Prostate carcinoma in transgenic Lewis rats - a tumor model for evaluation of immunological treatments
Introduction
Prostate cancer remains a worldwide health problem, currently the most diagnosed cancer in the United States, and with rising incidence and mortality documented in Asian countries (1). Much progress has been achieved in the treatment of advanced prostate cancer over the last decade. However there remains an urgent need to identify early stage disease with high risk for recurrence or progression, and an urgent need for new treatments that can delay or prevent the establishment of metastatic disease. The requirement for preclinical animal models to evaluate these new therapies, consequently, remains equally important.
Transgenic mouse models have provided important systems for these evaluations, and perhaps the most extensively studied model is an autochthonous model in which the simian virus 40 (SV40) large T antigen (TAg) is expressed downstream of the prostate-specific probasin promoter (TRAMP, transgenic adenocarcinoma of mouse prostate) (2). In this model, TRAMP mice develop epithelial hyperplasia at sexual maturity (approximately 8 weeks of age) which progresses to high-grade prostatic intraepithelial neoplasia (PIN) and well-differentiated adenocarcinomas by 18 weeks of age. Poorly-differentiated tumors are observed subsequently, and tumors with a neuroendocrine phenotype, and metastases to the lymph nodes and lungs can be observed TRAMP mice over 30 weeks of age. This progression of disease has provided a valuable model for studying various treatments interventions, and in particular has provided a model for the investigation of immune-based treatments (3-6).
While it is a useful animal model in which prostate cancers develop and progress quickly, enabling intervention studies, a disadvantage of the TRAMP model is the small size of the mouse prostate, limiting the amount of tissue available for analyses. In addition, the mouse prostate is anatomically different from primate prostate, and does not express some of the same tissue-specific markers used for human prostate cancer evaluation such as prostate-specific antigen (PSA) or prostatic acid phosphatase (PAP). Historically, rats have served as models for human prostate cancer and prostatitis given the observations that rats develop prostate inflammation with age and changes in hormone exposure (7-9) and can develop prostate tumors upon exposure to carcinogens (10). In addition, the rat prostate shares some features with human prostate, including the expression of a tissue-specific acid phosphatase (11). Consequently, to provide an autochthonous model of prostate cancer development in the rat, Shirai and colleagues developed a model similar to the TRAMP in which the SV40 TAg was under the probasin promoter in transgenic Sprague Dawley rats (12). These investigators demonstrated that these transgenic male rats (TRAP, transgenic rat adenocarcinoma of the prostate) demonstrated atypical epithelial cell proliferation in the prostate by 4 weeks of age, and by 15 weeks of age there was a 100% incidence of prostate adenocarcinomas (12). Unlike TRAMP mice, development of prostate carcinomas in these rats was exquisitely androgen-dependent since castration of TRAP rats at 5 weeks of age prevented the development tumors, and castration at 20 weeks of age promoted tumor involution (12,13). The TRAP rat model has been used as a model for several different intervention approaches (14,15).
The Sprague Dawley strain is an outbred strain, and hence has been poorly characterized genetically and immunologically. As an immunology model, the Lewis strain has been best characterized, including identification of the peptide binding specificities for strain-specific MHC class I and II molecules (16,17). Consequently, the Lewis strain of rats has been used as a model for autoimmune disease and tolerance (18) and antigen-specific vaccination (19). In addition, the development of age-dependent prostatitis in this strain has led it to be specifically evaluated as a model for T-cell mediated prostatitis (20,21). Finally, the presence of the tissue-specific PAP protein has led us to use the Lewis strain as a model for vaccine strategies targeting PAP (22,23). In particular, we have recently identified a RT1.Al MHC class I epitope specific for human PAP that is recognized in Lewis rats following human PAP-specific immunization (24). Given these findings, we sought to generate an autochthonous model of prostate cancer progression in the Lewis rat that might ultimately be useful as a tumor vaccine model. In the current study, we backcrossed the Sprague Dawley SV40 TAg+ rats onto the Lewis strain and characterized this strain (Lew-TRAP) with respect to tumor development. In addition, we developed prostate epithelial cell lines from this strain. Finally, we report that immunization of Lew-TRAP rats with a plasmid DNA vaccine encoding PAP can elicit PAP-specific, RT1.Al-restricted immune responses, demonstrating its usefulness as an experimental immunology model.
