and describe ongoing projects by its members.
Overview of ACCENT database
History
The ACCENT collaborative group was formed organically over several years, building on a history of collaboration and pooled analyses in colon cancer research. One early pooled analysis in adjuvant therapy was performed by the IMPACT group, who combined data from 3 trials testing 5-FU with leucovorin versus control in patients with stage II and III disease (2). Individual patient data from these trials was subsequently combined with data from two additional trials to conduct an analysis restricted to stage II patients (3). In 2000, Drs. Sargent and Goldberg from Mayo Clinic initiated a project to pool the data from these 5 trials with that of 2 additional North Central Cancer Treatment Group (NCCTG) trials to examine the benefit of adjuvant therapy in elderly patients (4). Following a meeting in Paris, France organized by Dr. Aimery de Gramont, where a potential surrogacy relationship between disease-free survival and overall survival was first discussed, the ACCENT group was founded in 2003 by adding data from an additional 6 trials conducted by the NCCTG and the National Surgical Adjuvant Breast and Bowel Project (NSABP). The critical mass of 13 trials generated considerable interest, and the ACCENT group was able to obtain the data from 5 additional completed trials, resulting in the initial set of 18 trials within ACCENT (5). In 2009, the database was updated with the data from 6 newly mature trials testing oxaliplatin or irinotecan added to 5-FU/LV, or oral fluoropyrimidines (6).
Database construction and management
Considerable time and effort is required to solicit, collect, combine, and prepare a database containing individual patient data from numerous trials, even within a specific cancer setting. First, a number of investigators with ownership of individual similar trials must be successfully encouraged to share data and to agree on policies guiding its sound scientific use. Second, the data itself must be transferred to a central organization tasked with the following: foreseeing the data items that will be useful in future research, making clear and feasible requests for these items, collecting the data electronically or otherwise (e.g., via post), cleaning and processing the data for consistency across studies, and in many cases, performing the proposed pooled analyses of interest. Third, the contributed data from each study must be examined in detail such that its comprehensiveness, missingness, and level of detail is fully understood, prior to attempts to combine it with data from other studies. Fourth, a number of individuals with knowledge of the data structure from each study must construct and agree upon rules by which the data may be generalized or re-coded for consistency across trials. This is especially challenging when vastly different levels of detail may be supplied for any single item, or in some cases, the data is not supplied at all. Finally, once rules have been established to optimize the use of data in future analyses, the data is combined across studies into an intermediate dataset according to these rules. As part of this last step, independent verification and detailed documentation (e.g., manual for assembly of the database and accompanying data dictionary) ensure the quality and consistency of future analyses and database expansion.
Several unique challenges are inherent to this process, which may take years to complete. For transferred data to be useable, an understanding must be reached between contributing and receiving institutions of the desired data formatting, definitions, and historic conditions from which each data element originated. For example, ACCENT contains data from many different countries that must be solicited and combined, resulting in potential language issues, both in correspondence and within the data. Also, each single trial typically yields many individual datasets, but each of these generally contains unique structure, format, or level of detail that is different from the datasets provided from any other trial. At this stage, it is critical to have prospective and comprehensive data management, such that theoretically similar sources of information (e.g., radiographic tumor measurements and baseline laboratory values) may be thoroughly understood and then carefully combined across trials without loss of the data’s original authenticity. An added layer of complexity is missing, erroneously recorded, or incomplete data elements common to any given trial; such issues must be handled consistently across trials to prevent the addition of noise or bias to a subsequent multi-trial analysis. Crucial throughout the process is regular, independent validation of data management principles and coding rules by multiple experienced data technicians, such that the clinical or laboratory variables of interest may ultimately be deemed usable for exploratory research. As new trials are added to the database, principles and techniques must be revisited and refined for consistency with past actions, with a detailed written record facilitating maintenance of the database as it grows over time.
