Immunoregulatory antigens—novel targets for cancer immunotherapy
Cancer drug development has traditionally focused on approaches that directly eliminate cancer cells—surgery, radiation therapy, chemotherapy, hormonal therapy, targeted therapy and more recently, immunotherapy (defined as an approach to harness the immune system to fight cancer). The latter has been based on the original immune surveillance hypothesis (1) that postulates that the immune system could recognize and reject cancer cells as being foreign, in the same way that it reacts against microbial agents and transplant organs. In order for immunotherapy to be successful, it needs to be able to activate and expand a pool of tumor-specific T cells either from the naïve repertoire or the existing tumor-specific T cells that may have been dormant or rendered anergic. To accomplish this goal many approaches and platforms have historically been explored: direct activation of antitumor immunity with cancer vaccines (comprising tumor antigens in various forms) or recombinant cytokines, or by infusing tumor-specific immune cells (2,3). However, despite the success in increasing the frequency and activity of tumor-specific T cells and demonstration of promising clinical outcomes in some studies (4,5) many were met with disappointing results, owing to failure to ensure that tumor-specific T cells could home to tumors and/or exert their function within the tumor. Indeed, the major learning from these failed studies is that tumor-induced immunosuppressive mechanisms in the tumor microenvironment (TME) are one of the main reasons for the limited success of current immunotherapeutic approaches (2). Thus, whilst the extensive refinement in immunotherapeutic tools and methodologies has ensured enhancement of the frequency and activity of tumor-specific T cells, this alone has been insufficient to break immune tolerance to the cancer.
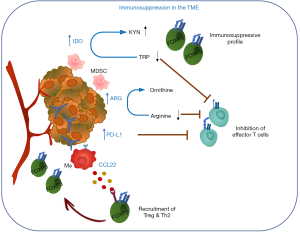
There are many regulatory mechanisms and negative feedback loops that ensure an appropriate termination of an immune response to maintain homeostasis and prevent chronic inflammation. These include the upregulation of inhibitory surface receptors and ligands, induction of distinct sets of metabolic enzymes or chemokines and cytokines that recruit regulatory immune cells or remodel immune cell subsets to a regulatory profile. These molecules are transiently induced in normal tissues in response to inflammation or stress but often hijacked by malignant cells and as a result constitutively expressed in various cancer tissues where they contribute to immunosuppressive TME and immune evasion of cancer. For example, the important role of metabolic enzymes such as indoleamine 2,3-dioxygenase (IDO: IDO1 and IDO2) and tryptophan 2,3-dioxygenase (TDO) in cancer tolerance has been well established (6) (Figure 1). Both IDO and TDO catalyze the degradation of the essential amino acid tryptophan (TRP) to kynurenine (KYN). TRP depletion and the accumulation of KYN metabolites lead to proliferative arrest of effector T cells as well as induction of regulatory T cells (Treg) differentiation and activity (7) and recruitment of myeloid-derived suppressor cells (MDSCs) (8). IDO is expressed in a number of human solid tumors and hematological malignancies and its activity has been shown to correlate with a poor prognosis and reduced survival (9). Arginase (ARG1 and ARG2) catalyzes the conversion of arginine to ornithine and urea in the hepatic urea cycle but also plays a role in the immune system (10). ARG1 is inducible in M2 macrophages, MDSCs, DCs and granulocytes and ARG-dependent arginine depletion leads to downregulation of the TCRζ chain and suppression of T cell and natural killer (NK) cell proliferation, which in a cancer setting supports cancer immune evasion and inhibition of immune effector functions. Both ARG1 and ARG2 have been found expressed in a variety of malignancies (11-14). The interaction of checkpoint proteins programmed death-1 (PD-1/CD279) and its ligand PD-L1 (CD274) represents another good example of an immune regulatory mechanism hijacked by cancer cells. PD-L1 expression is upregulated by pro-inflammatory stimuli (e.g., IFNγ) (15) and functions through induction of signalling pathways downstream of PD-1 (expressed on activated T cells) that inhibit T cell proliferation to prevent chronic inflammation (16). PD-L1 is aberrantly expressed on tumor cells as well as cells of the microenvironment in many different cancer types rendering effector T cells inoperative (17-19). Moreover, a renewed focus on the immunosuppressive adenosinergic pathway downstream of tumor hypoxia has led to development of novel antitumor strategies targeting ectonucleases and adenosine receptors (20). Finally, induction of Treg differentiation and the recruitment to the TME is another strategy employed by malignant cells to evade the host’s immune system. For example, CCL22, a macrophage-derived chemokine known to be involved in Treg recruitment through binding to its cognate receptor CCR4 expressed on the surface of Tregs and highly expressed in different tumor tissues (21-23), has been shown to correlate with Treg infiltration and is associated with histological features correlating with a poor prognosis in breast cancer (24).
