Clinical practice guidelines on cancer-related anemia (2012-2013 Edition)
1. Introduction
Cancer-related anemia (CRA), is a common comorbidity of malignant tumors. CRA may have a variety of potential causes, though they can be classified as either tumor-related factors (such as blood loss, hemolysis and bone marrow invasion) or factors associated with cancer treatment (such as chemotherapy-associated bone marrow suppression and cancer radiation therapy).
Several key guidelines or consensuses for CRA treatment have been published globally (1,2), but specific clinical guidelines for the treatment of CRA are still yet to be developed in China, particularly ones on the application of erythropoietin stimulating agents (ESAs). The foreign treatment guidelines (or consensuses) are useful as a reference. However, due to the difference in national conditions, awareness and application levels, as well as controversy over the clinical application of EPO treatment of CRA in clinical settings as shown in latest reports, it is necessary to develop the consensus on CRA treatment (hereinafter referred to as “the Consensus”) tailored to China’s national conditions for guidance of future clinical practice and research.
Following similar procedures to the formulation of other oncology guidelines, these guidelines are built on the recent research on CRA at home and abroad, incorporating relevant information from the existing guidelines on related therapies in other countries.
The following principles are observed during the development of these Guidelines:
(I) Multi-disciplinary experts and professionals (such the representatives from the departments of hematology, medical oncology, radiation oncology and the pharmaceutical sector) are involved in the process;
(II) Latest published literature (by December 2011) and prescription information provided by pharmaceutical companies are cited;
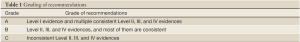
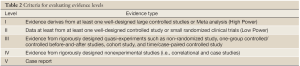
(III) The recommendation grade (as specified in the criteria in Table 1) of a specific clinical issue in these Guidelines is determined according to the evidence level of the source literature or information (as specified in Table 2).
Full table
Full table
These Guidelines are designed to provide clinicians with guidance for the diagnosis and treatment of CRA based on existing medical evidence.
2. Overview of CRA
2.1 Grading and classification of CRA
Anemia occurs when the red blood cell (RBC) count or hemoglobin (Hb) concentration per unit volume of peripheral blood is reduced so that the body may not deliver adequate oxygen to the cells in peripheral tissues. CRA is a subset of this condition that arises from the development or treatment course of tumors. It can be caused by multiple factors, including nutrient absorption disorders and the long-term, various therapies, in addition to the tumor per se.
From a pathophysiological point of view, CRA can be a result of decreased production and excessive destruction of red blood cells, blood loss or a combination of above factors, as well as such other complex causes as poor diet and inadequate iron intake (which is common in tumor patients). Insufficient generation of red blood cells can be caused by defects in EPO cells, hemoglobin synthesis disorders, bone marrow suppression or renal failure. Destruction of red blood cells is related to many causes, such as internal factors (e.g., enzyme defects) or chemotherapy drugs (e.g., antimetabolites), autoimmune hemolysis induced by the malignant lesion itself (e.g., chronic lymphocytic leukemia) or inflammatory cytokines generated by it (e.g., tumor necrosis factor). Blood loss may occur in the case of acute hemorrhage or tumor-associated coagulopathy (e.g., disseminated intravascular coagulation). In cancer patients, iron deficiency, reduced peripheral erythropoiesis may be a result of decreased vitamin B12 levels, reduced folic acid production and many other factors.
2.1.1 Severity-based grading system
Anemia is graded based on its severity according to two currently available international diagnostic criteria (3): the grading system of the U.S. National Cancer Institute (NCI) and the criteria of the World Health Organization (WHO). The NCI grading standards of anemia are adopted in most European and American countries, which slightly differ from the other system mainly in terms of the identification of mild to moderate anemia. China has also developed its own grading system based on clinical practice and therapeutic methods (Table 3). The NCI grading system is used in these Guidelines.

Full table
2.1.2 Cause-based classification (5)
2.1.2.1 CRA (non-chemotherapy-related)
CRA can be a result of tumor-related hemorrhage, tumor invasion of bone marrow, malnutrition caused by tumor, abnormal iron metabolism, kidney function impairment and compromised bone marrow function under the effect of various tumor-related cytokines. In most cases, the condition is a normocytic normochromic anemia with decreased serum iron and transferrin saturation, and normal or elevated serum ferritin.
In recent years, cancer-related inflammation is becoming an increasingly important issue. Cancer-related inflammation can trigger the release of inflammatory cytokines such as TNF, IL-1, and IFN-γ, which can not only suppress the production of EPO but also inhibit the release of storage iron and the proliferation of erythroid progenitor cells. Particularly, the inflammatory cytokines lead to increased hepcidin. Hepcidin can hinder the release of iron (which binds with macrophages in the reticuloendothelial system) into its transporter, the transferrin, resulting in the hematopoietic system’s blunt response to anemia. Table 5 lists the laboratory findings of non-chemotherapy-related anemia.

Full table
2.1.2.2 Chemotherapy-induced CRA
Bone marrow suppression is a common adverse reaction of chemotherapy and radiotherapy among cancer patients. The widespread use of cytotoxic drugs, particularly platinum-based agents, is an important contributing factor for tumor-related anemia, and the emergence of new chemotherapy drugs and their combined application is making anemia an increasingly significant challenge in clinical practice. These drugs can promote apoptosis of erythroid cells and cause kidney injury by damaging renal tubular cells, which in turn leads to reduced endogenous erythropoietin (EPO) and the resultant anemia.
2.1.3 Morphology-based classification
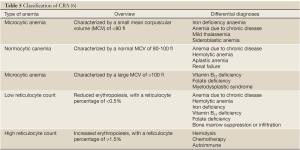
Based on the morphology of red blood cells, CRA can be classified as microcytic anemia, normocytic anemia and macrocytic anemia (Table 5). Other morphological basis for classification may include the amount of red blood cell hemoglobin and mean corpuscular hemoglobin (hypochromic or normochromic) or reticulocyte count, an indicator of the ability of bone marrow to produce blood cells. A low reticulocyte count suggests reduced red blood cell generation, while a high reticulocyte count represents increased red blood cell destruction and compensatory hyperplasia of the bone marrow red blood cell system.
2.1.3.1 Microcytic anemia
Iron deficiency and cachexia of end-stage cancer patients are the two common causes of tumor-associated microcytic anemia. Microcytic anemia is characterized by a small mean corpuscular volume (MCV) of <80 fl. In patients with iron deficiency, the anemia occurs as there is no sufficient iron for hemoglobin synthesis. Iron deficiency anemia is the most common type of anemia around the world, often found among children, pregnant women, adults and the elderly. In cancer patients, the most common cause of iron deficiency is inadequate intake (e.g., gastrectomy), excessive hemorrhagic loss of iron (e.g., bleeding of the uterus, gastrointestinal or urinary tract) and other tumor-related factors (e.g., anorexia). Differentiation is needed between iron deficiency microcytic anemia in cancer patients and other blood disorders, such as mild thalassemia and sideroblastic anemia. The occurrence of tumor-associated microcytic anemia is affected by cachexia and other symptoms in malignant patients, such as chronic infection and inflammation. The activation of immune and inflammatory cytokines and the release of cyokines such as tumor necrosis factors, interleukins and interferons at the end stage of cancer patients are also related to the development of microcytic anemia. EPO is reduced because of insufficient renal release as the kidney cells are damaged by the tumor or chemotherapy drugs, or the oxygen partial pressure receptors are compromised. Chronic infection will deplete the reservoir of stem cells and lower the overall response to EPO stimulation.
The reticulocyte count is an indicator of the hematopoietic ability of bone marrow and supplement to red blood cells, and a lower count in patients with tumor-associated microcytic anemia suggests hematopoietic dysfunction. This type of anemia should be differentiated from other blood disorders, such as aplastic anemia, bone marrow suppression caused by bone marrow dysplasia or chemotherapy, and anemia due to chronic renal failure.
2.1.3.2 Normocytic anemia
Normocytic manifestations, where red blood cells appear in normal sizes, are common in the early stages of CRA. With tumor progression and gradual accumulation of toxicity from anticancer therapy, microcytic anemia is often observed at the end stage.