Research proposals and agreements
As the database construction steps outlined above are performed, a process must be established for the pooled data to be utilized according to principles of open participation, scientific peer review, collaborative authorship, and support for proposed research. Specifically for the ACCENT database, any contributing or participating party may submit a formal research proposal that can be feasibly addressed by the data in the ACCENT database. These requests are circulated to all participating ACCENT investigators including clinical oncologists as well as biostatisticians, who assess the plausibility, design, and likely impact of the proposed research. Each trial lead investigator may decline to allow the data from their trial to be used in any analysis, or voice a scientific objection. Once consensus is reached for a given proposal, members interested in the topic form an authorship group, combining resources and attention that move the project to completion. Successful endeavors yield published manuscripts with authorship reflecting all scientific, statistical, and original data contributions.
Published research using ACCENT
To date, the trials comprising the ACCENT database have jointly facilitated the answering of several important questions in early stage colon cancer. We provide an overview of pooled analyses utilizing trials included in ACCENT, chronologically by publication date.
Efficacy of adjuvant therapy in colon cancer—IMPACT studies
One of the earliest combined analyses of trials now contained in ACCENT was conducted by the IMPACT group in 1995 (2). A pooled analysis was prospectively planned using data collected on 1,493 patients accrued to three international multi-center trials conducted by the Gruppo Interdisciplinare Valutazione Interventi Oncologia (GIVIO), the National Cancer Institute Canada Clinical Trials Group (NCIC-CTG), and the Fondation Française de Cancerologie Digestive (FFCD). These studies compared the same fluorouracil-based regimen versus surgery alone. Overall, significant reductions in both mortality (22%) and recurrence events (35%) were observed due to adjuvant therapy.
Following this analysis, individual patient data from these trials were pooled with two additional trials from the NCCTG Intergroup and the University of Siena group, in which another pooled analysis restricted to patients with stage II disease was performed (3). No significant benefit of adjuvant therapy was observed in the stage II setting.
Adjuvant chemotherapy in elderly patients
Historically, treatment of elderly patients with adjuvant chemotherapy following curative resection has been controversial, with limitations inherent to existing data obscuring or precluding practical guidelines. A profound limitation in existing data is that the very limited sample size of elderly patients within individual studies, prohibiting meaningful statistical inferences. The frequent exclusion of elderly patients from chemotherapy trials by design and failure to offer chemotherapy to elderly patients due to health-related or perceived risks exacerbates this problem. To address the question of whether established benefits of 5-FU based regimens could be generalized to elderly patients, in 2001 Sargent et al. published a pooled analysis of seven adjuvant therapy trials (GIVIO, NCIC-CTG, FFCD and four NCCTG studies) (4). With therapeutic benefit analyzed by age (pre-specified as < or ≥70 years), it was found that chemotherapy benefited patients aged 70 years and older to the same extent as younger patients.
Pooled prognostic and predictive models
In 2004, Gill et al. used the same seven adjuvant trials to identify clinical and disease factors prognostic for disease-free survival and overall survival and/or predictive of benefit from FU-based regimens (7). It was found that nodal status, tumor stage, and grade were independently prognostic for both disease-free and overall survival, while age was significant only for overall survival. Treatment benefit was consistent across age, sex, tumor location and stage, and tumor grade, while a significant stage-by-treatment interaction indicated greater benefit of adjuvant therapy for stage III than stage II patients. This early comprehensive prognostic model is now being updated to include additional trials and patient characteristics, as will be discussed in Section 5.
Disease-free survival as surrogate for overall survival (OS)
By 2003, formal efforts to solicit and pool data from additional adjuvant therapy trials in colon cancer yielded an additional 8 studies totaling more than 20,000 patients from 18 trials; these comprised the original ACCENT database and are listed in Table 1. The timely combination of these studies was motivated by the question of whether disease-free survival could be validated as a surrogate endpoint for overall survival, thus replacing OS as a primary endpoint in future adjuvant colon cancer trials. In 2005, the first official ACCENT collaboration led by Dr. Daniel Sargent confirmed that DFS with median of 3 years of follow-up was an appropriate surrogate for OS with median of 5 years of follow-up, through the robust findings across several meta-analytic surrogacy analyses (5). The platform (data collection, assembling, management, and analyses) established through this milestone project would be subsequently replicated by surrogacy explorations in other disease settings.