Checkpoint inhibition and beyond
A breakthrough in cancer immunotherapy arrived in 2010 when Dr. Stephen Hodi’s group in Boston demonstrated in a randomized controlled trial that treatment with ipilimumab, an antibody that targets the T cell checkpoint protein CTLA-4, significantly improved overall survival, and provided long-term survival benefit among patients with metastatic melanoma (25). The results led to the first approval from the US Food and Drug Administration (FDA) for an immune checkpoint blockade approach in 2011. This study clearly demonstrated that an anti-tumor immune response can be efficiently boosted in human, not by targeting tumor cells directly but by targeting the immune system in order to break the cancer tolerance [the “paradigm shift in oncology” (26)]. This initial demonstration was subsequently and quickly followed by clinical testing of similar approaches but most notably drugs blocking the distinct checkpoints PD-1 and its major ligand PD-L1, which have so far shown great promise in treating many diverse cancer types, including advanced melanoma, non-small cell lung carcinoma, Hodgkin’s lymphoma, Merkel cell carcinoma and tumors with a genetic marker of high mutational burden termed microsatellite instability (MSI) (27-29).
Whilst the clinical studies with checkpoint blockade approaches have undoubtedly made a major step forward in immuno-oncology, our understanding of the underlying mechanisms is still at an early phase, with many unanswered questions. Crucially, only a minority of patients with solid tumors exhibit maximal benefit from the checkpoint blockade, where significant clinical responses are restricted primarily to melanoma, non-small cell lung cancer (NSCLC), renal and bladder cancers but less successful in other cancers such as pancreatic, colorectal and ovarian cancer (28,30-32).
Among multiple factors that impact the outcome of checkpoint blockade treatment, accumulating evidence suggests that the maximal therapeutic effect of this approach is largely dependent on the presence of pre-existing tumor-specific CD8+ T cells (33), which in turn closely correlates with the presence of neoantigens as a result of cancer mutations (34-36). Cancers that exhibit active tumor-specific T cell immunity with infiltrating lymphocytes into tumor sites are often termed “hot” tumors, whereas those without such pre-existing responses are termed “cold” tumors (37). For example, only about half of patients with colorectal cancer show evidence of local tumor-specific T cell immunity (38,39).
As the clinical responses to checkpoint blockade are linked to the presence of T cell immunity to cancer-specific mutations, multiple approaches have been considered and tested to expand anti-tumor T cells in conjunction with checkpoint inhibition (3,40,41). Indeed, the success of combination therapies utilizing immune checkpoint inhibition in poorly immunogenic tumors in mouse models have been acknowledged for many years (42-44), and such have been successfully translated into clinical studies (45). In particular, there is a renewed focus on personalized therapies to target neoantigens derived from tumor mutations based on accumulating evidence that the number of mutations in individual tumors correlates directly with the effectiveness of checkpoint blockade (46-49). The necessity of combination therapeutic approaches in established cancer is highlighted also in other strategies that target immunosuppressive mechanisms, such as small molecule inhibitors (SMIs) that target IDO (9). In fact, accumulating evidence supports that combination of immunotherapeutic strategies as a key strategy to penetrate the complex relationship between established tumor and the immune system (3,41,50).
