Combinatorial strategies of radiotherapy and immunotherapy in nasopharyngeal carcinoma
Introduction
Head and neck cancers constitute a global healthcare burden; in 2012, more than 581,283 new diagnoses and 297,181 deaths were reported worldwide (1). Historically, the common etiological risk factors for head and neck cancers include smoking and chronic alcohol abuse; however, recent times have witnessed the emergence of a human papilloma virus (HPV)-associated head and neck cancer phenomenon, which has altered the landscape of this disease (2). Apart from contributing to an acute rise in head and neck cancer incidences, it is well established that HPV-associated head and neck cancers represent a distinct clinical disease compared to tumors induced by other carcinogens. In particular, HPV-associated oropharynx squamous cell carcinoma (HPV+ OPSCC) are more sensitive to chemotherapy and radiotherapy (RT) than HPV- tumors, which is likely due to intrinsic differences in tumor biology (3). Different molecular drivers of tumor aggression are also involved between these subtypes, since HPV+ OPSCC has a proclivity for distant metastatic than local relapses. As a result of the differential prognoses, HPV+ OPSCC are now staged by an exclusive TNM classification system, which interestingly, is not too dissimilar to the system employed in another viral-associated head and neck cancer—nasopharynx cancer (NPC) that is associated with the Epstein-barr virus (EBV) (4). Of note, the consideration of nodal metastasis in the neck is now consistent between both tumor types; the convergence of clinical phenotypes of HPV+ OPSCC and EBV+ NPC might suggest common molecular pathways linked to the host immune response that underpin tumorigenesis and aggression in head and neck cancers that are virally induced. However, the oncogenic potential of HPV is conventionally attributed to the inactivation of TP53 and RB1 tumor suppressor genes by the overexpression of the E6 and E7 viral proteins, respectively, while much less is known regarding the modulation of the host immune response during the malignant transformation process (5). Likewise, while clonal expansion of EBV-infected epithelium is invariably observed in NPC, the process for latent EBV activation infection in the epithelial cells is less well characterized. Nonetheless, recent evidence from comprehensive molecular profiling of NPC highlights that regulation of the inflammatory process is in fact a critical determinant of tumor progression; this centers on the nuclear factor NF-κβ pathway (6,7). Additionally, mutational status of the major histocompatibility complex (MHC) class of genes was shown to be associated with prognosis, thus supporting the importance of immune regulation on the tumor behavior in EBV + NPC. Collectively, these observations argue for the consideration of immunotherapeutic approaches in these viral-associated head and neck cancer subtypes.
Search strategy
To identify references for this review, we searched PubMed and MEDLINE databases for articles published in English between Jan 1, 2000, and December 1, 2017 with the following key terms: “radiation”, radiotherapy”, “radiosurgery”, “ablative radiotherapy”, “SABR”, “SBRT”, “immunotherapy”, “checkpoint blockade”, “CTLA-4”, “PD-L1”, “PD-1”, “nasopharyngeal carcinoma”, “Epstein-Barr virus” and “clinical trials”. Selected references were judged based on relevance to the clinical and mechanistic focus of this review, and mainly comprised of highly cited publications from the recent 5 years and randomized clinical trials. Older seminal work were also included if they were widely referenced and highly regarded. Abstracts of recent relevant medical conferences were also included to provide the latest updates.
Clinical evidence for immunotherapy in EBV + NPC
Several clinical trials have been conducted to test the efficacy of various immunotherapeutic strategies in NPC (Table 1). One such approach entails targeting the commonly expressed virus-coded proteins, such as Epstein-Barr virus nuclear antigen 1 (EBNA1) and latent membrane proteins (LMP1 and LMP2); this has led to strategies like adoptive transfer of LMP2-specific cytotoxic T-lymphocytes (CTLs) and dendritic cell-based vaccines to LMP2, which have demonstrated clinical efficacy in recurrent/metastatic NPC (8-10). For example, in a phase I trial, NPC patients were vaccinated with a modified vaccinia Ankara (MVA)-based vaccine that encoded a functionally inactive fusion protein of full length LMP2 and the C-terminal half of EBNA1, which resulted in markedly increased T cell expansion to EBNA1 and LMP2 in 74% of patients (ClinicalTrials.gov, NCT01094405).