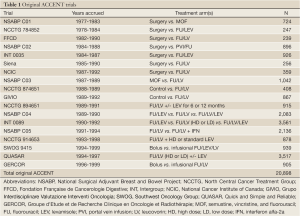
Full Table
In 2007, Sargent et al. expanded these initial surrogacy results to investigate shorter-term DFS endpoints, as well as dependency on stage of disease (8). In general, DFS with less than 3 years follow-up proved to be less accurate in predicting OS than 3-year DFS, although 1-year DFS demonstrated perfect negative predictive value: trials negative for DFS at 1 year were negative for 5-year OS. Furthermore, the surrogacy association across trials was stronger for stage III than stage II disease, indicating that use of DFS as a surrogate is most appropriate in trials comprised mostly or entirely of stage III patients.
Survival following recurrence
The early 2000s saw a lengthening of the time from patient recurrence to death. This has been attributed to increased availability of post-recurrent regimens, as well as new therapeutic options. This interest in post-recurrence survival prompted the ACCENT group to explore factors influencing survival following recurrence. Specifically, in 2008, O’Connell and colleagues examined five possible prognostic factors in a subset of ACCENT patients (approximately 33%) with documented recurrence: patient age at recurrence, time from randomization to recurrence, initial disease stage (II vs. III), initial adjuvant treatment (FU-based versus surgery alone), and era in which the patient was enrolled, where eras were defined as six-year-long periods from 1978 to 1999 (9). All factors were significant predictors of survival post-recurrence and in the expected directions, though notably, patients randomized to surgery alone experienced longer post-recurrence survival than patients randomized to FU-containing regimens.
In a parallel analysis motivated by newer therapies that had been found to prolong survival after recurrence, de Gramont et al. used the ACCENT trials to simulate the impact of longer post-recurrence survival on the association between 3 years follow-up on DFS and varying lengths of follow-up for OS (10). The authors found that improved survival after recurrence weakens the DFS/OS relationship, while the relationships strengthen with increased (>5 years) follow-up on OS. The group concluded that as a whole, these findings supported the use of DFS as a primary endpoint in modern adjuvant chemotherapy trials.
Evidence for cure by adjuvant therapy
While the ACCENT group had thoroughly studied the relationships between disease recurrence, adjuvant therapy, and overall survival in the first 5 years after surgery, until this point, longer-term outcomes and implications had not yet been explored. In 2009, Sargent et al. found that the overall survival benefit attributed to adjuvant chemotherapy was sustained over 8 years of available patient follow-up, with the recurrence risk in these patients never exceeding that of patients treated with surgery alone (11). Based on this fact, the authors concluded that a subset of chemotherapy-treated patients in ACCENT were in fact cured of their disease, with recurrence rates of less than 1% per year for patients followed beyond 8 years. However, it was found that improved DFS experienced by treated patients in the first two years was no longer significantly different from DFS among control patients beyond two years, indicating that adjuvant therapy’s primary benefit was a highly significant reduction of the risk of recurrence in the first 2 years following surgery.
DFS as endpoint for combination or oral adjuvant therapy
In light of new therapies showing promise in adjuvant colon cancer studies in 2009, the ACCENT database acquired a new cohort of six trials containing oral fluoropyrimidines, oxaliplatin, and irinotecan (Table 2). The additional data provided by these trials would not only aid the answering of important clinical and prognostic questions with greater power, but could also be examined to learn whether findings present under older treatment and trial paradigms would persist with modern trials and treatments. In 2011, Sargent et al. performed a re-evaluation of the surrogacy of DFS for OS in the new ACCENT trials and found the strength of the DFS/OS relationship to be somewhat diminished, especially for stage II patients (6). The authors maintained that while DFS remained an appropriate endpoint for stage III disease, modern treatment of patients in combination with newer drugs necessitated at least 6 years follow-up to assess OS benefit.
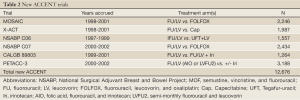
Full Table
Outcomes among black patients
In a prospectively planned study, Dr. Greg Yothers and colleagues noted that black patients in the ACCENT database demonstrated poorer overall survival than white patients (12). When investigating outcome disparities between patients treated with identical therapies in the ACCENT trials, the authors found worsened overall survival and recurrence-free survival persisted among blacks when subsets defined by sex, age, and stage were explored, but no difference in recurrence-free interval existed between black and white patients. These findings suggested that the disease process (risk of recurrence) did not differ by race, but that the poorer survival of black patients is most likely due to other factors.