Materials and methods
Animals
Transgenic Sprague Dawley rats with the SV40 TAg gene downstream of the prostate-specific probasin promoter were previously described (12). Breeders of this strain were generously provided by Dr. T. Shirai (Nagoya City University Medical School, Nagoya, Japan) via Dr. C. Lamartiniere (University of Alabama, Birmingham, AL, USA). Heterozygous SV40 TAg+ female breeders were crossed with non-transgenic Lewis strain males (Charles River, Wilmington, MA, USA) for 10 generations to develop a transgenic rat with the Lewis strain background (Lew-TRAP). At 21 days of age, offspring were weaned and ear punched for screening for the transgene. Tissue was digested with 50 mM NaOH at 95 °C for 1-3 hours. A PCR-based screening assay was performed to evaluate transgene incorporation, as previously described (12). Rats were fed a chow diet (8604 Teklab, Harlan Laboratories, Madison, WI, USA), consumed distilled water ad libitum, and maintained on a 12-hour dark/12-hour light schedule. They were housed in an American Association for the Accreditation of Laboratory Animal Care (AAALAC)-accredited facility. All experimental protocols were received and approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC).
Growth curve and tumor development
SV40 TAg+ transgenic and wild type (non-transgenic) littermates from Lewis and Sprague Dawley rat strains underwent necropsy at 4, 10, 12, 15, 20, 25, 30, and 35 weeks of age. Whole body mass, and mass of the genitourinary (GU) complex (prostate, seminal vesicles, and bladder), were measured. Prostate tissues were placed in 10% buffered formalin for histopathological evaluation. The presence of metastatic tumors was determined by gross observation. Where indicated, some groups of Lew-TRAP rats underwent surgical castration at 20 weeks of age prior to tumor development surveillance at 30 weeks of age.
Histopathology
Tissue sections containing both the ventral and dorsal lateral lobes of the prostate were stained with hematoxylin and eosin (University of Wisconsin Experimental Pathology Laboratory). Tumor development was scored in a blinded fashion with respect to animal age and genotype using the following grading scale: Grade 1 (benign), Grade 2 (prostatic intraepithelial neoplasia, PIN), Grade 3 (moderately-to-well-differentiated adenocarcinoma), and Grade 4 (poorly differentiated carcinoma), using the same criteria as used for grading murine prostate tumor models (25). For tumors containing multiple histopathological grades per section, the histopathological grade reported was based on the most advanced grade observed.
Immunohistochemistry
Immunohistochemistry was performed as previously described (6). For AR and SV40 immunohistochemistry, tissue sections were stained with the primary antibodies (AR: clone R/NR3C4, R&D systems, Minneapolis, MN, USA; SV40: clone PAb 101, BD Biosciences, San Jose, CA, USA) and developed using the LSAB+system-HRP (Dako, Carpinteria, CA, USA) and Metal Enhanced DAB substrate kit (Thermo Fisher Scientific, Rockford, IL, USA). Slides were imaged using an Olympus BX51 microscope (Olympus, Center Valley, PA, USA) in combination with SPOT analysis software (SPOT Imaging Solutions, Sterling Heights, MI, USA).
Generation of Lewis rat prostate tumor cell lines
Tissue samples of ventral prostate tumors were obtained from Lew-TRAP rats at 32 weeks of age. Tissues were minced into small sections and cultured in BRFF medium (Athena Enzyme Systems, Baltimore, MD, USA) supplemented with 20% fetal bovine serum and penicillin streptomycin at 37 °C in an atmosphere of 5% CO2. Cell lines were evaluated for SV40 and androgen receptor (AR) expression by Western blot (SV40: clone PAb 101 BD Biosciences, San Jose, CA, USA; AR: clone EP670Y Novus, Littleton, CO, USA) as previously described (6). The presence of prostatic acid phosphatase in tumor cell line lysates was determined by using a tartrate-inhibited phosphatase activity assay as described previously (26).
Immunization studies
Seven week old, male Lew-TRAP rats were immunized six times intradermally at weekly intervals with 100 µg of pTVG-HP or pTVG4. Two weeks after the last immunization (at fourteen weeks of age), the rats were euthanized. Spleens and sera were obtained under sterile conditions for immunological analyses.
