Risk of venous thromboembolism with the erythropoiesis-stimulating agents (ESAs) for the treatment of cancer-associated anemia: a meta-analysis of randomized control trials
Introduction
Anaemia is a common occurrence in patients with cancer, arising either as a result of the underlying malignant disease, as a consequence of myelosuppressive chemotherapy or radiotherapy, or a combination of both (1). Anaemia is associated with a multitude of symptoms including fatigue, dyspnea, depression, and other co-morbidities that have a profound impact on a patient’s condition and quality of life (QoL) (2). Furthermore, a meta-analysis of 60 published studies suggested that anaemia may be an independent prognostic factor for survival in patients with cancer (3).
The Erythropoiesis stimulating agents (ESAs), erythropoietin and darbepoetin, are widely used to treat anemia in patients with cancer. Most randomized trials and previous meta-analyses have shown that ESAs increase haemoglobin concentrations, reduce the need for transfusions (4-6), and reduce fatigue (7). However, they have been reported to increase the risk of venous thromboembolism (VTE) (5,8) and might stimulate tumour growth (9). Their safety has been discussed repeatedly at hearings of the US Food and Drug Administration and the European Medicines Agency (10,11).
Although two previous meta-analysis (5,8) reported that ESAs administration to patients with cancer was associated with increased risks of VTE, they did not conducted the subgroup analysis by stratified with study characteristics such as cancer types and hemoglobin level at baseline or target-line. Since then, several more large randomized clinical trials (RCTs) have been published. We therefore performed an updated meta-analysis of the ESA Phase III clinical trial experience to ascertain whether administration of ESAs increased the risk of VTE in patient with cancer and stratified by various cancer types.
Materials and methods
Publication search
The electronic databases PubMed and Web of Science were searched for studies to include in the present meta-analysis. An upper date limit of Dec 01, 2012 was applied; we used no lower date limit. Keywords included in our search were “Erythropoietin”, “Darbepoetin”, “cancer”, and was limited to “randomized controlled clinical trials”.
Abstracts and virtual meeting presentations containing the term “Erythropoietin” or “Darbepoetin” from the American Society of Clinical Oncology conferences (
Study selection
The goal of this study was to evaluate the risk of VTE with ESAs for the treatment of cancer-associated anemia. Therefore, we selected for analysis only those randomized clinical trials that directly compared patients with cancer treated with and without ESAs. Phase I and single-arm phase II trials were excluded due to their lack of control groups. Specifically, clinical trials that met the following criteria were included in the meta-analysis: prospective phase II and III randomised clinical trials in patients with cancer; random assignment of participants to ESAs treatment or control/placebo in addition to concurrent chemotherapy and/or radiotherapy; and available data including event or incidence of VTE and sample size for analysis. Trials with uncertain or marked inequality of characteristics between groups at baseline were also excluded. Two reviewers (P.Z and Q.W) independently determined study eligibility. Disagreements were resolved by consensus.
Data extraction
Data extraction was performed based upon patient characteristics, treatment information, results, and follow-up from these selected trials. Incidences of VTE were extracted from the safety profile in each trial. Two reviewers extracted the data independently (P.Z and Q.W). Any discrepancies between reviewers were resolved by consensus. VTE in these studies was assessed and recorded according to the National Cancer Institute’s common toxicity criteria (version 2 or 3;
Statistical analysis
The overall the relative risks (RRs) for VTE and 95% confidence intervals (CIs) were calculated using Reviewer Manager Version 5.0 provided by the Cochrane Collaboration (12). For the meta-analysis, we used fixed-effects (weighted with inverse variance) or random effects model (13). For each meta-analysis, the Cochran’s Q statistic and I2 score were first calculated to assess the heterogeneity among the proportions of the included trials (14). For the P value of Cochran’s Q statistic <0.1, the assumption of homogeneity was deemed invalid, and a random-effects model was reported. The causes of heterogeneity were also explored in this context. Otherwise, results from the fixed-effects model were reported. A two-tailed P value <0.05 was judged as statistically significant. We used the Begg’s and Egger’s tests to determine the presence of publication bias regarding primary endpoint (RR of high-grade hypertension) (15,16). A two-tailed P value of <0.05 was considered statistically significant.