Benefit and adverse events in young versus old patients
Until recently, limited data existed regarding the benefit or adverse events associated with adjuvant chemotherapy in very young patients. In 2012, patient-level data from the 24 trials contained in ACCENT were pooled to examine efficacy and toxicity outcomes by age, with young patients defined as age less than 40 years (5.2%) or 50 years (17.3%) (13). Overall, younger patients were found not to differ from older patients in terms of recurrence-free interval, but younger patients experienced improved OS and DFS relative to older patients. In a subset of 9 trials demonstrating benefit in the experimental arm, the DFS benefit was similar by age. Adverse events also differed by age, with younger patients experiencing less leukopenia and stomatitis, but more frequent nausea and vomiting. This analysis serves as a key example of the power of the ACCENT database; when the population of interest (young patients) comprises only a small percentage of those patients enrolled to any single trial, the answers to questions such as these could only be meaningfully obtained by combining such patients across many similar trials.
Comparative effectiveness of oxaliplatin versus non-oxaliplatin regimens
While 5-FU plus oxaliplatin regimens (e.g., FOLFOX) have demonstrated significant benefit in the treatment of early stage colon cancer, these promising results were obtained from a relatively younger, healthier population of patients eligible for participation in clinical trials. It remained unclear whether these benefits would extend to a more general patient population with more compromised disease or possible comorbidities. In 2012, Dr. Hanna Sanoff and colleagues compared pooled outcomes in ACCENT against those obtained from the Surveillance, Epidemiology, and End Results (SEER) registry linked to Medicare claims (SEER-Medicare), the New York State Cancer Registry (NYSCR), and National Comprehensive Cancer Network (NCCN) Outcomes Database, and the Cancer Care Outcomes Research & Surveillance Consortium (CanCORS). Overall, Sanoff et al. confirmed that the benefits of oxaliplatin-enhanced adjuvant chemotherapy extended to the general population, including older and minority patients, and to those with a higher level of comorbidities (14).
Body mass index and overall survival
While high body mass index (BMI) has been established as a strong risk factor in colon cancer, until recently, the prognostic and predictive impact of BMI on outcomes of patients treated with adjuvant therapy remained unclear. In 2012, Dr. Frank Sinicrope and other investigators performed a pooled analysis of patient outcomes from ACCENT, including time to recurrence (TTR), DFS, and OS, as a function of baseline BMI (15). Of the 25,291 patients examined, obese and underweight patients showed worse OS than normal weight or overweight patients; however, this pattern was significant only for men. In addition, men with severe (class 2 or 3) obesity at randomization demonstrated significantly shorter DFS than normal-weight men. Underweight men and women showed a shorter TTR and reduced DFS, but this relationship was more significant in men than in women. Notably, BMI was not found to be predictive of benefit from adjuvant therapy. Similar to the age-based ACCENT analysis discussed previously, the BMI analysis of Sinicrope et al. was made more feasible by the combination of patients from small subgroups across many trials.
Endpoint estimation via log-normal modeling and other statistical endeavors
In addition to facilitating large-scale predictive and prognostic clinical analyses in early stage colon cancer, the ACCENT database has served as an important resource for developing or testing advanced multi-trial statistical analysis techniques. One such recent endeavor by Dr. Judy-Anne Chapman and colleagues (16) involved alternative modeling of time-to-event endpoints (e.g., TTR, DFS, and OS) assuming the parametric log-normal distribution, versus standard semi- and non-parametric Cox proportional hazards and Kaplan-Meier approaches, respectively. In theory, parametric models may offer more
precise estimation of quantities of interest (such as treatment effect) when the distribution is carefully chosen to reflect features of the observed data. The authors concluded that for a subset of ACCENT patients, the log-normal distribution demonstrated limited improved performance over Cox proportional hazards modeling.