The new kid on the block in cancer immunotherapy: immunoregulatory antigens as cancer vaccine targets
As described above, conventional cancer therapies specifically targeted cancer cells themselves, and vaccination strategies are aimed at eliciting an antigen-specific T cell response against various tumor antigens [e.g., preferentially elevated and amplified compared to normal tissue (e.g., Her2/neu), lineage-specific (e.g., MART-1, gp100), oncoviral antigen (e.g., EBV, HPV), or a mutated antigen (e.g., Mum-1, CDK4) that is unique to the cancer or even the patient]. However, efficacy of these treatments has been limited in part due to an immunosuppressive TME providing the malignant cells a means to escape elimination by specific immune effector cells. The recent success of immunotherapeutic approaches targeting immunosuppression in the TME, i.e., checkpoint inhibitors (anti-PD-1, anti-PD-L1, anti-CTLA4) or SMIs (i.e., IDO inhibitor 1-MT) strongly support the role of immunosuppression in cancer progression and underline the need to remove the “immunological break” to enable immune effector cells to attack the cancer. Taking this into account, an intriguing novel approach to cancer immunotherapy has been postulated—namely, a cancer vaccination to direct immune responses against immunoregulatory cells that impede effective anti-tumor T cell responses. This is based on the recent demonstration of the existence of naturally occurring, pro-inflammatory T cells against immunoregulatory proteins, such as IDO, PD-L1 and TDO present in the periphery and among TILs of cancer patients and, to a lesser degree, in healthy individuals (51,52). Thus, contrary to the central dogma in immunology that T cells expressing a TCR with a high affinity towards a self-peptide/HLA complex undergo clonal deletion in the thymus, “self-reactive” repertoires of T cells were found, and not restricted to autoimmune pathologies (53). Thus, high frequency of peripheral CD4+ and CD8+ T cells that recognize various immunoregulatory proteins [IDO (54-56), TDO (57), PD-L1 (58), FOXP3 (59), CCL22 (60) and Arginase (Martinenaite, submitted)] are readily detectable ex vivo in blood from both cancer patients and healthy individuals. Surprisingly, these T cells exhibit cytotoxic activity against both target-expressing cancer cells or target peptide-loaded cells in vitro (54,58,60), as well as CD25hi FOXP3+ CD127− Tregs (59). The T cells also indirectly augment effector function of other T cells, as simultaneous stimulation of anti-IDO T cells boosted anti-viral immunity against CMV or influenza antigens as well as the response to melanoma-associated antigen MART-1 in vitro (54). In addition, co-stimulation of anti-PD-L1 T cells augments T cell response to a dendritic cell (DC) vaccine (61).
Thus, current hypotheses based on the available data support the notion that these self-reactive T cells against immunoregulatory targets may represent yet another level of immune regulation by “regulating the regulators” i.e., counteracting the immune-suppressive feedback provided by Tregs, MDSCs, regulatory B cells or specific DC subtypes. The expansion of these T cells by vaccination could lead to effective anti-tumor responses by direct killing of immunoregulatory cells contributing to immune suppression (Figure 2). Indeed, preclinical data in various mouse tumor models indicate that vaccination with synthetic peptides encoding immunoregulatory antigens is sufficient to (I) activate and expand immunoregulatory antigen-specific T cells and (II) confer protection from cancer in vivo (unpublished data, personal communication). Given that this approach targets boosting of a pre-existing T cell pool, a simple vaccination approach is unlikely to be met by immunological tolerance.
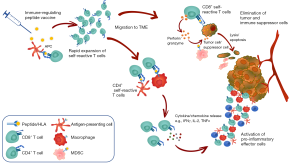
If successfully targeted, a therapeutic vaccination approach to activate these self-reactive T cells can, like the other approaches that target immune suppression (by checkpoint inhibition or SMIs targeting immunosuppressive molecules), contribute to anti-tumor immunity by overcoming the immune suppression and thereby potentiating effective anti-tumor T cell responses. However, unlike other approaches, because these T cells can directly kill the target cells, it could also lead to epitope spreading towards the potential target cells (62) and immunological memory. Importantly, numerous cancer cell types have elevated expression of immunoregulatory proteins and thus cancer cells themselves could also be directly targeted by immunoregulatory antigen-specific T cells. Furthermore, given that these T cells are naturally present in vivo, a mechanism that ensures immune homeostasis to keep these T cells in check must exist—therefore the risk of triggering autoimmune-related adverse events is potentially minimal. Indeed, mice vaccinated with immunoregulatory antigens have shown no signs of toxicity (unpublished data), and data from clinical studies conducted so far demonstrate the safety of this approach (see below).