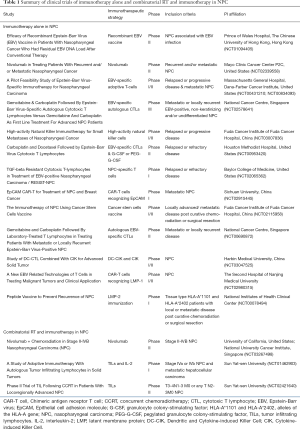
Full table
More recently, there has been great enthusiasm in an alternative immunotherapeutic strategy of targeting common immune checkpoint signals that are modulated by regulatory T cells (Treg) and programmed death-1 and -ligand 1 interaction (PD-1 and PD-L1). Inhibition of these signals has resulted in better than expected responses in several treatment-naïve and -refractory tumor types (11,12). Likewise in NPC, modest responses of 20–30% from nivolumab and pembrolizumab (anti-PD-1 antibodies) have been observed in patients with treatment-resistant recurrent disease, which is unsurprising given that EBV-associated overexpression of LMP1 and IFN-γ is known to drive the corresponding expression of PD-L1 on the tumor epithelial cells (13). Of note, KEYNOTE-028 was a phase 1b trial investigating the efficacy of pembrolizumab (an anti-PD1 antibody) in patients with recurrent NPC; in this trial, patient recruitment was limited to individuals harboring tumors with ≥1% PD-L1 expression. Response rates (including partial responses and stable disease) were 26% (7 of 27) and 52% (14 of 27), respectively (14). Likewise, a companion phase II trial looking at another anti-PD1 antibody—nivolumab also demonstrated comparable response rates; 19% (8 of 43) and 33% (14 of 43) of patients exhibited partial response and stable disease, respectively (15). Collectively, these preliminary results arising from both tumor antigen-specific and agnostic immunotherapeutic strategies favor the notion of EBV+ NPC as a highly immune-enriched tumor, and therefore supports extending the consideration of immunotherapy to the following clinical scenarios: (I) in combination with chemotherapy in treatment-naïve metastatic NPC, and (II) in patients with advanced disease, whom are presently managed by definitive chemo-radiotherapy alone. The latter is particularly appealing given the emerging scientific evidence supporting the interplay between RT and the tumor-host immune response; this thus widens the possibility of harnessing the immune response to target radioresistant and occult metastatic tumor clones (16,17). However, it would also seem that the nature of immune response depends on the RT quality and dose (18), and as such would imply a need to cater the therapeutic approach to the specific clinical scenario. For example, there is early evidence supporting the concept that a subset of patients with oligometastatic NPC may be “cured” by combining definitive chemo-RT with local therapy to the few metastatic lesions, either with surgery or ablative RT (19). In this instance, a different synergy between RT and immunotherapy may be desired, whereby immunotherapy is employed to induce an abscopal systemic immune anti-tumor cytotoxicity (20). A comprehensive understanding of the RT-induced immune response is thus needed, so as to design the optimal therapeutic strategy that is individualized to the specific clinical indication.
The RT-induced immune repertoire
The conventional model of how RT exerts its anti-tumor effects is based on direct and indirect induction of DNA damage, and the inability of the cell to repair lethal DNA double-strand breaks (DSBs). These cellular responses can be modeled closely using the linear-quadratic (L-Q) equation to derive the biological effective dose (BED), but the accuracy of this dose conversion is affected when larger doses per RT fraction (≥6 Gy) are utilized (21,22). This would thus suggest that the RT-response is modulated by other molecular mechanisms at different RT doses (23). Experimental evidence now supports the concept that RT alters the immune contexture of a tumor, promoting a host anti-tumor immune reaction through a number of mechanisms. For example, the accumulation of micronuclei, formed as a result of cells harboring residual DSBs and progressing through mitosis, induces a cytosolic DNA damage response that activates type-I interferon (IFN-I) via the cyclic GMP-AMP (cGAMP) synthase (cGAS) and its downstream adaptor stimulator of interferon genes (STING) pathway (18,24,25). Activation of IFN-I then leads to optimal dendritic cell recruitment and cross-priming of effector T cell, with the end result being a tumor microenvironment (TME) that is immune-enriched and mimics an in situ anti-tumor vaccine. Additionally, RT induces immunogenic cell death (ICD), whereby immunostimulatory tumor-associated antigens and damage-associated molecular patterns (DAMPs) such as ATP, calreticulin and HMGB-1 are released from dying tumor cells, thus facilitating the recruitment, activation and maturation of dendritic cells (26). T cell attracting chemokines such as CXCL-9 and CXCL-10 are also released during ICD, and these signals increase the density of tumor-infiltrating lymphocytes (TILs) within the tumor (27). Apart from stimulating the immune microenvironment, RT can also induce a temporary overexpression of MHC Class-I and Fas receptors on the tumor cells, which increases their vulnerability to effector T cell killing.