Analysis of immune responses
Antigen-specific T-cell proliferation
Splenocytes harvested from immunized animals were evaluated using methods previously described (22,24). Specifically, 2×105 cells were cultured with 2 µg/mL human PAP protein (Chemicon Int., Temecula, CA, USA), 2 µg/mL individual peptides (HP201-215 or RP200-214), or 10 µg/mL phytohemaglutinin (PHA) (Sigma, St. Louis, MO, USA) for 72 hours at 37 °C in an atmosphere of 5% CO2. Then, cultures were pulsed with 1 µM BrdU (BD Biosciences, San Jose, CA, USA) for eight to twelve hours. Antigen-specific, proliferating (BrdU+) CD4+ or CD8+ T cells were detected using an intracellular flow cytometric staining method (BD Flow kit, BD Biosciences) according to the manufacturer’s standard protocol, and BrdU incorporation was measured and analyzed as previously described (22).
Interferon gamma (IFNγ) enzyme-linked immunosorbent assay (ELISA)
Splenocytes from immunized rats were cultured with the human PAP protein, individual peptides (HP201-215 or RP200-214), or PHA for 72 hours as described above. The presence of IFNγ in the culture media was determined using a quantitative capture ELISA specific for rat IFNγ as described previously (22,24). Results are reported as the mean IFNγ concentration and standard deviation from multiple replicates.
Antigen-specific antibodies
The presence of antibodies specific to human PAP (or ovalbumin, negative control) in the sera of immunized rats was determined by indirect ELISA, as described previously (22).
Results
Probasin/SV40 TAg+ transgenic rats on the Lewis background strain develop age-dependent prostate tumors with high penetrance
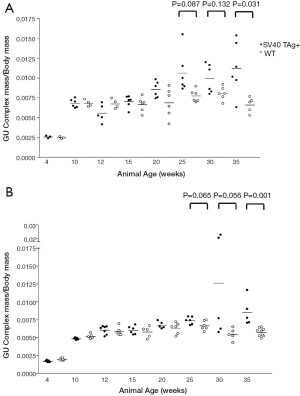
We sought to develop an autochthonous model of prostate tumor development in the Lewis rat strain, a strain that has been well characterized immunologically with respect to MHC restriction (16,17), autoimmune disease development (18,27), experimental models of prostatitis (8,21), and immunological evaluation following vaccination (19,23,24). A transgenic rat model of prostate cancer, in which the SV40 TAg is expressed under the prostate-specific probasin promoter, TRAP, has been previously described (12). However, the strain used was the outbred Sprague Dawley strain, and investigators demonstrated that the development of prostate tumors differed with respect to genetic strain background from F1 crosses (28). In order to characterize prostate tumor development in the Lewis strain, we crossed transgenic TRAP female breeders on the Sprague Dawley background with male Lewis strain rats for 10 generations prior to inbreeding within the transgenic Lewis strain. Tumor development was then characterized both grossly and histologically with respect to age. As shown in Figure 1, there were detectable differences in GU complex mass with respect to total body mass between the SV40 TAg+ transgenic and non-transgenic littermates in both the Lewis and Sprague Dawley strain backgrounds by 20 weeks of age, and this was significantly different by 35 weeks of age. Tumor development in the prostate tissues (both ventral and dorsolateral lobes) from the Lewis and Sprague Dawley SV40+ transgenic rats and their littermates were histopathologically scored in blinded fashion (Figure 2 and Table 1). As demonstrated, areas of atypical hyperplasia and PIN were identifiable as early as 4 weeks of age in Lew-TRAP rats, with invasive carcinoma identified by 12 weeks of age and poorly differentiated tumors seen as early as 25 weeks of age (Table 1). The overall disease progression was similar for the Lewis strain as for the Sprague Dawley strain. Tumors were not seen in the seminal vesicles of the transgenic rats regardless of age (data not shown). In addition, no overt evidence of metastasis was noted, however ~20% of the Lew-TRAP rats over 30 weeks of age had locally invasive disease with hydronephrosis. The presence of taste bud tumors was observed in <5% of animals, as has been reported for Sprague Dawley TRAP rats (28).