Hemolytic anemia is a normocytic anemia that mostly induced by anticancer agents or other drugs. In tumor patients, this type of anemia can be associated with autoimmune hemolytic anemia (e.g., cold agglutinin antibody), enzyme defects (e.g., glucose-6-phosphatase), tumor destruction (e.g., non-Hodgkin’s lymphoma), chemotherapy drugs (e.g., cisplatin platinum), total body irradiation (e.g., bone marrow transplant) or tumor and risks during its treatment (e.g., disseminated intravascular coagulation). In addition, non-chemotherapy drugs for the treatment of cancer can also cause anemia (e.g., lorazepam).
Reduced generation of blood cells as a direct result of chemotherapy or radiotherapy may appear to be normocytic anemia because of pluripotent stem cells depletion due to treatment-induced bone marrow suppression. The impact of red blood cell destruction on metabolism may be caused by complex physical factors, such as tumor metastasis, bone marrow infiltration or fibrosis and rare bone marrow necrosis. Tumor may compromise metabolism by damaging protein synthesis so severely that the bone marrow is unable to effectively generate a sufficient number of red blood cells and maintain their normal size.
The generation process of EPO and red blood cell is essential to stimulating the formation and maturation of bone marrow erythrocytes. Renal cell hypoxia can stimulate feedback regulation of bone marrow and the release of EPO. In tumor patients, damaged kidney function due to the tumor itself or anticancer agents may lead to reduced erythropoiesis and the resultant anemia.
2.1.3.3 Macrocytic anemia
Red blood cell dysmaturity can result in megaloblastic macrocytic anemia (MCV >100 fl), which is mainly due to impaired DNA synthesis of red blood cell precursors with insufficient vitamin B12 and folic acid levels. Vitamin B12 is necessary for the metabolism of folic acid, while there is very limited resevoir of folic acid in the body. Hence, adequate dietary intake of vitamin B12 and folic acid becomes essential. Alcoholism, liver disease, malabsorption syndrome and and loss of appetite may all lead to folate deficiency. In addition, vitamin B12 malabsorption can be related to lack of intrinsic factor secreted by gastric parietal cells (e.g., total gastrectomy), vegetarian (who do not eat dairy products and meat), bacterial infection of the gastrointestinal tract, or in some cases, long-term use of certain drugs (e.g., cimetidine). Some anticancer drugs may also lead to folic acid deficiency, such as folate metabolism antagonists (e.g., hydroxyurea, methotrexate, and pemetrexed). The differential diagnosis of macrocytic anemia includes vitamin B12 or intrinsic factor deficiency, folic acid deficiency, ACD, and myelodysplastic syndrome (MDS).
2.2 Epidemiological surveys on CRA
The prevalence of CRA, including the distribution of patients with different severity of anemia, the distribution of anemia in patients with different tumors or different tumor stages, and the distribution of anemia in patients receiving different anti-tumor treatments, differs in different regions and among different races. Therefore, it is of vital importance to study such differences through epidemiological investigation and thus take corresponding interventions. Currently, epidemiological surveys on CRA have been conducted in Europe, Australia, and China.
2.2.1 Epidemiological surveys on CRA in Europe (7)
So far, the prevalence of anemia and its related data are both obtained from results of clinical trials on anemia treatment or cytotoxic drugs, in which these data are based on the per-protocol patients who were diagnosed of anemia as their hemoglobin value were lower than normal. For example, the European Cancer Anemia Survey (ECAS), as a prospective epidemiological study, investigated the prevalence, severity, and management of anemia in a large and representative sample of cancer patients in 24 European countries. In the survey, a six-month evaluation was carried out and the observational data (n=15,367) including demographic data, tumor type, performance status, Hb level, cancer treatment, and anemia treatment were obtained. The incidence of anemia at enrollment was 39.3% (Hb <10.0 g/dL, 10%), and it became 67.0% (Hb <10.0 g/dL, 39.3%) during the survey. Hb level was closely related with low physical status of patients.
The survey showed that the prevalence of anemia in cancer patients was 53.7% (Hb <10.0 g/dL, 15.2%), in which 38.9% received anti-anemia treatment (17.4% with EPO treatment, 14.9% with blood transfusion, and 6.5% with oral iron therapy). The mean Hb was 9.7 g/dL for patients starting to receive treatment. This study showed that there was a relatively high incidence of anemia in cancer patients. Anemia was highly relevant with physical status, and meanwhile many patients with anemia did not receive treatment.
2.2.2 Epidemiological survey on CRA in China
A multi-center epidemiological survey retrospectively analyzed the anemia in cancer patients in 15 hospitals in China from 1995 to 2006. By reviewing the patient’s medical history and filling out survey forms, the researchers collected the demographic data, disease status, previous anti-tumor therapies, interventions (blood transfusions, EPO or iron) for anemia, as well as blood count, liver and kidney functions, and body iron status at baseline period. Data on anti-cancer therapy (radiotherapy, chemotherapy, and surgery), anemia treatment (blood transfusions, EPO, or iron supplementation), as well as blood count, liver and kidney functions, and body iron status were also collected at the follow-up period. Statistical analysis was performed on the basis of these data. In this study, data from 2,034 patients with solid tumors were collected. Subjects were classified according to tumor types, which included gastric cancer (54.6%), non-small cell lung cancer (24.6%), small cell lung cancer (18.6%), and other tumors (2.2%). According to gender composition, 58.9% were males and 41.1% were females. Tumor stages: stage I, 25.3%; stage II, 12,8%; stage III, 33.3%; stage IV, 20.4%; and no specific stage, 12.2%. Anti-cancer therapies included chemotherapy (71%), radiotherapy (25.3%), and concurrent chemo-radiotherapy (6.9%); 10.6% did not receive any treatment.
The preliminary results showed that the proportion of patients with anemia was 37.3%, among which 21.3% were mild [mean Hb: (11.09±0.58) g/dL], 9.3% moderate [mean Hb: (9.12±0.58) g/dL], and 6.6% severe [mean HB: (6.68±1.09) g/L].
According to these two surveys, that proportion of patients with anemia in China was similar with that in Europe, in which the proportion of patients with mild anemia (21.3%) was slightly lower than that in Europe (29.3%), but that with severe anemia was higher in China (6.6%) than in Europe (1.3%). Preliminary results from the Chinese survey also showed that the proportion of female patients with anemia (41.4%) was significantly higher than that of males (34.3%). Compared with patients with other tumor types, patients with gastric cancer had the highest proportion of severe anemia, and the differences were statistically significant. The distribution of anemia severity was similar among all tumor types.
Complete records of the chemotherapy and anemia status were available among 435 patients. Analysis on the changes of anemia status along with the chemotherapy cycles showed that the proportion of anemia increased with the launching of chemotherapy. In fact, the proportion of patients with anemia and without anemia changed from 64.4% and 35.6% at baseline to 66.0% and 34.0% at the forth cycle of chemotherapy. This finding was similar with that from European survey.
2.3 Clinical manifestations of CRA
The prevalence of CRA ranges 10-40% and differs from other anemia types. First, compared with those with iron deficiency anemia, CRA patients tend to have lower EPO at any severity (8). Second, the CRA patients have a remarkably decreased feedback relationship between Hb and EPO, which is even more significant during chemotherapy. Finally, tumor patients are more likely to experience anemia symptoms, i.e., the symptoms are more likely to occur in tumor patients with high Hb levels; on the contrary, the non-tumor patients suffer from anemia symptoms only when the Hb level is low.
2.3.1 Relationship betweeen CRA and quality of life
The relationship is tight between CRA and quality of life in tumor patients. Many studies have demonstrated that CRA and fatigue are important causes of the decreased quality of life (QoL) in tumor patients (9,10). Also, quite a few clinical studies have found that, in patients who have developed anemia, the QoL is improved after the anemia is effectively corrected.