Perhaps unsurprisingly, the RT-immune response is comprised of a network of immune-stimulatory and -inhibitory signals; upregulation of immune checkpoint proteins, such as PD-1/PD-L1 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), along with increased levels of immunosuppressive Treg within the tumor have been observed post-RT (28). Contrary to the intrinsic radiosensitivity of other lymphoid cells, the Treg subpopulation is radioresistant due to an increased ratio of antiapoptotic-to-proapoptotic proteins (29). This balance of immune-stimulatory and -inhibitory signals probably explains why RT alone is usually unable to induce anti-tumor immune-mediated cytotoxicity. It is therefore logical that combinatorial strategies are needed to more effectively exploit the anti-tumor immune response by RT. Alluding to the clinical scenarios highlighted in the preceding section, we summarize some potential RT-immunotherapeutic strategies that may improve the therapeutic ratio of RT in these patients, along with the corresponding mechanistic concepts for each approach.
Overcoming radioresistance in de novo and recurrent T3-4 NPC
With the advent of precision RT techniques like intensity-modulated RT, the community has witnessed substantial improvements in tumor control even for patients with locally advanced NPC (30). However, about 10–20% of patients still harbor radioresistant disease; a proportion of which may be attributed to inadequate RT dose intensity because of proximity to critical normal organs. To explore the role of immunotherapy as a potential radiosensitizer, several laboratories have combined RT with immune checkpoint blockade therapies, and demonstrated improved tumor control in preclinical in vivo 4T1 breast cancer and MC38 colorectal cancer models (31,32). Importantly, these preliminary findings translated to the clinic; notably, in a recent report of the PACIFIC study (a randomized controlled phase III trial of sequential treatment with a PD-L1 inhibitor, durvalumab post-platinum-based chemoRT in stage III non-small cell lung cancer), treatment intensification with durvalumab significantly prolonged progression-free survival (16.8 vs. 5.6 months in the placebo arm) in these patients (33). Translating these findings to locally advanced NPC, it is appealing to test if immunotherapy can be an effective adjunct to RT, especially in T3-4 cases where dose intensity is compromised to preserve normal organ function. Adjuvant immunotherapy post-chemoRT could also activate the systemic immunity to target occult metastatic tumor clones.
On this note, depending on the desired synergistic effects between RT and immunotherapy, we argue that sequencing and type of immunotherapy both play crucial roles in optimizing clinical responses (Figure 1). Foremost, neoadjuvant immunotherapy serves to “prime” the tumor immune microenvironment, enriching for effector and memory T cells within the tumor to exert their anti-tumor cytotoxic effects. This may be particularly relevant in NPC, since the baseline microenvironment of these tumors is typically characterized by syncytial sheets of tumor epithelial cells that are admixed with rich lymphocytic infiltrates. In support of this sequencing approach, Young et al. demonstrated the most pronounced responses when anti-CTLA-4 was given as a neoadjuvant therapy before RT in mice bearing CT26 colorectal tumors; complete tumor regression and 100% survival were achieved in all tumor-bearing mice when treated with anti-CTLA-4 7 days before RT. In contrast, complete tumor cures were observed in only 50% (3 of 6) of the tumor-bearing mice which received anti-CTLA-4 1 or 5 days post-RT; median survival of the mice was 92 and 53 days, respectively (34). Binding of CTLA-4 to B7 ligand of antigen presenting cells (APCs) also triggers inhibitory signals causing anergy of effector T cells and clonal contraction (35). Targeting CTLA-4 therefore not only reverses these immunosuppressive effects, but also contributes to further immune “priming” by Fc-dependent depletion of Treg cells within the tumor. Together, these effects induce an immuno-conversion from a “cold” to a “hot” tumor.