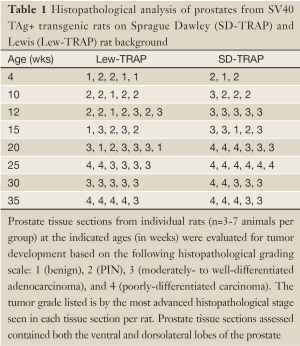
Full table
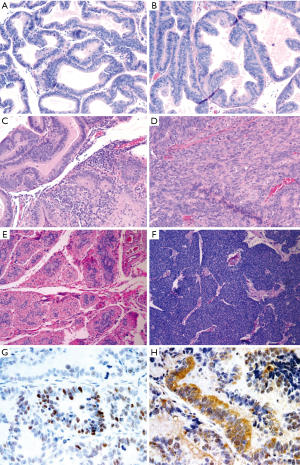
Prostate carcinomas in Lew-TRAP rats are primarily androgen-dependent
Lew-TRAP rats (n=5) were castrated at 20 weeks of age and then euthanized at 30 weeks of age for tumor evaluation. Adenocarcinomas were not observed in 4 out of 5 transgenic rats, suggesting that the tumor development is mostly androgen dependent, at least to 20 weeks of age (Figure 2E). A poorly differentiated tumor was observed in 1 of 5 animals (Figure 2F).
Prostate epithelial cell lines derived from Lew-TRAP rats
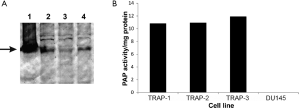
Prostate tumors obtained from the ventral lobes of prostates of 32-week-old animals were cultured in vitro to establish transplantable cell lines. Tumor cell lines were established that express the SV40 TAg (Figure 3A) and the androgen receptor (not shown), demonstrating it is of prostate origin. In addition, these cell lines were found to express prostatic acid phosphatase as determined by the expression of tartrate-inhibited phosphatase activity in cell lysates (Figure 3B). Subcutaneous implantation of cell lines in wild type Lewis rats did not lead to tumor growth (data not shown).
Immunization of Lew-TRAP rats with a DNA vaccine encoding PAP elicited a PAP-specific immune response
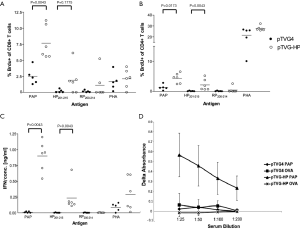
Lewis rats have been evaluated as a model of age- and vaccine-dependent prostate inflammation (20,21). We have previously characterized immune responses to the human prostatic acid phosphatase (hPAP) antigen in Lewis rats, and identified an MHC class I restricted epitope derived from hPAP in this strain (22-24). Consequently we sought to characterize whether the presence of prostate tumors in Lew-TRAP rats affected the ability to develop PAP-specific immune responses, and hence whether this transgenic model might be further developed as a tumor immunology model. Seven-week-old, male Lew-TRAP rats were immunized six times intradermally with 100 µg of pTVG-HP or pTVG4 (control vector) as previously described (24). As shown in Figure 4, PAP-specific T-cell immune responses were detected by antigen-specific proliferation (panels A and B) and by IFNγ-specific release (panel C). Of note, responses were specifically observed to the RT1.Al MHC class I epitope HP201-215. Antibodies to hPAP protein were also detected, as we reported previously for wild type Lewis rats (Figure 4D) (22). The findings suggest that the Lew-TRAP rat could be used as a prostate cancer immunology model, and specifically as a model to investigate the efficacy of immunotherapies targeting the PAP antigen.
Discussion
We report here the development of an autochthonous transgenic model of prostate cancer in the Lewis rat, derived from a transgenic strain previously characterized in the Sprague Dawley outbred strain (12,28). Tumors in this strain shared several characteristics with those of the parental strain; however, there were notable differences. For example, unlike what had been observed using F1 hybrids from other strains, tumors developed in these animals with 100% penetrance by 25 weeks of age, and with a similar progression to the parental Sprague Dawley strain (28). Similar to the Sprague Dawley strain, these tumors were predominantly androgen-sensitive, as castration at 20 weeks prevented the progression of tumors in the majority of animals. However, while this was not extensively evaluated, we did observe tumor recurrence with a poorly differentiated phenotype in 1 of 5 animals, a finding not previously observed with the Sprague Dawley strain, and more analogous to what has been observed with TRAMP mice (29). This difference may be due to the advanced age of the animals, at a time when poorly differentiated tumors might have arisen. However, given this finding in an inbred strain, it is also conceivable that the development of poorly-differentiated tumors arise under the pressure of androgen deprivation rather than necessarily deriving from a different cell type, as has been previously suggested (12). This remains to be determined, however, as we did not further evaluate these poorly-differentiated tumors for markers of tissue specificity or differentiation (such as androgen receptor, chromogranin A, synaptophysin expression), and we did not evaluate the effects of castration at an earlier age. Similar to the Sprague Dawley strain, and in contradistinction to the murine TRAMP model, we did not identify gross metastatic disease.