Results
Study characteristics
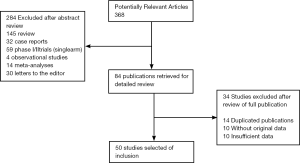
Our search yielded a total of 368 potentially relevant clinical studies on ESAs and treatment of cancer in the literature (Figure 1). After excluding review articles, phase I studies, single-arm phase II studies, case reports, meta-analyses, and observational studies (Figure 1), 50 phase III randomized controlled clinical trials (10,17-40) were included in our meta-analysis(41-60). Table 1 presents the principal characteristics of these studies including study-year, drug, patient numbers, treatment duration, concomitant treatments, and cancer types. Epoetin alfa or epoetin beta was evaluated in 43 trials with 8,723 patients and darbepoetin in 7 trials with 2,909 patients. Duration of ESA treatment ranged from 4 to 52 weeks. Concomitant treatment varied between trials as follows: chemotherapy (29 trials), radiotherapy (3 trials), chemoradiotherapy (9 trials), palliative radiotherapy (1 trial), no treatment (4 trials), and treatment not reported (4 trials). Twenty-eight trials included 8,184 patients with a single cancer diagnosis (lung cancer (8 trials), breast cancer (6 trials), head and neck cancer (4 trials), cervical cancer (3 trials), ovarian cancer (4 trials), lymphoma (1 trial), CLL (1 trial) and multiple myeloma (1 trial)).
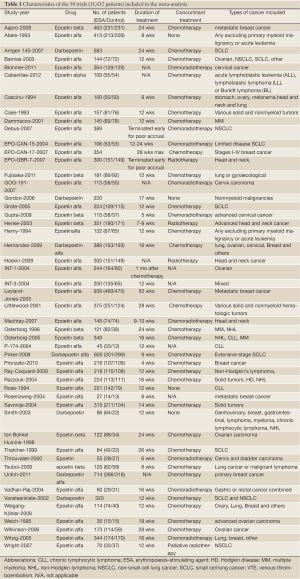
Full table
RR of venous thromboembolism
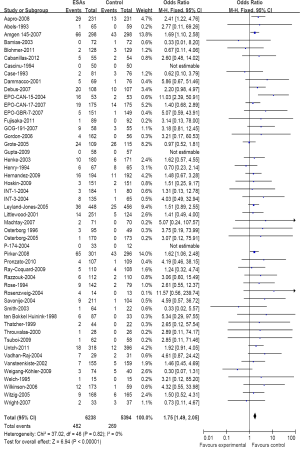
A meta-analysis was performed to calculate the overall RR of VTE (combination of all grade) associated with ESAs in comparison with controls for 50 trials included 11,632 patients. Among those patients receiving ESAs, the summary incidences of all-grade VTE were 7.62%. These trials identified a significantly increased risk of VTE among patients treated with ESA (482 events among 6,238 patients treated with ESA vs. 269 events among 5,394 control patients; RR=1.75; 95% CI, 1.49-2.05) (Figure 2), suggesting a 75% greater risk for developing VTE with ESAs compared with a control. This association also was not dominated by a small number of trials. There was no significant heterogeneity when evaluating all 50 trials (heterogeneity: Chi2=37.02; I2=0%; P=0.82).