Other advanced statistical explorations have flourished with access to the ACCENT database, where complex multi-trial surrogacy evaluation methods and novel adaptive trial designs have been successfully motivated by actual data. These include but are not limited to: a comparative assessment of trial-level surrogacy evaluation methods by Shi et al. (17), a Bayesian adjusted trial level surrogacy evaluation method by Renfro et al. (18), a Bayesian adaptive trial design for a newly validated surrogate endpoint by Renfro et al. (19), and others.
Current ACCENT projects
To date, the ACCENT group has continuously accumulated valuable clinical, treatment, outcome, and genetic markers data from pivotal trials over an extended period of time, i.e., over 20 years. With the pooling of data from modern trials, the ACCENT database provides a uniquely powerful ability to identify prognostic and predictive markers, develop better risk classification strategies, and motivate personalized cancer treatment through identification of targeted disease populations based on molecular markers. Currently, ongoing ACCENT projects include: (I) assessment of the impact of patient sex (male versus female) on long-term outcomes such as overall survival, time to recurrence, and recurrence-free survival; (II) development of the lymph node ratio (number of positive lymph nodes to number of nodes examined) as an improved prognostic calibrator of recurrence in stage III colon cancer patients; (III) examination of the association of tumor mismatch repair (MMR) status with clinical and pathologic features, as well as the prognostic impact of MMR within patient subgroups using the ACCENT database’s large sample size; (IV) incorporation of the rich baseline and follow-up data within ACCENT to generate a prognostic nomogram for patients with stage III disease, in an attempt to provide more accurate prognoses compared to traditional American Joint Committee on Cancer (AJCC) staging.
Conclusions
Since its establishment in 2003, the ACCENT collaboration has significantly impacted the efficiency of trial conduct, design and interpretation in stage II and III colon cancer. Continued evaluation of data from clinical trials completed in order to define the optimal endpoint in varying settings is an ongoing process, which will require re-examination after each set of newly completed trials. The future opportunities for mining the ACCENT database to yield novel insights are ever present. Ultimately, these insights gained by thoughtful examination of clinical trial data will facilitate timely assessment of new adjuvant therapies, with the overall objective of significantly improved outcomes for patients with colon cancer.
Acknowledgements
This work was supported by the NCCTG Grant from the National Cancer Institute, U10 CA180882.
Disclosure: The authors declare no conflict of interest.
References
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8. [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345:939-44. [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356-63. [PubMed]
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7. [PubMed]
- Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005;23:8664-70. [PubMed]
- Sargent D, Shi Q, Yothers G, et al. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer 2011;47:990-6. [PubMed]
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797-806. [PubMed]
- Sargent DJ, Patiyil S, Yothers G, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol 2007;25:4569-74. [PubMed]
- O’Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol 2008;26:2336-41. [PubMed]
- de Gramont A, Hubbard J, Shi Q, et al. Association between disease-free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant chemotherapy for colon cancer: simulations based on the 20,800 patient ACCENT data set. J Clin Oncol 2010;28:460-5. [PubMed]
- Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872-7. [PubMed]
- Yothers G, Sargent DJ, Wolmark N, et al. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials. J Natl Cancer Inst 2011;103:1498-506. [PubMed]
- Hubbard J, Thomas DM, Yothers G, et al. Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set. J Clin Oncol 2012;30:2334-9. [PubMed]
- Sanoff HK, Carpenter WR, Martin CF, et al. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J Natl Cancer Inst 2012;104:211-27. [PubMed]
- Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 2013;119:1528-36. [PubMed]
- Chapman JW, O’Callaghan CJ, Hu N, et al. Innovative estimation of survival using log-normal survival modelling on ACCENT database. Br J Cancer 2013;108:784-90. [PubMed]
- Shi Q, Renfro LA, Bot BM, et al. Comparative assessment of trial-level surrogacy measures for candidate time-to-event surrogate endpoints in clinical trials. Computational Statistics and Data Analysis 2011;55:2748-57.
- Renfro LA, Shi Q, Sargent DJ, et al. Bayesian adjusted R2 for the meta-analytic evaluation of surrogate time-to-event endpoints in clinical trials. Stat Med 2012;31:743-61. [PubMed]
- Renfro LA, Carlin BP, Sargent DJ. Bayesian adaptive trial design for a newly validated surrogate endpoint. Biometrics 2012;68:258-67. [PubMed]