Tumor antigens to date have been categorized into distinct classes, depending on their expression profile, distribution and mutational status (3,4)—however the focus has always been on antigens expressed by the tumor itself. Given that immunoregulatory antigens can be new targets for cancer immunotherapy, we propose that they could be considered as a new class of cancer vaccine antigens. The obvious advantages of targeting these antigens over other types of antigens are that (I) it negates the requirement for identification of relevant antigens that are specific for the cancer type; (II) the targets are genetically stable, unlike the approach that relies exclusively on antigen expression by tumor cells, and (III) by targeting immune suppression it can potentiate anti-tumor effector T cell responses—thus this approach has a potential to work as a monotherapy in certain cancer settings.
This novel approach to target immune suppression in cancer has already been tested in two clinical trials thus far, where a peptide vaccine targeting IDO-specific T cells was administered as monotherapy in stage III/IV NSCLC patients (63) and in combination with ipilimumab in metastatic melanoma (64) respectively. Additional trials have recently started to evaluate the safety of a vaccine targeting PD-L1-specific T cells in multiple myeloma (NCT03042793) and a combination vaccine that targets both IDO- and PD-L1-specific T cells with nivolumab in metastatic melanoma (NCT03047928). In all of these trials the vaccinations were well tolerated by all patients with no severe toxicity, for administration of up to 5 years in the NSCLC study (ESMO 2017, manuscript in preparation). Given that therapeutic peptide-based vaccinations historically demonstrated minimal toxicity, the safety profile of the immunoregulatory peptide vaccines is unsurprising. It is reassuring to confirm that there are no signs of autoimmunity in the treated patients (manuscript in preparation). In addition, the first clinical trial demonstrated promising clinical results: with a median OS of 25.9 months and long-lasting PR + SD observed in 47% of treated patients. In fact, a follow-up study reveals that two of 15 treated patients remain alive and are maintaining PR and SD status 5 years after receiving the first vaccination, with detectable IDO-specific T cells in the blood (ESMO 2017, manuscript in preparation).
Concluding remarks & future perspectives
Today there is ample evidence to support the existence of self-reactive, immunoregulatory antigen-specific T cells and a rationale to target these T cells as a cancer immunotherapy strategy. An obvious risk for such an approach—potential long-term toxicity due to vaccine-induced autoimmune mechanisms—appears to be minimal, illustrated both in mouse in vivo studies and in human safety clinical trials. Important questions remain as to how and when these T cells are induced or become activated, and to what extent they contribute to immune regulation in physiological conditions. Investigations to address some of the most clinically relevant questions are ongoing in preclinical studies, including (I) what is the relative contribution of direct tumor killing by immunoregulatory antigen-specific T cells (which will inform us the necessity of target expression by tumor cells), (II) would direct and/or indirect modes of target killing lead to antigen epitope spreading and long-term memory, and (III) elucidating the potential benefits of combination strategies with other therapeutic modalities. In future clinical studies, immune monitoring processes encompassing multiple bioassays to detect changes in immune phenotype both at the TME and in the blood, coupled with the clinical efficacy parameters, will provide further guidance to identify patient groups that maximally benefit from this approach.
Acknowledgements
We would like to thank Mrs. Dorrit Andersen and Dr. Eva Ehrnrooth at IO Biotech ApS, Copenhagen. Denmark, for their advice and input into regulatory and clinical aspects of immunotherapy, respectively. We would also like to thank Dr. Alexander Muller at Lankenau Institute for Medical Research, PA, USA and Dr. Samir Khleif at Augusta University, GA, USA for personal communication of mouse in vivo studies, and Professor Inge Marie Svane at CCIT, Copenhagen University Hospital, Herlev, Denmark, for personal communication of NSCLC follow-up study.