In their randomized trial, Littlewood et al. (11) found that the QoL of tumor patients with anemia was significantly improved in the EPO-α treatment group (compared with the placebo group). The researchers applied three QoL scales in their study: the Linear Analogue Self-assessment (LASA), the Functional Assessment of Cancer Therapy-Anemia (FACT-An), and the MOS item short from health survey (SF-36). LASA is a self-assessment tool, with a graduation of 8 mm. It is designed for measuring the physical status, daily living activity, and overall QoL. FACT-An is a questionnaire. It contains 55 tumor-related items, including a sub-scale for assessing fatigue-related anemia. SF-36 contains 11 items, which is mainly used to assess the QoL-related mental and physical factors. However, it is not specifically designed for tumor patients. During the study, both the LASA scale (physical status, P<0.01; daily living activity, P<0.01; and overall QoL, P<0.01) and FACT-An (P<0.01) showed the QoL scores significantly increased after EPO-α treatment when compared with the placebo group. Meanwhile, the SF-36 scale also showed similar findings. Tumor-related fatigue often co-exists with anemia (in fact, they are correlated) and severely affects QoL, which may explain why the management of fatigue and anemia has became an integral part of tumor treatment.
2.3.2 Relationship between CRA and hypoxia and anti-tumor treatment
CRA can exaggerate tumor hypoxia. An increasing number of evidences have proved that hypoxia can cause proteomic changes that affect tumor spread and thus induce the malignant progression; meanwhile, hypoxia can also lower the effectiveness of anti-tumor therapies and thus impact on the prognosis. Hypoxia can induce changes in the proteome and genome of tumors, which, by increasing the level of heat shock protein, or, by increasing the amount of cells that may lower the tumor apoptosis potentials or enhance tumor proliferation potentials, may lead to radiotherapy resistance. When the tumor has an oxygen partial pressure below 25-30 mmHg, the radiosensitivity remarkably decreases (12). Hypoxia can also induce the tumor cells’ resistance to chemotherapeutic drugs. For example, hypoxia may result in the decline in the cytotoxicity of drugs and cause the acid intoxication of tissues, which may be exaggerated along with glycolysis. In addition, hypoxic stress protein and missed apoptotic potential can also produce chemotherapy drug resistance (12).
3. Treatment of CRA
3.1 Therapeutic transfusion
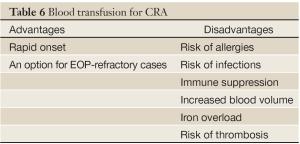
For many years, transfusion of whole blood or erythrocytes is the main treatment for CRA. It has many advantages: it can rapidly elevate hemoglobin levels and is able to treat patients that developed EPO resistance (Table 6). However, there are also many disadvantages of blood transfusions for cancer-related anemia. First of all, many repeated blood transfusions are prone to induce allergic reactions, acute hemolytic transfusion reaction, allograft immune response, and post-transfusion pulmonary oedema. Secondly, since 1980s, transfusion-transmitted virus infection has becaming an increasingly important concern. Although the important progress in the screening and related technologies has been achieved and the transfusion safety has been significantly improved, the risk for visus infection still exists during transfusions. Hepatitis is highly prevalent in China and post-transfusion hepatitis is a critical issue for blood transfusion therapy in the clinical practice. Currently, epidemiological survey has revealed that the incidence of hepatitis B is around 10% among the voluntary blood donors and the anti-HCV positive rate is around 2% (13). Finally, although the hemoglobin level increases rapidly after the transfusion, it may drop to the pre-transfusion level shortly due to the persistence of malignancy or passive erythropoietic response to chemotherapeutic agents. Therefore, the hemoglobin level fluctuate in a large range during the treatment, with a short maintenance time.
Overall, in principle, transfusion therapy should not be initiated in a CRA patient before his/her hemoglobin levels is decreased to 7 or 8 g/dL or lower. In patients whose Hb is below 7 g/dL or requires correction for hypoxia, in patients with EPO-refractory chronic symptomatic anemia, or in patients with severe anemia that do not have time and opportunity to receive EPO treatment, blood transfusion may be considered.
Full table
3.2 EPO therapy
Since 1990s, erythropoietins (ESA) has became the most important therapy for cancer-related anemia.
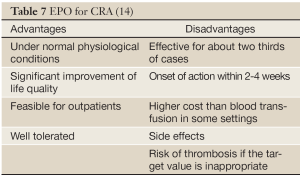
EPO is one of the most commonly used and most frequently studied erythropoietins. The main advantages of EPO therapy are: under normal physiological conditions; significant improvement of life quality; feasible for outpatients; and well tolerated (Table 7).
It is currently believed that both EPO and blood transfusions are the key means for treating anemia in cancer patients. However, the main goal of EPO therapy is to reduce blood transfusion. Many evidence-based studies have revealed that EPO can improve the life quality of patients with anemia and reduce their demands for blood transfusion. A meta analysis (15) in 2005, retrospectively reviewed 27 randomized clinical trials, in which 3,287 patients were treated with or without EPO (or Darb-EPO) from 1985 to 2001. The results showed that administration of EPO significantly reduced the demand of blood transfusion (HR=0.67, 95% CI: 0. 62-0.73). In 2006, the same group of authors updated their results of meta analysis regarding EPO therapy for cancer-related anemia (16). The updated data included 57 randomized clinical trials with and without EPO (or Darb-EPO) from 1985 to 2005, with a total of 9,353 cases that included the 3,287 cases in the previous study. Again, the results from 42 trials (n=6,510) showed that administration of EPO significantly reduces the requirement for blood transfusion (HR=0.64). Meanwhile, 22 trials (n=4,307) found that, for the patients with Hb<12 g/dL, Hb level was significantly improved after EPO administration (HR=3.43). In addition, 42 trials of survival (n=8,167) showed HR=1.08. Obviously, EPO significantly reduces the demand for blood transfusion without affecting the survival (HR=1.08).
Full table
3.3 Relationship between risk and benefit related to EPO therapy and blood transfusion for cancer patients
3.31 Effect of EPO therapy on the survival
Some recent retrospective studies suggest that EPO therapy for anemia can reverse the status of hypoxia in tumor patients, promote the effectiveness of anti-tumor therapy, and improve quality of life. Accordingly, some other prospective clinical studies have assessed the role of prophylactic EPO that pursues the aim at maintaining a high level of Hb, correcting hypoxic status of tumor cells, and thus improving the treatment efficacy. However, these studies have also provoked controversies on the potential effects of prophylactic EPO on the survival and tumor-free period (17-21).
3.3.1.1 ENHANCE study regarding the role of EOP therapy for radiotherapy-related anemia (17)
In a multicenter, randomized, double-blind, placebo-controlled trial (17), totally 351 patients with cancers (including carcinoma of the oral cavity, oropharynx, hypopharynx or larynx) were enrolled. The hemoglobin level at enrollment was <12 g/dL in women and <13 g/dL in men. All patients received curative radiotherapy at 60 Gy for completely and histologically incomplete resected disease, or 70 Gy for macroscopically incompletely resected advanced disease.
In addition, 171 received subcutaneous placebo, while 180 were assigned to subcutaneous EPO beta 300 IU/kg. The treatment was provided three times weekly, initiated 10-14 days before radiotherapy and lasted till the end of radiotherapy. The results showed that progression-free survival (PFS), locoregional progression-free survival (LPFS), and overall survival in EPO group was significantly inferior to those in placebo group (P=0.0008, 0.007, and 0.02, respectively). Further analysis (20) showed that the study had some problems in the group assignment and protocol implementation. First, the EPO treatment group and the control group were not well balanced for clinical characteristics. In ENHANCE study, the smoking rate in the EPO therapy group and the control group was 66% and 53%, respectively. In patients with hypopharynx cancer, however, it was 55% vs. 40% respectively between EPO therapy group and the control group, due to different proportions of patients with stage IV in two groups (85% vs. 70%). Secondly, the EPO prophylaxis for patients with an Hb level of 12-13 g/dL resulted in a high level of Hb, which reached 15.4 g/dL in the 9th week (vs. 12.9 g/dL in the control group). In addition, 40% of patients were excluded because they failed to follow the criteria of standard treatment. The cases that were legible for analysis were only 56.1% (101/180) in EPO group but 66.1% (113/171) in the control group.