Venous thromboembolism risk and tumor type
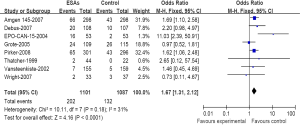
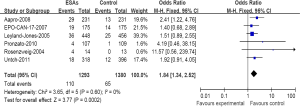
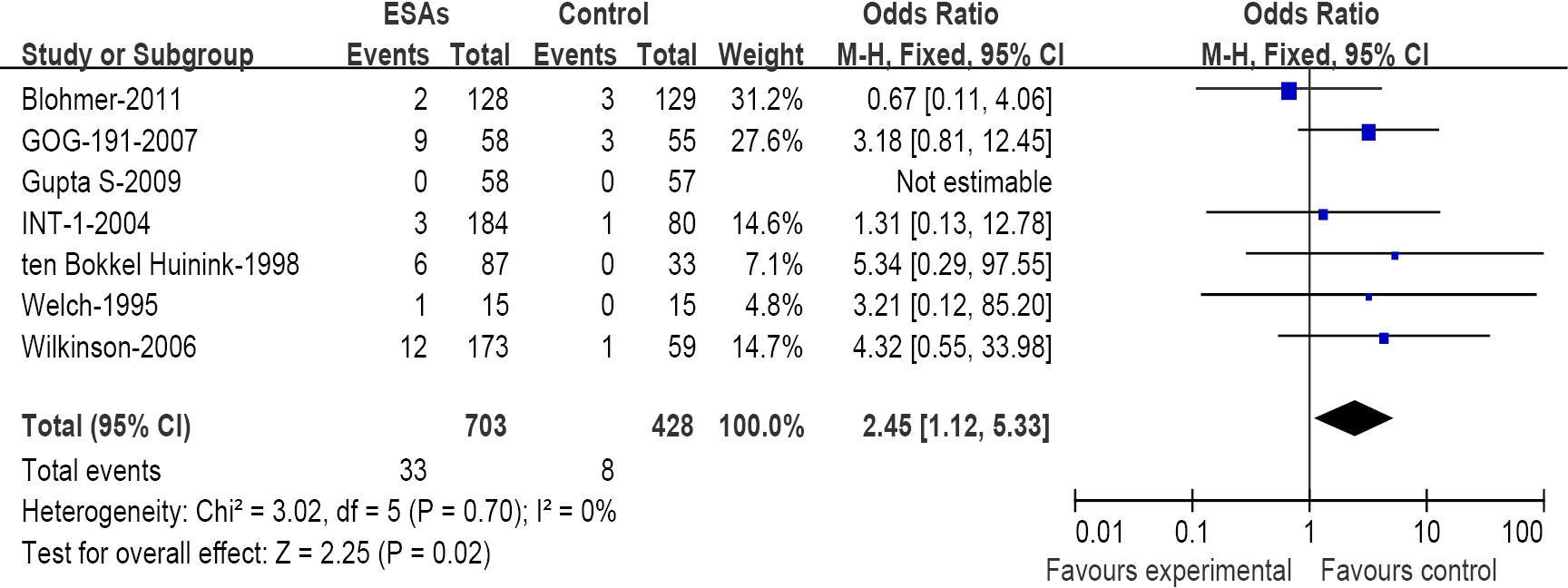
We have further determined the risk of VTE with ESAs separately according to their histology to investigate the relationship between tumor type and VTE. The incidence and risks of VTE with ESAs vary among different tumor types (Table 2). The highest incidence in ESAs and control was observed among patients with lung cancer (Figure 3) (18.34% and 12.14%); meta-analysis showed that the RR of VTE was 1.67 (1.31-2.12). The highest RR of VET was found in patients with ovarian and cervical cancer (Figure 4) for 2.45 (1.12-5.33), however the incidence was relative lower for (4.69% and 1.86%); While for patients with breast cancer (Figure 5), the RR of VTE was 1.84 (1.34-2.52), the incidence of VTE was 8.50% vs. 4.71%; For patients with head and neck cancer, the RR of VTE was relative higher with 2.14 (0.98-4.67), but was no significance statistically.