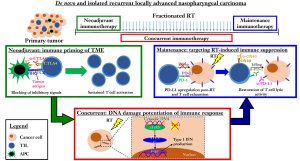
Next, concurrent RT and immunotherapy exploits a separate mechanism that is linked to RT-induced DNA damage (Figure 1). As previously explained, the accumulation of cytosolic dsDNA fragments in the irradiated tumor cell activates IFN-I via the cGAS-STING pathway (18,25). This phenomenon is cumulative with increasing RT dose (per fraction), but nonetheless appears to have a plateau effect beyond 10 Gy. In fact, it was recently shown by Vanpouille-Box and colleagues that above this dose, three-prime repair exonuclease 1 (Trex1) is induced, which causes rapid degradation of cytosolic DNA, precluding the activation of cGAS-STING pathway and subsequent IFN-I production (24). This highlights the importance of developing a sound mechanistic understanding of the RT-induced immune response, so that the optimal RT dose and fractionation regime can be designed for maximum therapeutic efficacy in combination with immunotherapy.
RT-induced ICD also has the potential to drive immuno-conversion by modulating the microenvironment to enhance T cell infiltration, but this however often associated with corresponding upregulation of PD-L1 expression on surviving tumor cells (36). PD-L1 upregulation has been attributed to DSB-dependent damage signaling mediated by ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related protein (ATR) and checkpoint kinase 1 (Chk1) kinases (37). Binding of PD-L1 on tumor cells to PD-1 on effector T cells results in T cell exhaustion and immune suppression despite of the increased T cell infiltration post-RT. Hence, maintenance immunotherapy can be deployed to target such RT-induced immune suppression in “hot” tumors. For example, a study by Dovedi et al. found that inhibition of PD-1/PD-L1 axis concurrently or immediately after RT reverses T cell exhaustion and increases T cell lytic activity (26,38). Young et al. also found that immunotherapy with agonistic anti-OX40 worked best when given one day after RT during the post-RT window of increased antigen presentation (34). This was because resting T cells did not express OX-40; the expression of OX-40 was transiently induced on activated T cells following antigen stimulation, with peak expression observed between 24 h to 5 d following initial stimulation (39). The binding of OX-40 on effector T cells with the natural OX-40L ligand or agnostic anti-OX40 antibody promoted their survival and expansion (40). Treg cells also constitutively express OX-40, and OX-40 stimulation on Treg cells have been demonstrated to inhibit their suppressor functions, thereby enhancing immune response (41). In summary, each of the above sequential approaches potentiates the RT response through independent pathways, but much more work is needed to define the optimal sequencing, type and cycles of immunotherapy to achieve the maximum potentiation of RT. Moreover, while it would seem that combination RT-immunotherapy is better tolerated compared to chemo-RT, some retrospective studies have also highlighted the potential for increased neurological toxicities post-stereotactic radiosurgery for brain metastases (42). Ongoing clinical trials (Table 1) investigating for different combinatorial strategies will yield insights on other novel pathways of immune modulation by RT, including the differential effects on tumor and normal tissues.
Exploiting the systemic abscopal effect of immunotherapy in metastatic NPC
In widespread metastatic NPC, RT is often administered for symptom relief using profound or moderate hypofractionation regimens (single 8–10 Gy or 20–30 Gy in 5 fractions); progressively, in patients with oligometastatic disease, local therapy to the metastases using either stereotactic ablative RT (30–50 Gy in 3–5 fractions) or surgical excision is being considered, given the long-term control observed in some patients (43). Such ablative doses of RT have been shown to induce cellular and tissue effects amounting to an immuno-conversion of the tumor into an in situ vaccine; this process is believed to be crucial in driving an acute systemic anti-tumor response, resulting in what is known as an abscopal effect—regression of tumors outside of the radiation field. However, abscopal responses induced by RT alone are infrequent (<10%) (44); reasons for this include physical anatomical barriers and interlesional (tumoral) antigen heterogeneity that may prevent an anti-tumor T cell response in the non-irradiated lesions. Nonetheless, unpublished data from our group and others have revealed a systemic immune response post-RT that is characterized by acute shifts in CD8 and CD4 (mainly effector memory subset of) T cells within 1 week of irradiation; interestingly, these observations are also diverse between patients. These findings raise the possibility of manipulating this systemic immune response to induce the abscopal effect; in this space, combining immune checkpoint blockade is the most commonly tested strategy at present, albeit with some success (45,46). For example, Demaria et al. investigated the combinatorial treatment of RT and anti-CTLA-4 in mice bearing 4T1 mammary carcinoma, and observed that combining both therapies resulted in the greatest reduction of the number of lung metastases (5 metastatic nodules per mouse) compared to RT or anti-CTLA-4 treatment alone (17 and 14 nodules, respectively) (47). This corresponded to a significantly longer mean survival of 49 days for the group that received combinatorial treatment, compared to 41 and 43 days for the individual treatments, respectively. Mechanistically, the improved metastatic control is attributed to the conversion of tumors into an in situ vaccine by RT, which involves the release of tumor-associated antigens as a result of RT-induced ICD (Figure 2). These tumor-associated antigens are taken up by APCs, which then traffic to draining lymph nodes for presentation and T cell activation. By combining RT with CTLA-4 blockade, sustained activation and proliferation of tumor-specific T cells are achieved, which then traffic to the distant metastases outside of the radiation field, causing immune-mediated killing of these lesions.