3.3.1.2 BEST study on the prophylactic EPO for radiotherapy-induced anemia (18)
BEST study was a randomized, placebo-controlled clinical trial, including a total of 939 patients with metastatic breast cancer (Hb>13 g/dL). Its target value for hemoglobin was 12-14 g/dL. The one-year survival rate in EPO group was 70%, while 76% in placebo group (P=0.017). An increased risk of death was found in the first 4 months during the treatment in EPO group. The rate of disease progression was 6% vs. 3% between EPO therapy group and the placebo group. The incidence of thrombosis was 1% vs. 0.2% between these two groups. The majority of these female patients with metastatic breast cancer were anemia-free at the beginning of EPO therapy; However, the EPO therapy to maintain a high level of Hb might lead to a decrease in survival.
Nevertheless, there is argument for this study, such as unbalanced clinical characteristics in the patients between EPO therapy group and the control group. In the BEST study, the percentage of performance status (PO) >0 was 68% vs. 52% between EPO therapy group and the control group, whereas the percentage of the patients with stage III and IV was 42% vs. 37% between these two groups. Prophylactic EPO for patients with an Hb level below 12-13 g/dL in the BEST study that resulted in an extraordinarily high level of Hb (12-14 g/dL) may be a main cause of shorter survival.
3.3.1.3 Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomized trials (20)
The results of this meta-analysis were published in Lancet in May 2009. Data from a total of 13,933 patients with cancer in 53 trials were analyzed. The results revealed that 1,530 patients died during the active study period and 4,993 overall. Erythropoiesis-stimulating agents increased mortality during the active study period [combined hazard ratio (cHR) 1.17, 95% CI: 1.06-1.30] and worsened overall survival (HR: 1.06, 95% CI: 1.00-1.12), with little heterogeneity between trials (I2=0%, P=0.87 for mortality during the active study period and I2 =7.1%, P=0.33 for overall survival). A total of 10,441 patients on chemotherapy were enrolled in 38 trials. The cHR for mortality during the active study period was 1.10 (95% CI: 0.98-1.24) and 1.04 (95% CI: 0.97-1.11) for the overall survival. Few evidences have shown that there was a difference between trials of patients given different anticancer treatments (P for interaction=0.42).
However, the inclusion criteria of this analysis for clinical trials remain questionable, in which only 53% of trials had placebo control, only 30% with random sampling procedure, and only 70% completed. Among all the enrolled trials, 4% were early terminated and 26% incomplete. The inclusion bias will inevitably lead to invalid interpretations during the entire meta-analysis.
3.3.1.4 Meta-analysis of randomized, double-blind, and placebo-controlled clinical trials of darbepoetin α in the patient with chemotherapy-induced anemia (22)
The results of this meta-analysis were published in June 2009. It concluded the treatment with darbepoetin α did not increase the mortality (HR=0.97, 95% CI: 0.85-1.10), and also had no influence on the progress-free survival (HR=0.93, 95% CI: 0.84-1.04) and time to disease progression (HR=0.92, 95% CI: 0.82-1.03). However, as expected, treatment with darbepoetin α increased the risk of thrombotic events (HR=1.57, 95% CI: 1.10-2.26). The overall survival (OS) and progress-free survival were not affected by the baseline level of Hb. It seemed that the patients with Hb level >12 or 13 g/dL had a longer overall survival and progress-free survival. It was also noticed that blood transfusions and the increased ratio of HB that relied on blood transfusions (14 days >1 g/dL; 28 day >2 g/dL) were correlated with an increased risk of death and disease progression, which were observed in both treatment groups. However, for the patients that did not receive blood transfusion, an increased ratio of Hb did not increase the risk of adverse outcomes. Compared to the placebo, darbepoetin α significantly reduced the risk related to one or more blood transfusions.
Meanwhile, this meta-analysis also noted the problems in the above two meta-analyses: the criteria for the clinical studies in meta-analysis were not strict, leading to many invalid cases and negative results.
Obviously, EPO can be used for the treatment of cancer-related anemia by reducing the demand of blood transfusion and improving the quality of life. For the safety reason, however, EPO is not recommended in the cancer patient that dose not have a clinical sign of anemia or have not received chemotherapy. Further investigations on the effects of EPO on the overall survival, progress-free survival, and disease progression are warranted.
3.3.2 Blood transfusion and virus infections
In 2007, the US Food and Drug Administration (FDA) affiliated Oncologic Drugs Advisory Committee (ODAC) held a special meeting on the clinical application of EPO, during which it was stated that blood transfusion-associated virus infections (mainly HIV infection) had dramatically dropped in the past two decades due to the improvement of blood test techniques (5); on the other side, the excessive application of EPO for the prevention of anemia may shorten the survival. Therefore, the ODAC pointed out that EPO should be mainly applied for radiotherapy-induced anemia in tumor patients; meanwhile, the prophylactic use of EPO should be strictly controlled. In China, however, blood transfusion-related virus infections are mainly viral hepatitis (especially hepatitis C), which should be carefully considered.
The most common transmission routes for HCV infection are blood transfusion and use of plasma/blood products. About 80-90% of the post-transfusion hepatitis cases are hepatitis C (22). The high risk populations for hepatitis C include individuals who have received blood transfusion, hemodialysis, organ transplantation, and non-disposable syringes or ENT instruments without strict disinfection. The China Hepatitis C Prevention Forum, which was held in 2007 under the sponsorship of Chinese Foundation of Hepatitis Prevention and Control (23), noted that, according to a viral hepatitis epidemiological survey in China in 1992, the positive rate of anti-HCV antibody in the general population was 3.2%, and about 38 million people were carriers of HCV. According to the reports of National Notifiable Diseases released by the Ministry of Health, the number of new HCV cases increases annually (22).
It was therefore concluded that, after the tumor progression and the risk of post-transfusion viral hepatitis is well balanced, the recommendation grades of EPO, the main approach for CRA, should be higher than those in the Western countries.
3.4 Treatment of CRA in China
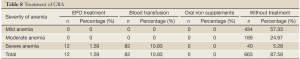
Currently, the treatment of CRA in China is far from satisfactory. According to the results of China Anemia Survey (Table 8), most CRA cases did not receive appropriate treatment, and only a few patients with Hb levels below 8 g/dl received anemia correction. Of over 2,000 tumor patients, 757 had anemia, among whom 82 (10.83%) had received RBC suspension transfusion due to CRA, while only 12 (1.59%) had a record of EPO treatment.
Most Chinese literature on EPO for CRA are focused on recombinant human erythropoietin injection, which has shown promising effecacies in elevating the Hb level in CRA patients and lowering the demands for blood transfusion.
Full table
3.4.1 Phase III clinical studies on the role of recombinant human erythropoietin injection for CRA
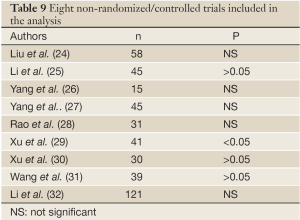
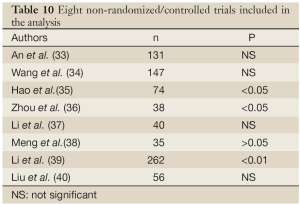
Quite a few studies on the role of recombinant human erythropoietin injection for CRA have been carried out in China. These studies can be classified into two catagories: non-randomized studies (n=8) (Table 9), focusing on the change of CRA patients before and after treatment (24-32), and randomized controlled studies (n=8) (Table 10), comparing the difference of Hb after treatment between the treatment group and control group (33-40). The latter had more reliable evidence-based findings. In another typical study, Chu et al. (41) reported their findings from a multi-center, randomized, controlled phase III trial, in which 121 tumor patients with an Hb level ≤10.5 g/dL or with a drop of Hb level ≥1.5 g/dL after chemotherapy were randomized into chemotherapy alone group and themotherapy plus EPO (150-300 IU/kg) group. EPO was administered three times weekly, with 4-8 weeks in each treatment course. The resulst showed that the combination group had significantly higher Hb level: the Hb level increased by ≥2.0 g/dL in 62% of the patients in the combination group, which was significantly higher than that in the chemotherapy alone group (3%); meanwhile, the requirement for blood transfusion was also significantly lower in the combination group (7%) than in the chemotherapy alone group (20%).