Full table
Publication bias
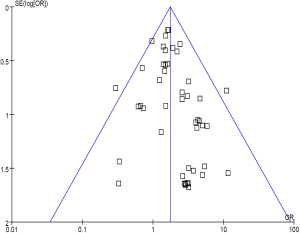
No evidence of publication bias was detected for the primary end point of this study (RR of venous thromboembolism) by either the Begg or Egger test (Begg test, P=0.43; Egger test, P=0.59) (Figure 6).
Discussion
VTE is a major complication of cancer, and one of the leading causes of death in cancer patients (61). The association of VTE with ESAs presents a challenge for recognition because many RCTs may not be powered to reveal a significant relationship. In our meta-analysis, we involved 50 RCTs including a total of 6,238 cancer cases with ESAs and 5,394 patients with controls and demonstrated that ESAs are associated with a significantly increased risk of VET [RR=1.75 (95% CI, 1.49-2.05); P<0.00001] in patients with a variety of cancer types. The highest increased risk is observed in patients with ovarian and cervical cancer [RR=2.45 (95% CI, 1.12-5.33)]. The highest incidence of VTE in ESAs and control is observed among patients with lung cancer for 18.34% and 12.14%.
Our data were consistent with the results of a previous meta-analysis (8) published in 2008 that showed the increased risks of VTE in ESAs to patients with cancer. Bennett CL et al. study included only 38 studies, and the data were insufficient to determine the risks of VTE for subgroups divided according to types of cancer. We have improved upon that previous meta-analysis by including more recent related RCTs and by generally using a more comprehensive search strategy, screening and study selection, were performed independently and reproducibly by two re-viewers.
Expression of erythropoietin and erythropoietin receptors has been demonstrated in a variety of human cancers (62). Erythropoieitn stimulation of cancer cells in vitro activates signal transduction pathways, including phosphatidylinositol 3-kinase-Akt and JAK-STAT (Janus kinase Signal Transducer and Activator of Transcription) (63). In head and neck squamous cell carcinoma and melanoma, activation of the erythropoietin/erythropoietin receptor signaling axis results in measurable cellular effects, including proliferation, antiapoptosis, and invasion (64-66). Erythropoietin-mediated functions may result from autocrine/paracrine signaling or recruitment of both endogenous and exogenous erythropoietin by the tumor (67,68). Clearly, many issues remain to be clarified regarding the specific actions of ESAs in human cancer cells. Further research is needed to clarify cellular and molecular mechanisms and pathways of the effects of ESAs on thrombogenesis and their potential effects on tumour growth.
Our study has the following limitations. First, we could not detect the association with the available data between the relative risk for VTE events and hemoglobin level at baseline or target line. Second, there was lack of standard definitions of VTE. It does not distinguish distal from proximal VTE, and accidental finding of VTE. Also, the majority of trials included in this meta-analysis reported VTE events in combined grades; in addition, the ability to detect VTE may vary among institutions in which these trials were performed, and may cause bias of the reported incidence rates. Third, the study may have a potential publication bias even though it was not detectable by our analysis. Fourth, this is a meta-analysis at the study level, and confounding factors at the patient level cannot be properly assessed and incorporated into the analysis. Finally, we did not report separately on epoetin vs. darbepoetin, because the American Society of Clinical Oncology/American Society of Hematology guidelines considered the products as belonging to a single class (69).
In conclusion, our study has shown that the ESAs are associated with a significantly increased risk of VTE in cancer patients who receive chemotherapy and/or radiotherapy. The risks of VTE may vary with tumor type. It is imperative for physicians and patients to recognize the risk. In the event of VTE, a nticoagulation is indicated, and ESAs may be continued if benefits of the drug outweigh the risk. Future studies are needed to investigate the prevention and management of VTE associated with ESAs.