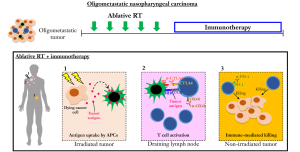
Similarly, combinatorial treatment of RT and anti-PD1 antibodies has been observed to produce abscopal effects in mouse models of melanoma, renal cell carcinoma and other cancers, along with clinical evidence supporting this approach (48,49). A study by Sharabi et al. showed that PD-1 blockade in combination with RT increases the number of tumor-specific effector memory T cells (50). In another proof-of-principle trial studying the combination of RT and granulocyte-macrophage colony-stimulating factor (GM-CSF), abscopal effect was reported in 26.8% (11 of 41) of patients with metastatic solid tumors (51). Here, GM-CSF, a potent cytokine, promotes differentiation of dendritic cells, facilitating greater capture of tumor-associated antigens and migration of antigen-loaded dendritic cells to the lymph nodes, leading to enhanced presentation to T cells (52). In summary, it can be observed that combinatorial RT and immunotherapy serves to create an enhanced and sustained anti-tumor immune response that can aid in the control of distant tumors via various distinct mechanisms depending on the specific immunotherapy that is utilized. Nonetheless, given the complex interplay between the host immune system and the tumor immune microenvironment, it is unsurprising that mechanisms of resistance to combinatorial immune checkpoint blockade and RT have been described. Referring to an elegant study by Twyman-Saint and colleagues, they demonstrated that tumors eventually developed a compensatory escape mechanism via PD-1/PD-L1 overexpression to resume tumor-associated immune blockade following treatment with anti-CTLA4 and RT; treatment with dual checkpoint blockade and RT consequently resulted in a significantly prolonged duration of response and survival (53). It is therefore necessary to obtain a complete understanding of the molecular pathways underpinning response and resistance to immunotherapy and combinatorial strategies (54).
The optimal RT dose and fractionation required to stimulate systemic anti-tumor immunity and the abscopal effect is still under active investigation. RT regimens of 8 Gy ×3 fractions or 6 Gy ×5 fractions in combination with immunotherapy were reported to result in an increased likelihood of abscopal responses compared with a single 20 Gy dose. This may be explained by the activation of the DNA exonuclease Trex1 and rapid degradation of cytosolic DNA at high radiation doses, precluding cGAS-STING activation and IFN-I production. In clinical practice, most ablative RT regimens used to target metastatic lesions fall within the range of 6–8 Gy per fraction (55,56), which would be supported by the findings from preclinical models. Ongoing clinical and companion translational studies will help to inform on the optimal dose and fractionation.
Future directions: selecting the “right” patient and immunotherapeutic agent
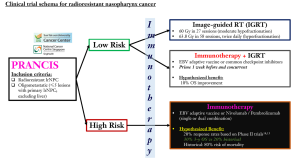
Looking ahead, it is obvious that optimizing patient selection is crucial given the immune diversity of cancer patients. Moreover, the combination of RT and immunotherapy may not be suitable for all patients, given the potential for increased toxicities (42). In the space of radioresistant NPC, our group has developed a novel clinical tool—PRANCIS (Predicting RAdioresistant Nasopharynx CacrcInoma Survival) that accurately predicts survival and treatment-related complications with a repeat course of RT (http://prancis.medlever.com) (57). Using PRANCIS, patients could be stratified into two risk-groups with disparate survival outcomes of 75% (low-risk) vs. 25% (high-risk) at 3 years post-RT. Here, we present a novel clinical trial concept where PRANCIS can be utilized for optimal patient selection to either a combinatorial immunotherapy-RT arm or immunotherapy alone (Figure 3). In addition, we further allow for the choice of immunotherapy between adaptive vaccine against EBV-encoded proteins and checkpoint inhibitors, given the efficacy of both agents in the metastatic setting (14,15). As such, we may gain a deeper understanding on the differential immune-related pathways between adaptive vaccines and checkpoint inhibitors when combined with RT that underpin the exaggerated clinical responses. It is plausible that radioresistant NPC tumors harbor a substantially higher mutational burden than treatment-naïve tumors, and therefore may respond preferentially to checkpoint inhibitors (7).