Full table
Full table
3.4.2 Phase IV clinical studies on the role of recombinant human erythropoietin injection for CRA
A large number of Phase IV clinical studies on the role of recombinant human erythropoietin injection for CRA have been published in recent years. Of these studies, 19 are relatively valuable, and most of them (n=14, 74%) were published by authors from tertiary hospitals. These articles have a relatively uniform conclusion that recombinant human erythropoietin injection are effective and safe in improving the Hb level. These 19 clinical reports include 1 multi-center research, 12 parallel studies with negative controls, 5 paraplle controled studies that compared pulse therapy with conventional therapy, and 3 studies with self controls. A total of 1,322 patients were enrolled in these 19 studies (Table 11).
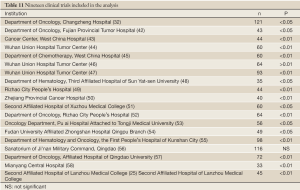
Full table
All the patients enrolled in these 19 studies had CRA, which were due to chemotherapy, surgery, tumor, or tumor-related malnutrition. Most patients had solid tumors, and only a few were suffered from hematologic cancers. According to their primary sites, these tumors mainly include gastrointestinal tumors (including gastric cancer, colorectal cancer, colon cancer, and esophageal cancer), followed by lung cancer, gynecologic tumors (including breast cancer, ovarian cancer, and cervical cancer), hematologic cancers (including acute leukemia, chronic myeloid leukemia, malignant lymphoma, multiple myeloma, and myelodysplastic syndrome), and bladder cancer.
Hb level was used as a key indicator for efficacy assessment in all studies. In addition, 7 studies also used Hct and 9 used the demands for blood transfusion. Notably, the score of quality of life was used for efficacy assessment in 7 studies. Previously, although anemia-related symptoms severely affected the quality of life of the patients, chemotherapy-associated anemia was somehow neglected and tended to be under-treated. Nevertheless, in developed countries, the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) has been introduced to systematically assess the fatigue and quality of life in patients treated with chemotherapy. These studies have demonstrated the definite correlation among chemotherapy-associated anemia, fatigue, and quality of life. Meanwhile, the use of erythropoietin can remarkably raise the Hb level, improve the FACT-F and FACT-General scores, and improve the quality of life. Currently, the QoL score has commonly been applied as an indicator for efficacy assessment in clinical observasions, reflecting that the QoL in cancer patients has increasingly been the focus of clinical practice in China.
Yan et al. randomized 60 tumor patients receiving chemotherapy into two groups: the treatment group was given recombinant human erythropoietin (10,000 IU, sc, tiw) and iron supplementation. No recombinant human erythropoietin was provided in the control group. After treatment, the Hb level increased by 22.7 g/L in the treatment group and decreased by 9.5 g/L in the control group (P<0.01). Meanwhile, the sleep quality score in the treatment group was significantly improved after treatment (P<0.01) (45).
Cheng et al. equally randomized 60 cancer patients with post-operative anemia into treatment group and control group. Similarly, the treatment group was given recombinant human erythropoietin (10,000 IU, sc, qod), which lasted 8 weeks. Non-responsive patients or those with laboratory evidences of iron deficiency during treatment were supplemented with ferrous succinate (0.1 g, orally administered, tid). No recombinant human erythropoietin was provided in the control group, although the other interventions were same as in the treatment group. After the intervention, Hb level increased by >20 g/L in 22 patients (73.3%) in the treatment group and only in 4 patients (13.3%) in the control group (P<0.01). Similar benefit was also found in the demand for blood transfusion. In the treatment group, 5 of 6 patients who had been transfusion-dependent before treatment became transfusion-independent after treatment; in addition, the treatment also was superior to the control group in terms of blood transfusion volume (600 vs. 1,600 mL, P<0.05) and number (3.396 vs. 26.60, P<0.05).
Therefore, recombinant human erythropoietin has definite efficacy in improving CRA. As shown in these studies, for either the dosages recommended in the manufacturer’s instructions or the high dose used for pulse-maintenance treatment, recombinant human erythropoietin remarkably increased the Hb level and hematocrit after 2-4 weeks of treatment (P<0.01 or P<0.05). As shown in clinical studies that have investigated serum EPO, quality of life, number of blood transfusions, and blood transfusion volume, the use of recombinant human erythropoietin significantly improved EPO level and QoL score (P<0.01 or P<0.05) and significantly decreased the volume and time of blood transfusion (P<0.05).
Most clinical observations (n=12) also arranged iron supplementation, when appropriate, for their patients during the administration of recombinant human erythropoietin, which reflects that the assumption that the co-administration of iron supplements and EPO can increase the responsiveness of Hb has gradually been recognized by oncologists. However, most studies did not describe the timing and dosages of their iron supplements in details. Although intravenous iron has higher bio-availability and less adverse reactions and has been widely accepted in the developed countries, it is seldom used in mainland China. Among these 19 studies, none mentioned intravenous iron supplementation.
Most studies only reported mild adverse reactions (e.g., pain at the injection site, fever, fatigue, and dizziness) after the administration of EPO. Of these 1,322 subjects, only 7 (0.5%) experienced drug-induced hypertension. All these adverse reactions resolved after symptomatic management. No severe reverse event such as allergy and venous thrombosis was reported. The overall incidence of adverse reactions was less than 10%. Notably, 7 studies used protocols with a dosage higher than that recommended in the manufacturer’s instructions, such as 40,000 IU on d1,4,7,10,13,20,27,34, and 41; or, 20,000 IU, qd on d1-2; or, 10,000 IU, qd on d3-8, followed by 10,000 IU, tiw, etc. All these protocoles also did not cause severe adverse events such as thrombosis. It is reasonable to assume that EPO treatment is safe only if: routinely monitor the changes of Hb and Hct levels during the treatment (once weekly during the initiation period and every two weeks during the maintenance period); adjust the dose accordingly to avoid excessive RBC production (ensure that Hct is below 36 vol%); and, suspend the use of EPO if necessary.
3.4.3 Phase IV clinical studies on the role of recombinant human erythropoietin injection for CRA
Currently, when applied for treating chemotherapy-induce anemia, the starting dose of EPO in the United States reaches 40,000 IU or even 60,000 IU (59-61), which is remarkably higher than the standard starting dose several years ago. The recommended starting dose of EPO approved by US FDA for treating CRA is 150 IU/kg (subcutaneous injection, three times weekly). After 4 weeks of administration, the dose can be increased to 300 IU/kg (three times weekly) if the patient is unresponsive. An alternative dose can be 40,000 IU taken via subcutaneous injection once weekly, which is expected to reach a similar efficacy with the injections at 3 times per week (62). A pharmacokinetic study compared the efficacy of EPOGEN 150U/kg 3 times weekly with the once weekly Epogen-treatment and found that the latter had higher Cmax (3- to 7-fold), longer Tmax (2- to 3-fold), higher AUC0-168 h (2- to 3-fold), and lower clearance rate (50%).
Most Western countries have adopted EPO as key option for treating chemotherapy-induced anemia (Hb level). Currently the initial dose of EPOGEN is 40,000 or 60,000 IU weekly in foreign countries, which has shown good clinical efficacies. In a double-blinded placebo-controlled trial participated by 338 patients, EPOGEN was given once weekly, and the longest duration of EPOGEN treatment was 4 months; the results showed that the incidences of adverse reactions between the treatment group and control group were similar; meanwhile, the treatment also did not cause remarked impact on blood viscosity (63). In China, the EPO injection 10,000 IU [Yibiao(r)] was developed by 3SBio, Shenyang, Liaoning Province, China. It was licensed by the State Food and Drug Administration in 2001 for treating CRA. Clinical trials have confirmed that this product has similar efficacy in treating CRA as its foreign counterparts.
A Japanese study demonstrated that weekly administration of EPO 36,000 IU significantly increased hemoglobin level and ameliorated the decline of QOL in CIA patients (64).