Acknowledgements
This work was supported in part by a grant from “Twelve-Five Plan” the Major Program of Nanjing Medical Science and Technique Development Foundation (Molecular Mechanism Study on Metastasis and Clinical Efficacy Prediction of Non-small Cell Lung Cancer) (Lk-Yu) and Third Level Training Program of Young Talent Project of Nanjing Health (P-Zhan).
Disclosure: The authors declare no conflicts of interest.
References
- Bokemeyer C, Oechsle K, Hartmann JT. Anaemia in cancer patients: pathophysiology, incidence and treatment. Eur J Clin Invest 2005;35:26-31.
- Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293-306.
- Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91:2214-21.
- Seidenfeld J, Piper M, Flamm C, et al. Epoetin treatment of anemia associated with cancer therapy: a systematic review and meta-analysis of controlled clinical trials. J Natl Cancer Inst 2001;93:1204-14.
- Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708-14.
- Wilson J, Yao GL, Raftery J, et al. A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess 2007;11:1-202, iii-iv.
- Minton O, Richardson A, Sharpe M, et al. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008;100:1155-66.
- Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008;299:914-24.
- Henke M, Mattern D, Pepe M, et al. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol 2006;24:4708-13.
- FDA Briefing Document: Safety Concerns Asso-ciated with Aranesp (darbepoetin alfa) Amgen, Inc.and Procrit (epoetin alfa) Ortho Biotech, L.P., for the Treatment of Anemia Associated with Cancer Chemotherapy: Hearings Before the SubCommittee on Oncologic Drugs Advisory Committee, Center for Drug Evaluation and Research. Available online: http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037B2_04_FDA-Aranesp-Procrit.htm#_ednref45. Accessed January 10, 2008.
- Brower V. ESAs further restricted, but debate continues. J Natl Cancer Inst 2008;100:1344-51.
- Review Manager (RevMan) (Computer program). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34.
- Aapro M, Leonard RC, Barnadas A, et al. Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol 2008;26:592-8.
- Amgen DA 145 Study. April 19, 2007. Available online: http://wwwext.amgen.com/media/media_pr_detail.jsp?year=2007&releaseID=987476. Accessed January 9, 2008.
- Abels R. Erythropoietin for anaemia in cancer patients. Eur J Cancer 1993;29:S2-8.
- Blohmer JU, Paepke S, Sehouli J, et al. Randomized phase III trial of sequential adjuvant chemoradiotherapy with or without erythropoietin Alfa in patients with high-risk cervical cancer: results of the NOGGO-AGO intergroup study. J Clin Oncol 2011;29:3791-7.
- Bamias A, Aravantinos G, Kalofonos C, et al. Prevention of anemia in patients with solid tumors receiving platinum-based chemotherapy by recombinant human Erythropoietin (rHuEpo): a prospective, open label, randomized trial by the Hellenic Cooperative Oncology Group. Oncology 2003;64:102-10.
- Cabanillas ME, Kantarjian H, Thomas DA, et al. Epoetin alpha decreases the number of erythrocyte transfusions in patients with acute lymphoblastic leukemia, lymphoblastic lymphoma, and Burkitt leukemia/lymphoma: results of a randomized clinical trial. Cancer 2012;118:848-55.
- Cascinu S, Fedeli A, Del Ferro E, et al. Recombinant human erythropoietin treatment in cisplatin-associated anemia: a randomized, double-blind trial with placebo. J Clin Oncol 1994;12:1058-62.
- Case DC Jr, Bukowski RM, Carey RW, et al. Recombinant human erythropoietin therapy for anemic cancer patients on combination chemotherapy. J Natl Cancer Inst 1993;85:801-6.
- Dammacco F, Castoldi G, Rödjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol 2001;113:172-9.
- Debus J, Hindermann S, Morr H, et al. Epoetin alfa (EPO) and survival in patients with non-resectable NSCLC: interim re-sults.German Medical Science. Available online: http://www.egms.de/en/meetings/dkk2006/06dkk257.shtml. Accessed December 17, 2007.