Footnote
Conflicts of Interest: MH Andersen and MB Zocca are shareholders and employees of IO Biotech ApS, who has the purpose of developing commercial IDO and PD-L1 vaccines for cancer treatment. MH Andersen is an author of various patent applications based on the use of immunoregulatory antigens for vaccination. The other authors have no conflicts of interest to declare.
References
- Burnet F. Immunological surveillance. Oxford: Pergamon Press, 1970.
- Guo C, Manjili MH, Subjeck JR, et al. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res 2013;119:421-75. [Crossref] [PubMed]
- Butterfield LH. Cancer vaccines. BMJ 2015;350:h988. [Crossref] [PubMed]
- Melief CJ, van Hall T, Arens R, et al. Therapeutic cancer vaccines. J Clin Invest 2015;125:3401-12. [Crossref] [PubMed]
- Melero I, Gaudernack G, Gerritsen W, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 2014;11:509-24. [Crossref] [PubMed]
- Zhai L, Spranger S, Binder DC, et al. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin Cancer Res 2015;21:5427-33. [Crossref] [PubMed]
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013;34:137-43. [Crossref] [PubMed]
- Kumar V, Patel S, Tcyganov E, et al. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol 2016;37:208-20. [Crossref] [PubMed]
- Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer 2017;76:167-82. [Crossref] [PubMed]
- Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol 2009;158:638-51. [Crossref] [PubMed]
- Heuvers ME, Muskens F, Bezemer K, et al. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer 2013;81:468-74. [Crossref] [PubMed]
- Ochoa AC, Zea AH, Hernandez C, et al. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 2007;13:721s-6s. [Crossref] [PubMed]
- Bedoya AM, Tate DJ, Baena A, et al. Immunosuppression in cervical cancer with special reference to arginase activity. Gynecol Oncol 2014;135:74-80. [Crossref] [PubMed]
- Zaytouni T, Tsai PY, Hitchcock DS, et al. Critical role for arginase 2 in obesity-associated pancreatic cancer. Nat Commun 2017;8:242. [Crossref] [PubMed]
- Seo SK, Seo DI, Park WS, et al. Attenuation of IFN-gamma-induced B7-H1 expression by 15-deoxy-delta(12,14)-prostaglandin J2 via downregulation of the Jak/STAT/IRF-1 signaling pathway. Life Sci 2014;112:82-9. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004;101:17174-9. [Crossref] [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [Crossref] [PubMed]
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [Crossref] [PubMed]
- Vijayan D, Young A, Teng MWL, et al. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer 2017;17:709-24. [Crossref] [PubMed]
- Wiedemann GM, Knott MM, Vetter VK, et al. Cancer cell-derived IL-1alpha induces CCL22 and the recruitment of regulatory T cells. Oncoimmunology 2016;5:e1175794. [Crossref] [PubMed]
- Gobert M, Treilleux I, Bendriss-Vermare N, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 2009;69:2000-9. [Crossref] [PubMed]
- Liu W, Wei X, Li L, et al. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-beta pathway in human non-small cell lung cancer. Biochem Biophys Res Commun 2017;488:196-203. [Crossref] [PubMed]
- Li YQ, Liu FF, Zhang XM, et al. Tumor secretion of CCL22 activates intratumoral Treg infiltration and is independent prognostic predictor of breast cancer. PLoS One 2013;8:e76379. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Shekarian T, Valsesia-Wittmann S, Caux C, et al. Paradigm shift in oncology: targeting the immune system rather than cancer cells. Mutagenesis 2015;30:205-11. [Crossref] [PubMed]
- Colle R, Cohen R, Cochereau D, et al. Immunotherapy and patients treated for cancer with microsatellite instability. Bull Cancer 2017;104:42-51. [Crossref] [PubMed]
- Festino L, Botti G, Lorigan P, et al. Cancer Treatment with Anti-PD-1/PD-L1 Agents: Is PD-L1 Expression a Biomarker for Patient Selection? Drugs 2016;76:925-45. [Crossref] [PubMed]
- Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol 2015;67:4-17. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 2014;21:231-7. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Martin SD, Coukos G, Holt RA, et al. Targeting the undruggable: immunotherapy meets personalized oncology in the genomic era. Ann Oncol 2015;26:2367-74. [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Yarchoan M, Johnson BA 3rd, Lutz ER, et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017;17:569. [Crossref] [PubMed]
- Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [Crossref] [PubMed]
- Koch M, Beckhove P, Op den Winkel J, et al. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann Surg 2006;244:986-92; discussion 992-3. [Crossref] [PubMed]
- Deschoolmeester V, Baay M, Van Marck E, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol 2010;11:19. [Crossref] [PubMed]
- Patel A, Kaufman HL, Disis ML. Next generation approaches for tumor vaccination. Chin Clin Oncol 2017;6:19. [Crossref] [PubMed]
- Dammeijer F, Lau SP, van Eijck CHJ, et al. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine Growth Factor Rev 2017;36:5-15. [Crossref] [PubMed]
- van Elsas A, Sutmuller RP, Hurwitz AA, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med 2001;194:481-9. [Crossref] [PubMed]
- Duraiswamy J, Kaluza KM, Freeman GJ, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013;73:3591-603. [Crossref] [PubMed]
- Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 2013;13:5. [PubMed]
- Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: A new era in cancer immunotherapy. J Intern Med 2018;283:110-20. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- van der Burg SH, Arens R, Ossendorp F, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016;16:219-33. [Crossref] [PubMed]
- Andersen MH. Anti-regulatory T cells. Semin Immunopathol 2017;39:317-26. [Crossref] [PubMed]
- Andersen MH. Immune Regulation by Self-Recognition: Novel Possibilities for Anticancer Immunotherapy. J Natl Cancer Inst 2015.107. [PubMed]
- Yu W, Jiang N, Ebert PJ, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity 2015;42:929-41. [Crossref] [PubMed]
- Sorensen RB, Hadrup SR, Svane IM, et al. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 2011;117:2200-10. [Crossref] [PubMed]
- Sorensen RB, Kollgaard T, Andersen RS, et al. Spontaneous cytotoxic T-Cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res 2011;71:2038-44. [Crossref] [PubMed]
- Munir S, Larsen SK, Iversen TZ, et al. Natural CD4+ T-cell responses against indoleamine 2,3-dioxygenase. PLoS One 2012;7:e34568. [Crossref] [PubMed]
- Hjortso MD, Larsen SK, Kongsted P, et al. Tryptophan 2,3-dioxygenase (TDO)-reactive T cells differ in their functional characteristics in health and cancer. Oncoimmunology 2015;4:e968480. [Crossref] [PubMed]
- Munir S, Andersen GH, Met O, et al. HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res 2013;73:1764-76. [Crossref] [PubMed]
- Larsen SK, Munir S, Woetmann A, et al. Functional characterization of Foxp3-specific spontaneous immune responses. Leukemia 2013;27:2332-40. [Crossref] [PubMed]
- Martinenaite E, Munir Ahmad S, Hansen M, et al. CCL22-specific T Cells: Modulating the immunosuppressive tumor microenvironment. Oncoimmunology 2016;5:e1238541. [Crossref] [PubMed]
- Munir Ahmad S, Martinenaite E, Hansen M, et al. PD-L1 peptide co-stimulation increases immunogenicity of a dendritic cell-based cancer vaccine. Oncoimmunology 2016;5:e1202391. [Crossref] [PubMed]
- Gulley JL, Madan RA, Pachynski R, et al. Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. J Natl Cancer Inst 2017.109. [PubMed]
- Iversen TZ, Engell-Noerregaard L, Ellebaek E, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res 2014;20:221-32. [Crossref] [PubMed]
- Bjoern J, Iversen TZ, Nitschke NJ, et al. Safety, immune and clinical responses in metastatic melanoma patients vaccinated with a long peptide derived from indoleamine 2,3-dioxygenase in combination with ipilimumab. Cytotherapy 2016;18:1043-55. [Crossref] [PubMed]