A domestic clinical trial compared the effectiveness and safety of EPO 10,000 IU three times weekly with those of EPO 36,000 IU once weekly in treating chemotherapy-induced anemia (65). A total of 206 patients with non-myeloid malignancies accompanied with chemotherapy-induced anemia were divided into two groups: EPO 36,000 IU once weekly group (treatment group) and control group (EPO 10,000 IU, three times weekly). During the 8-week treatment, the obvious effective rate of increase in Hb was 52.0% and 51.9% in the control group and EPO 36,000 IU group (P>0.05), and the overall response rate (obvious effective rate + effective rate) was 73.5% and 67.3%. CMH test showed that the difference between the two groups was not significant (P>0.05). The maximal value of increase in Hb from baseline was 112 g/L in the control group and 113 g/L in the treatment group. The difference of Hb value between the maximum and the baseline was 22 and 23 g/L (both P>0.05), respectively, with no significant difference between the two groups. The median time of Hb increase >10 g/L from the baseline was 18 and 16 d, respectively (P>0.05). The median time of Hb increase >20 g/L from the baseline was 31 and 32 days respectively (P>0.05). The percentages of blood transfusion were 2.9% and 8.7%, respectively, in the two group during the treatment, with no significant difference in the times and volume of blood transfusion (P>0.05). The total incidence rates of adverse events were 21.6% in the treatment group and 18.3% in the control group; the incidence rates of drug-associated adverse events were 2.9% in the control group and 1.9% in the treatment group; and the incidences of severe adverse events were 3.9% in the control group and 1.0% in the treatment group. As shown in this clinical trial, within 8 weeks, the overall response rate of EPO (36,000 IU) once a week for anemia is similar in comparison with routine EPO (10,000 IU) three times a week. It can significantly decrease injection frequency, alleviate patient’s pain, and will not increase the adverse events. The drug-associated adverse events are mild and transient, and most of them can resolve spontaneously (65).
3.4.4 Studies on QoL (66)
A limited number of domestic studies have explored the impact of EPO treatment for CRA on the QoL. Wen et al. (66) have reported a prospective controlled study, in which 43 patients with advanced lung cancer were randomized into the chemotherapy alone group and chemotherapy combined with EPO (10,000 IU) group. EPO was administered three times weekly, with 4-8 weeks in each treatment course. Finally, the Karnofsky performance score (KPS) and QoL were signlificantly improved in the combination group (P<0.05). Li et al. (32) also reported a prospective non-randomized controlled study, in which 121 patients with CRA were treated with EPO α, and their anemia-related QoL was assessed with FACT-An. After the treatment, the FACT-An score was significantly increased, and linear regression analysis showed the change of Hb level was significantly correlated with FACT-An before and after EPO treatment.
4. Guidelines on clinical practice of cancer-related anemia
Based on the above literature and foreign guidelines on CRA, the Experts Committee on Cancer- and Treatment-Related Anemia, CSCO, proposed the following consensus:
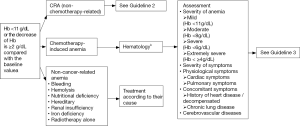
4.1 Guideline 1 - Diagnosis and assessment of CRA (Figure 1)
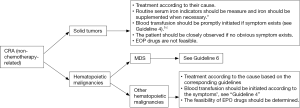
4.2 Guideline 2 - Treatment of CRA (non-radiotherapy-related) (Figure 2)
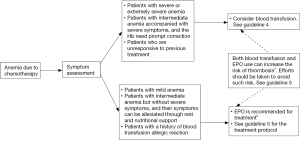
4.3 Guideline 3 - Treatment of chemotherapy-related anemia (Figure 3)
4.4 Guideline 4- Indications of blood transfusion in tumor patients (Figure 4)
Target Hb level in asymptomatic patients:
For chronic anemia patients with stable hemodynamics but without acute coronary syndrome: the aim of blood transfusion is to maintain the Hb level at 7-9 g/dL.
Target Hb level in symptomatic patients:
For patients with acute hemorrhage, along with the evidence of unstable hemodynamics or inadequate oxygen delivery: the aim of blood transfusion is to correct the unstable hemodynamics or maintain adequate oxygen delivery.
Symptomatic (including tachycardia, tachypnea, and orthostatic hypotension) anemia (Hb level <10 g/dL): the aim of blood transfusion is to maintain the Hb level at 8-10 g/dL, so as to avoid the occurrence of these symptoms.
The target Hb level in patients with acute coronary syndrome or acute myocardial infarction: the aim of blood transfusion is to maintain the Hb level >10 g/dL.
4.5 Guideline 5 - Prevention of thrombosis due to blood transfusion and/or EPO treatment (Figure 5)
EPO drugs for CRA should be stopped once the Hb level reaches 12 g/dL to avoid a high-Hb status. See “Guideline 5” for recommendations on populations with an increased risk of thrombosis (1). These patients should be treated with low-molecular-weight heparin upon a daily dose of 2,000-4,000 IU (once or twice daily). The treatment should typically last 1-2 weeks.
Patients who develop thrombosis can be treated with tPA, low-molecular-weight heparin, or fondaparinux sodium (decasodium).
In case of thrombosis, Thalidomide and lenalidomide as well as targeted therapies can be applied.
Oral administration of aspirin at a dosage of 40-100 mg/d can also be used for the prevention of deep vein thrombosis. See NCCN guidelines on tumor and thrombosis treatment (2012 edition).
4.6 Guideline 6 - Treatment of MDS-related anemia
4.6.1 International Prognostic Scoring System (IPSS) (Table 12)

Full table
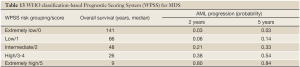
4.6.2 WHO classification-based Prognostic Scoring System (WPSS) for MDS (Table 13)
Full table
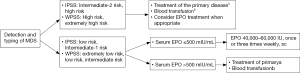
4.6.3 Treatment of MDS types and MDS-related anemia (Figure 6)
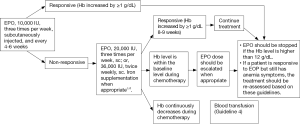
4.7 Guideline 7 - Administration and Dosage of EPOa,b (Figure 7)
4.8 Guideline 8 - Treatment decision and drug selection pertaining to the functional iron deficiency after EPO therapy (Figure 8)

4.9 Guideline 9 - Iron supplementation
4.9.1 Serum ferritin and iron supplementation (4)
Iron deficiency can be divided into three stages: iron depletion (ID), iron deficient erythropoiesis (IDE), and iron deficiency anemia (IDA). The diagnostic criteria of iron deficiency anemia include: (I) Serum iron <8.95 μmol/L (50 mg/dL); and (II) serum ferritin <12 μg/L. Also, it should have the features of microcytic hypochromic anemia. Patients with mild iron deficiency anemia are typically treated with intravenous iron (100 mg, once per week). It usually takes 2 weeks for the Hb level returns to the normal level. For patients with severe iron deficiency anemia, intravenous iron (100 mg, once per week, for four weeks) can be applied; when the Hb level is increased by 20 g/dL, the drug can be continuously provided till the iron deficiency anemia returns normal. Before the administration of intravenous iron, skin test should be performed under medical supervision.
In patients with renal failure due to cancer or chemotherapy, the continuous use of ESAs may result in functional iron deficiency (ferritin <800 ng/mL and transferrin saturation <50%) The iron stored in the reticuloendothelial system (RES) can be massively transported into the bone marrow and depleted during the rapid production of erythrocytes under the stimulation of ESAs. The decreased iron storage can not support the further hematopoiesis, and thus the effectiveness of ESAs will decrease over time. Absolute iron deficiency occurs when the iron storage further drops (ferritin ≤300 ng/mL and transferrin saturation <15%). If this condition exists before the initiation of ESAs treatment, iron supplementation should be applied firstly.
4.9.2 Indications of iron supplementation
- Oral iron supplements: Oral iron has the advantage of being simple, but it is limited by the fact that only 10% of the iron will be absorbed by human body and meanwhile the gastrointestinal irritation can also be problematic. Some patients may be allergic to oral iron supplements. Ferrous sulfate and ferrous fumarate are the most common types of iron supplement. Other available forms include ferrous succinate, ferrous gluconate, and ferrous lactate.