- FDA Briefing Document. May 10, 2007: Onco-logic Drugs Advisory Committee. Continuting reas-sessment of the risks of erythropoiesis-stimulating agents (ESAs) administered for the treatment of anemia associated with cancer chemotherapy. Available online: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4301b2-02-FDA.pdf. Accessed January 11,2008.
- Fujisaka Y, Sugiyama T, Saito H, et al. Randomised, phase III trial of epoetin-β to treat chemotherapy-induced anaemia according to the EU regulation. Br J Cancer 2011;105:1267-72.
- Gupta S, Singh PK, Bisth SS, et al. Role of recombinant human erythropoietin in patients of advanced cervical cancer treated "by chemoradiotherapy". Cancer Biol Ther 2009;8:13-7.
- Grote T, Yeilding AL, Castillo R, et al. Efficacy and safety analysis of epoetin alfa in patients with small-cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2005;23:9377-86.
- Gordon DH, Nichols G, Ben-Jacob A, et al. Treating anemia of cancer with darbepoetin alfa adminis-tration every 4 weeks: final results from a phase 2, ran-domized, double-blind, placebo-controlled study in cancer patients not receiving chemotherapy and/or radiotherapy. Presented at: American Society of He-matology; December 9, 2006; Orlando, FL. Abstract 1304.
- Hernandez E, Ganly P, Charu V, et al. Randomized, double-blind, placebo-controlled trial of every-3-week darbepoetin alfa 300 micrograms for treatment of chemotherapy-induced anemia. Curr Med Res Opin 2009;25:2109-20.
- Hoskin PJ, Robinson M, Slevin N, et al. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol 2009;27:5751-6.
- Henke M, Laszig R, Rübe C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003;362:1255-60.
- Henry DH, Abels RI. Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double-blind and open-label follow-up studies. Semin Oncol 1994;21:21-8.
- Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 2005;23:5960-72.
- Littlewood TJ, Bajetta E, Nortier JW, et al. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2001;19:2865-74.
- Machtay M, Pajak TF, Suntharalingam M, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03). Int J Radiat Oncol Biol Phys 2007;69:1008-17.
- Osterborg A, Boogaerts MA, Cimino R, et al. Recombinant human erythropoietin in transfusion-dependent anemic patients with multiple myeloma and non-Hodgkin’s lymphoma--a randomized multicenter study. The European Study Group of Erythropoietin (Epoetin Beta) Treatment in Multiple Myeloma and Non-Hodgkin’s Lymphoma. Blood 1996;87:2675-82.
- Osterborg A, Brandberg Y, Hedenus M. Impact of epoetin-beta on survival of patients with lymphoproliferative malignancies: long-term follow up of a large randomized study. Br J Haematol 2005;129:206-9.
- Pirker R, Ramlau RA, Schuette W, et al. Safety and efficacy of darbepoetin alpha in previously untreated extensive-stage small-cell lung cancer treated with platinum plus etoposide. J Clin Oncol 2008;26:2342-9.
- Pronzato P, Cortesi E, van der Rijt CC, et al. Epoetin alfa improves anemia and anemia-related, patient-reported outcomes in patients with breast cancer receiving myelotoxic chemotherapy: results of a European, multicenter, randomized, controlled trial. Oncologist 2010;15:935-43.
- Razzouk BI, Hockenberry M, Hinds PS, et al. A double-blind, placebo-controlled study of once-weekly epoetin alfa in children with cancer undergoing myelosuppressive chemotherapy. J Clin Oncol 2004;22:Abstr 8527.
- Rose E, Rai K, Revicki D, et al. Clini-cal and health status assessments in anemic chronic lymphocytic leukemia (CLL) patients treated with epo-etin alfa (EPO). Blood 1994;84:526a.
- Rosenzweig MQ, Bender CM, Lucke JP, et al. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage 2004;27:185-90.