Conclusions
RT has evolved in the last decade to achieve the optimal delivery of radiation for maximum local tumor control, along with minimizing toxicities; this is particularly evident in NPC. The advent of immunotherapy has now triggered the growing enthusiasm of using RT to invoke both local and systemic immune response. Current evidence for the potential efficacy of combinatorial treatment with RT and immunotherapy is largely derived from in vitro and animal models, which may not necessarily translate to responses in the clinic. Robust hypothesis-testing clinical trials with companion translational investigation on biological surrogates of response and eventual resistance are therefore needed to drive these experimental regimes into the clinic. Here, we argue that combinatorial RT-immunotherapy may benefit NPC patients across disease states; in particular, the role of immunotherapy in the locally advanced subset is appealing, since these patients succumb to radioresistant local and systemic metastatic relapses. In addition, companion accurate predictive tools are needed to identify suitable patients who are likely to benefit most from this combinatorial approach.
Acknowledgements
We thank all members of the M.L.K.C. laboratory for contributions to the scientific content.
Funding: MC is supported by the National Medical Research Council Singapore Transition Award (#NMRC/TA/0030/2014), and the Duke-NUS Oncology Academic Program Proton Research Program.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. In: GLOBOCAN 2012 v1.0. Lyon, France: International Agency for Research on Cancer. 2013. Aavailable online: http://globocan.iarc.fr/Default.aspx
- Fakhry C, Gillison ML. Clinical Implications of Human Papillomavirus in Head and Neck Cancers. J Clin Oncol 2006;24:2606-11. [Crossref] [PubMed]
- Fakhry C, Westra WH, Li S, et al. Improved Survival of Patients With Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J Natl Cancer Inst 2008;100:261-9. [Crossref] [PubMed]
- O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol 2016;17:440-51. [Crossref] [PubMed]
- Ganguly N, Parihar SP. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J Biosci 2009;34:113-23. [Crossref] [PubMed]
- Zheng H, Dai W, Cheung AK, et al. Whole-exome sequencing identifies multiple loss-of-function mutations of NF-κB pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci 2016;113:11283-8. [Crossref] [PubMed]
- Li YY, Chung GT, Lui VW, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat Commun 2017;8:14121. [Crossref] [PubMed]
- Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother 2010;33:983-90. [Crossref] [PubMed]
- Chia WK, Wang WW, Teo M, et al. A phase II study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol 2012;23:997-1005. [Crossref] [PubMed]
- Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 2014;22:132-9. [Crossref] [PubMed]
- Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 2016;7:73068-79. [PubMed]
- Xu R, Wang F, Li Q, et al. Recombinant humanised anti-PD-1 monoclonal antibody (JS001) treatment for patients with refractory or metastatic nasopharyngeal carcinoma: preliminary results of an open-label, phase 1b/2, clinical study. Lancet Oncol 2017;18:S1. [Crossref]
- Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014;5:12189-202. [Crossref] [PubMed]
- Hsu C, Lee SH, Ejadi S, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol 2017;35:4050-6. [Crossref] [PubMed]
- Ma BB, Goh BC, Lim WT, et al. Abstract CT076: Multicenter phase II study of nivolumab in previously treated patients with recurrent and metastatic non-keratinizing nasopharyngeal carcinoma - Mayo clinic Phase 2 Consortium P2C-MN026, NCI9742, NCT02339558. Cancer Res 2017;77:CT076. -CT. [Crossref]
- McBride WH, Ganapathy E, Lee MH, et al. A perspective on the impact of radiation therapy on the immune rheostat. Br J Radiol 2017;90:20170272. [Crossref] [PubMed]
- Weichselbaum RR, Liang H, Deng L, et al. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol 2017;14:365-79. [Crossref] [PubMed]
- Formenti SC. Optimizing Dose Per Fraction: A New Chapter in the Story of the Abscopal Effect? Int J Radiat Oncol Biol Phys 2017;99:677-9. [Crossref] [PubMed]
- Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet 2016;387:1012-24. [Crossref] [PubMed]
- Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516-24. [Crossref] [PubMed]
- Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:847-52. [Crossref] [PubMed]
- Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 2008;18:240-3. [Crossref] [PubMed]
- Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 2014;88:254-62. [Crossref] [PubMed]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [Crossref] [PubMed]
- Harding SM, Benci JL, Irianto J, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017;548:466. [Crossref] [PubMed]
- Van Limbergen EJ, De Ruysscher DK, Olivo Pimentel V, et al. Combining radiotherapy with immunotherapy: the past, the present and the future. Br J Radiol 2017;90:20170157. [Crossref] [PubMed]
- Formenti SC, Demaria S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J Natl Cancer Inst 2013;105:256-65. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Liu S, Sun X, Luo J, et al. Effects of radiation on T regulatory cells in normal states and cancer: mechanisms and clinical implications. Am J Cancer Res 2015;5:3276-85. [PubMed]
- Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 77:16-21. [Crossref] [PubMed]
- Ruocco MG, Pilones KA, Kawashima N, et al. Suppressing T cell motility induced by anti–CTLA-4 monotherapy improves antitumor effects. J Clin Invest 2012;122:3718-30. [Crossref] [PubMed]
- Pilones KA, Kawashima N, Yang AM, et al. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res 2009;15:597-606. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Young KH, Baird JR, Savage T, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One 2016;11:e0157164. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015;1:1325-32. [Crossref] [PubMed]
- Sato H, Niimi A, Yasuhara T, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun 2017;8:1751. [Crossref] [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res 2014;74:5458-68. [Crossref] [PubMed]
- Croft M, So T, Duan W, et al. The Significance of OX40 and OX40L to T cell Biology and Immune Disease. Immunol Rev 2009;229:173-91. [Crossref] [PubMed]
- Linch SN, McNamara MJ, Redmond WL. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front Oncol 2015;5:34. [Crossref] [PubMed]
- Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood 2007;110:2501-10. [Crossref] [PubMed]
- Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and Stereotactic Radiosurgery Versus Stereotactic Radiosurgery Alone for Newly Diagnosed Melanoma Brain Metastases. Am J Clin Oncol 2017;40:444-50. [Crossref] [PubMed]
- Chan OS, Ngan RK. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol 2014;50:791-7. [Crossref] [PubMed]
- Hong JC, Salama JK. The expanding role of stereotactic body radiation therapy in oligometastatic solid tumors: What do we know and where are we going? Cancer Treat Rev 2017;52:22-32. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 41:503-10. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Demaria S, Kawashima N, Yang AM, et al. Immune-Mediated Inhibition of Metastases after Treatment with Local Radiation and CTLA-4 Blockade in a Mouse Model of Breast Cancer. Clin Cancer Res 2005;11:728-34. [PubMed]
- Park SS, Dong H, Liu X, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res 2015;3:610-9. [Crossref] [PubMed]
- Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res 2015;75:2232-42. [Crossref] [PubMed]
- Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1–Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 2015;3:345. [Crossref] [PubMed]
- Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795-803. [Crossref] [PubMed]
- Yan WL, Shen KY, Tien CY, et al. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017;9:347-60. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Syn NL, Teng MWL, Mok TSK, et al. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 2017;18:e731-e41. [Crossref] [PubMed]
- Chua KLM, Sin I, Fong KW, et al. Stereotactic body radiotherapy for early stage lung cancer-historical developments and future strategies. Chin Clin Oncol 2017;6:S20. [Crossref] [PubMed]
- Chua KLM, Tan DBH, Chua MLK, et al. The promise of stereotactic body radiotherapy-next phase of integration into oncological practice. Chin Clin Oncol 2017;6:S8. [Crossref] [PubMed]
- Li YQ, Tian YM, Tan SH, et al. Prognostic Model for Stratification of Radioresistant Nasopharynx Carcinoma to Curative Salvage Radiotherapy. J Clin Oncol 2018;36:891-9. [Crossref] [PubMed]