- Parenteral iron preparations: they can be thoroughly absorbed by human body, with rapid onset of action and without gastrointestinal irritation. However,compared with the oral supplements, they are less inconvenient during administration. Parenteral iron preparations include iron dextran, ferric gluconate, and iron sucrose. Iron sucrose is recommended when taking into account the patient tolerance and pharmacokinetics.
- Parenteral iron preparations can be used in patients who can not tolerate or are non-responsive to oral iron supplements, and are also indicated for functional iron deficiency in patients with chronic renal failure; patients who are receiving EPO treatment can also receive parenteral iron supplementation.
- A test dose should be given prior to starting iron dextran therapy, especially in patients with a history of drug allergy. The recommended iron dextran is low-molecular-weight iron dextran.
4.9.3 Administration of parenteral iron (Table 14)

Full table
4.9.4 Precautions of iron supplementation
Of these two iron supplements, iron dextran was approved firstly, and deeper research has been conducted on its efficacy and toxicities. Clinical practices have demonstrated that a single administration of iron dextran is associated with higher incidences of adverse events. For a new patient, an initial test dose of 25 mg should be administered firstly, after which the patients are observed for possible adverse effects. and then the patient should be observed for 1 hours for any adverse reaction. Then the remaining dose is injected. Research has shown that the adverse reactions of iron dextran are mainly due to the high-molecular-weight iron dextran. Therefor, clinically the low-molecular-weight iron dextran is recommended. Iron dextran has also been approved for intramuscular (IM) injection; however, considering its disadvantages including injection site pain, skin pigmentation, and slow drug absorption, IM injection is not recommended. Iron sucrose and iron dextran are usually well tolerated by most patients; however, iron sucrose has lower reported incidence of adverse events than iron dextran. In fact, a test dose is not required during the administration of iron sucrose. however, for safety reason, test dose is also listed in the above table. The common adverse reactions associated with intravenous iron supplements include: low blood pressure, nausea, vomiting or diarrhea, pain, high blood pressure, dyspnea, itching, headaches and dizziness.
Acknowledgements
Experts Committee on Cancer and Treatment-Related Anemia, CSCO
Director: Jun Ma and Jie-jun Wang
Deputy Directors: Li Zhang, Yuan-kai Shi, Jun Zhu, Jian-min Wang, and Shun Lu
Consultants: Yan Sun, Mei-lin Liao, Zhongzhen Guan
Members
Ji-feng Feng (Department of Oncology, Jiangsu Provincial Cancer Hospital), Bing Hu (Department of Oncology, Anhui Provincial Hospital), Cheng Huang (Department of Oncology, Fujian Provincial Cancer Hospital), Jin Li (Shanghai Fudan University Cancer Hospital), Xian-ling Li (Department of Oncology, Liaoning Provincial Cancer Hospital), Ji-wei Liu (Department of Oncology, the First Affiliated Hospital of Dalian Medical University), Wen-chao Liu (Xi’an Xijing Hospital), Xiao-qing Liu (Department of Oncology, Beijing 307 Hospital), Yun-peng Liu (Department of Oncology, the First Affiliated Hospital of Dalian Medical University), Shun Lu (Shanghai Chest Hospital), You Lu (Cancer Center, West China Hospital, Sichuan University), Jun Ma (Harbin Institute of Blood), Xue-zhen Ma (Qingdao Cancer Hospital), Shu-kui Qin (Nanjing Bayi Hospital), Yuan-kai Shi (CAMS Cancer Institute & Hospital), Jian-min Wang (Changhai Hospital, the Second Military Medical University), Jie-jun Wang (The Second Military Medical University Affiliated Changzheng Hospital), Yajie Wang (Changhai Hospital, the Second Military Medical University), Zhehai Wang (Shandong Provincial Cancer Hospital), Lin Wang (Nanjing Bayi Hospital), Yang Yao (Shanghai 6th People Hospital), Shi-ying Yu (Wuhan Tongji Hospital), Ming-zhi Zhang (Department of Medical Oncology, the First Affiliated Hospital of Zhengzhou University), Li Zhang (Sun Yat-sen University Cancer Center) Jun-yi Zhang (Guangzhou Nanfang Hospital), Yi-ping Zhang (Department of Medical Oncology, Zhejiang Cancer Hospital), Yue Zhang (Department of Integrative Medicine, Jilin Provincial Cancer Hospital), and Jun Zhu (Beijing Cancer Hospital)
Compiled by: Jun Ma, Jie-jun Wang, Li Zhang, and Shu-kui Qin
Revised by: Shu-kui Qin and Yao Wang
Disclosure: The authors declare no conflict of interest.
References
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cancer-and chemotherapy-induced anemia. 2011.V.2. 2011 (2011-08-01). Available online: http://www.nccn.org/index.asp
- Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol 2010;28:4996-5010.
- Groopman JE, Itri LM. Chemotherapy-induced anenmia in adults: incidence and treatment. J Natl Cancer Inst 1999;91:1616-34.
- Zhang ZN, Shen T. editors. Hematopathy diagnosis and curative standard. Beijing: science publishing house, 2007:5-6.
- Del Mastro L, Gennari A, Donati S. Chemotherapy of non-small-cell lung cancer: role of erythropoietin in the management of anemia. Ann Oncol 1999;10:S91-4.
- Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology 2005;68:3-11.
- Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS):A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer, 2004;40:2293-306.
- Miller CB, Jones RJ, Piantadosi S, et al. Decreased erythropoietm response in patients with the anemia of cancer. N Eng J Med 1990;322:1689-92.
- Vogelzang NJ, Breitbart W, Cella D, et al. For the fatigue coalition patient, caregiver, and oncologist perception ofcancer-related fatigue: results of a tripart assessment survey. Semin Hemat 1997;34:4-12.
- Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 1999;91:1616-34.
- Littlewood TJ, Bajetta E, Nortier JW, et al. Effects of epoetin alfa on hematologic parameters and quality oflife in cancer patients receiving nonplatinum chemotherapy: Results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2001;19:2865-74.
- Höckel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001;93:266-76.
- Wei ZW, Li FC. Epidemiology study of four results of pre-transfusion test in 2286 patients. Zhi Ye Yu Jian Kang 2004;20:63-5.
- Ludwig H, Fritz E. Anemia in cancer patients. Semin·Oncol 1998;25:2-6.
- Bohlius J, Langensiepen S, Schwarzer G, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst 2005;97:489-98.
- Bohlius J, Wilson J, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2006;3:CD003407.
- Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled triaI. Lancet 2003;362:1255-60.
- Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol 2005;23:5960-72.
- Kaanders JH, van der Kogel AJ. Erythropoietin to treat anaemia in patients with head and neck cancer. Lancet 2004;363:78-79;author reply 81-2.
- Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer:a meta-analysis of randomised trials. Lancet 2009;373:1532-42.
- Ludwig H, Crawford J, Osterborg A, et al. Pooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J Clin Oncol 2009;27:2838-47.
- China Foundation for Hepatitis Prevention and Control. Prevention and cure of Hepatitis C. 2010 [2010-08-01]. Available online: http://www.cfhpc.org
- Xu ZD, editor. Lemology. Shanghai: Shanghai medical university press 1997:23-37.
- Liu L, Ding Q, Lu H, et al. Recombinant human erythropoietin in the treatment of chemotherapy-induced anemia in patients with advanced NSCLC. Hua Zhong Ke Ji Da Xue Xue Bao: Yi Xue Ban 2004;33:219-21.
- Li GX. Recombinant human erythropoietin for the treatment of chemotherapy-related anemia. Zhong Guo Zhong Liu 2004;13:191-3.
- Yang Y, Wang WX, Jiang QY, et al. Recombinant human erythropoietin for the treatment of cancer-related anemia. Zhong Guo Lin Chuang Kang Fu 2003;7:1486-7.
- Yang WH, Wang CY, Wang SX, et al. The application of recombinant human erythropoietin in anemia associated with malignancy. Zhong Guo Fu Chan Ke Lin Chuang 2002;7:1486-7.
- Rao AH. Clinical observation on 31 patients of tumor-related anemia with recombinant human erythropoietin. Xian Dai Shi Yong Yi Xue 2007;19:559-61.