- Ray-Coquard I, Dussart S, Goillot C, et al. A risk model for severe anemia to select cancer patients for primary prophylaxis with epoetin alpha: a prospective randomized controlled trial of the ELYPSE study group. Ann Oncol 2009;20:1105-12.
- Savonije J, Van Groeningen C, Van Bochove A, et al. Early intervention with epoetin-alfa during platinum-based chemotherapy. J Clin Oncol 2004;22:abstr 8111.
- Smith RE Jr, Tchekmedyian NS, Chan D, et al. A dose- and schedule-finding study of darbepoetin alpha for the treatment of chronic anaemia of cancer. Br J Cancer 2003;88:1851-8.
- ten Bokkel Huinink WW, de Swart CA, van Toorn DW, et al. Controlled multicentre study of the influence of subcutaneous recombinant human erythropoietin on anaemia and transfusion dependency in patients with ovarian carcinoma treated with platinum-based chemotherapy. Med Oncol 1998;15:174-82.
- Thatcher N, De Campos ES, Bell DR, et al. Epoetin alpha prevents anaemia and reduces transfusion requirements in patients undergoing primarily platinum-based chemotherapy for small cell lung cancer. Br J Cancer 1999;80:396-402.
- Throuvalas N, Antonadou D, Boufi M, et al. Erythropoietin Decreases Transfusion Requirements During Radiochemotherapy. Proc Am Soc Clin Oncol 2000;19: abstr 1558.
- Tsuboi M, Ezaki K, Tobinai K, et al. Weekly administration of epoetin beta for chemotherapy-induced anemia in cancer patients: results of a multicenter, Phase III, randomized, double-blind, placebo-controlled study. Jpn J Clin Oncol 2009;39:163-8.
- Untch M, von Minckwitz G, Konecny GE, et al. PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer--outcome on prognosis. Ann Oncol 2011;22:1999-2006.
- Vadhan-Raj S, Crane C, Buesos-Ramos CE, et al. Randomized, double-blind, placebo-controlled trial of epoetin alfa (Procrit) in patients with rectal and gas-tric cancer undergoing chemo-radiotherapy (CT/RT) followed by surgery: early termination o f the trial due to increased incidence of thrombo-embolic events (TEE). Blood 2004;104:abstr 2915.
- Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002;94:1211-20.
- Witzig TE, Silberstein PT, Loprinzi CL, et al. Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol 2005;23:2606-17.
- Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 2007;25:1027-32.
- Welch RS, James RD, Wilkinson PM, et al. Recombinant human erythropoietin and platinum-based chemotherapy in advanced ovarian cancer. Cancer J Sci Am 1995;1:261-6.
- Wilkinson PM, Antonopoulos M, Lahousen M, et al. Epoetin alfa in platinum-treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. Br J Cancer 2006;94:947-54.
- Weigang-Köhler K, Vetter A. Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie 2009;32:168-74.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632-4.
- Hardee ME, Arcasoy MO, Blackwell KL, et al. Erythropoietin biology in cancer. Clin Cancer Res 2006;12:332-9.
- Hardee ME, Rabbani ZN, Arcasoy MO, et al. Erythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivity. Mol Cancer Ther 2006;5:356-61.
- Acs G, Acs P, Beckwith SM, et al. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res 2001;61:3561-5.
- Lai SY, Childs EE, Xi S, et al. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene 2005;24:4442-9.
- Kumar SM, Yu H, Fong D, et al. Erythropoietin activates the phosphoinositide 3-kinase/Akt pathway in human melanoma cells. Melanoma Res 2006;16:275-83.
- Kumar SM, Acs G, Fang D, et al. Functional erythropoietin autocrine loop in melanoma. Am J Pathol 2005;166:823-30.
- Lai SY, Grandis JR. Understanding the presence and function of erythropoietin receptors on cancer cells. J Clin Oncol 2006;24:4675-6.
- Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol 2008;26:132-49.