- Xu JP, Zhang XR, Xu JP, et al. Clinical Observation of Epiao in treating the radiotherapy-induced anemia. Liao Ning Yi Xue Za Zhi 2002;16:263-4.
- Xu F, Zhang L, Xiang XJ, et al. High-dose induction therapy followed by maintenance with recombinant human erythropoietin for 30 Patients with tumor-related anemia. Ai Zheng 2006;25:1120-2.
- Wang ZQ, Zhang L, Xu F. Efficacy Study of 31 cases of recombinant human erythropoietin in weekly treatment of chemotherapy-induced anemia. Xin Yi Xue 2006;37:160-2.
- Li R, Wu Q, Lu S, et al. Recombinant human erythropoietin-α in the treatment of malignant solid cancer-related anemia: a multicenter clinical trial. Zhong Liu 2008;28:132-5.
- An YH, Sun YW. Recombinant human erythropoietin at different doses for in cancer-associated anemia: clinical efficacy evaluation on 131 cases. Zhong Guo Yi Yuan Yong Yao Ping Jia Yu Fen Xi 2007;7:17-9.
- Wang YB, Chen WJ, Zhen WE, et al. Randomized comparison the clinical trial of high or common-dose erythromycin treatment in anemic patients with cancer. Shi Yong Zhong Xi Yi Jie He Lin Chuang 2007;7:1-4.
- Hao HK, Cai D, Han TQ, et al. Randomized multicentre trial of the influence of recombinant human erythropoietin on the perioperative patients. Fu Dan Xue Bao: Yi Xue Ban 2007;34:243-5.
- Zhou M, Wang YD, Han GD, et al. Application of erythropoietin in patients with esophageal carcinoma. Ling Nan Xian Dai Lin Chuang Wai Ke 2004;4:131-3.
- Li B, Chen XZ, Zhang HW, et al. The curative effect of erythropoietin in patients treated with chemotherapy. Zhe Jiang Zhong Xi Yi Jie He Za Zhi 2004;14:407-8.
- Meng JH, Huang RW, Lin DJ, et al. The tentative observation of erythropoietin in preventing chemotherapy-induced anemia in patients with hematological tumour. Guang Dong Yi Xue 2004;25:1329-30.
- Li Y, Long YP. Clinical study of recombinant human erythropoietin in gastrointestinal cancer patients with anemia. Xiong Bu Wai Ke 2002;15:236-7.
- Liu SJ. Preliminary study of erythropoietin treatment on gynecological anemia. Chen Du Yi Yao 2004;30:326-7.
- Chu DT, Zhang XR, Li LQ, et al. Effect of recombinant human erythropoietin in treating chemotherapy-induced anaemia. Zhong Hua Yi Xue Za Zhi 2001;17:1086-8.
- Liu J, Fan NF, Lin RB. Clinical observation of weekly recombinant human erythropoietin in treating chemotherapy-induced anaemia. Fu Jian Yi Yao Za Zhi 2010;32:122-5.
- Cao D, Jiang M, Qiu M, et al. High dose of rHuEPO in the treatment of chemotherapy-induced anemia in patients with cancer. Hua Xi Yao Xue Za Zhi 2005;20:563-4.
- Chen J, Wu G, Peng G, et al. Clinical observation of erythropoietin in treating the malignant tumour anemia. Zhong Liu Fang Zhi Za Zhi 2005;32:511-4.
- Yan X, Hou M, Lu JJ, et al. Effect of recombinant human erythropoietin on anaemic symptom and sleep quality in tumor patients. Zhong Guo Lin Chuang Kang Fu 2005;9:22-4.
- Liu L, Ding Q, Dai XF, et al. New regimen of recombinant human erythropoietin for anemic patients with malignancy with combination chemotherapy. Lin Chuang Xue Ye Xue Za Zhi 2007;20:31-6.
- Liu L, Ding Q, Song YQ, et al. Study on prevention of recombinant human erythropoietin (rhEPO) on chemotherapy-related anemia and influence on QOL. Shi Yong Ai Zhen Za Zhi 2005;20:624-8.
- Meng JH, Huang RW, Lin DJ, et al. The tentative observation on erythropoietin for the chemotherapy-related anemia. Guang Dong Yi Xue 2004;25:1329-32.
- Chen ZF, An BG, Su Z, et al. Study on the efficacy of recombinant human erythropoietin with less dosages or common dosages on anemia with malignancies. Zhong Guo Yi Yuan Yao Xue Za Zhi 2009;23:2023-5.
- Li B, Chen XZ, Zhang HW, et al. The efficacy of erythropoietin for the radiotherapy-induced anemia. Zhe Jiang Zhong Xi Yi Jie He Za Zhi 2004;14:407-8.
- Chen LS, Zhang QR, Chen XG, et al. Study on serum erythropoietin levels in patients of hematologic malignancies with aneamia and application of recombinant human erythropoietin. Bai Xue Bing·Lin Ba Liu 2009;18:681-5.
- Chi YH, Wang H, Chen Y. Clinical effect of therapy with high-dose recombinant human erythropoietin on cancer-related anemia. Xian Dai Zhong Liu Yi Xue 2008;16:1413-7.
- Zhang LJ, Fei Y, Feng G, et al. Clinical observation on 29 cases of recombinant human erythropoietin treatment on chemotherapy-related anemia. Zhong Guo Quan Ke Yi Xue 2005;16:1413-7.
- Zou J, Sun LH, Meng YH, et al. Clinical study of recombinant human erythropoietin for treatment of chemotherapy-induced anaemia. Ji Lin Yi Xue 2010;31:4264-8.
- Shen WX, Chen MB, Shen G, et al. Clinical study of the anemic management with rhEPO in malignancy patients receiving chemotherapy. Nan Jing Yi Ke Da Xue Xue Bao: Zi Ran Ke Xue Ban 2008;28:1054-7.
- Ji L, Chen WW. Analysis on 115 cases of recombinant human erythropoietin for the prevention and treatment of chemotherapy-induced anaemia. Zhong Guo Wu Zhen Xue Za Zhi 2008;8:2913-5.
- Sun YW, Mou L, An YH. The Observation on the Curative Effect of high doses of recombinant human erythropoietin treatment on chemotherapy-related anemia. Zhong Guo Yi Yao 2008;3:542-5.
- Feng G. Recombinant Human Erythropoietin for the Treatment of Cancer Related Anemia. Zhong Liu Yu Fang Yu Zhi Liao 2009;22:139-143.
- Boccia RV. Erythropoietic Agents in Chemotherapy-induced Anemia: A Review of Recent Therapeutic Progress, Issues, and Concems. Current Hematology Reports 2004;3:397-405.
- Gabrilove JL, Cleeland CS, Livingston RB, et al. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality oflife are similar to three-times-weekly dosing. J Clin Oncol 2001;19:2875-82.
- Cortesi E, Mancuso A, Ceratti A, et al. Effectiveness and safety of an induction therapy with epoetin alfa in anermc cancer patients recelvlng concomitant chemotherapy. The Oncologist 2004; 9:459-68.
- Demetri GD, Kris M, Wade J, et al. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol 1998;16:3412-25.
- Ariganello O, Mancuso A, Molfetta MD, et al. A new induction schedule of epoetin alfa 40000IU in anemic patients with advanced lung cancer. Lung Cancer 2004;46:119-24.
- Tsuboi M, Ezaki K, Tobinai K, et al. Weekly Administration of Epoetin Beta for Chemotherapy-induced Anemia in Cancer Patients: Results of a Multicenter, Phase III, Randomized, Double-blind, Placebo-controlled Study. Jpn J Clin Oncol 2009;39:163-8.
- Wang HY, Zhang XR, Sun Y. A multicenter randomly controlled clinical trial to evaluate the efficacy and safety of rhEPO 136000IU) in the treatment of cancer chemotherapy-related anemia. Zhong Guo Zhong Liu Lin Chuang Yu Kang Fu 2009;16:222-6.
- Wen FG, Liu J, Li ZJ, et al. Impact of recombinant human erythropoietin hormone combined with chemotherapy on the qulity of life in patients with lung cancer. Zhong Guo Lin Chuang Za Zhi 2003;7:1871.