The goal of our study was to generate an autochthonous model of prostate cancer in the Lewis strain of rats, an inbred strain particularly amenable to immunological analyses. Using a plasmid DNA vaccine encoding PAP, we found that these rats were able to be immunized, with immune responses analogous to what we have observed in non-tumor bearing Lewis rats (22,24). In particular, MHC class I epitope-specific T cells were identified specific for the Lewis strain (24). In addition, we generated prostate cancer epithelial cell lines from this strain. While we were unable to use these as transplantable tumor cell lines in wild type Lewis rats, the presence of tumor cell lines potentially provides an additional in vitro model. The ability of these cell lines to form tumors in Lew-TRAP rats, which should be tolerant of the SV40 TAg, remains to be determined.
In summary, we report the generation of a prostate cancer tumor model in Lewis rats, generated by backcrossing of the SV40 TAg+ transgenic model developed in the Sprague Dawley outbred rat strain. We found that animals developed prostate tumors with 100% penetrance with age, that tumors were androgen sensitive, and that animals could be immunized with a human prostate-specific tumor antigen. Future studies will explore, using vaccines targeting rat prostate-specific tumor antigens, whether this can be used as a model to characterize anti-tumor immune responses within prostate tumors directly.
Acknowledgements
Grant support was provided by the National Institutes of Health (R01 CA142608 and P20 CA103697) and by the US Army Medical Research and Materiel Command Prostate Cancer Research Program (W81XWH-08-1-0341).
Disclosure: The authors declare no conflicts of interest.
References
- Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer 2005;41:834-45.
- Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A 1995;92:3439-43.
- Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res 2000;60:2444-8.
- Ahmad S, Casey G, Sweeney P, et al. Prostate stem cell antigen DNA vaccination breaks tolerance to self-antigen and inhibits prostate cancer growth. Mol Ther 2009;17:1101-8.
- Anderson MJ, Shafer-Weaver K, Greenberg NM, et al. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol 2007;178:1268-76.
- Olson BM, Johnson LE, McNeel DG. The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunol Immunother 2012. [Epub ahead of print].
- Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate 1988;12:271-86.
- Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol 1988;140:1049-53.
- Morón G, Maletto B, Rópolo A, et al. Effect of aging on experimental autoimmune prostatitis: differential kinetics of development. Clin Immunol Immunopathol 1998;87:256-65.
- Shirai T, Imaida K, Masui T, et al. Effects of testosterone, dihydrotestosterone and estrogen on 3,2’-dimethyl-4-aminobiphenyl-induced rat prostate carcinogenesis. Int J Cancer 1994;57:224-8.
- Roiko K, Jänne OA, Vihko P. Primary structure of rat secretory acid phosphatase and comparison to other acid phosphatases. Gene 1990;89:223-9.
- Asamoto M, Hokaiwado N, Cho YM, et al. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res 2001;61:4693-700.
- Said MM, Hokaiwado N, Tang M, et al. Inhibition of prostate carcinogenesis in probasin/SV40 T antigen transgenic rats by leuprorelin, a luteinizing hormone-releasing hormone agonist. Cancer Sci 2006;97:459-67.
- Seeni A, Takahashi S, Takeshita K, et al. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac J Cancer Prev 2008;9:7-14.
- Takahashi S, Takeshita K, Seeni A, et al. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Prostate 2009;69:644-51.
- Reizis B, Schild H, Stefanović S, et al. Peptide binding motifs of the MHC class I molecules (RT1.Al) of the Lewis rat. Immunogenetics 1997;45:278-9.
- Reizis B, Mor F, Eisenstein M, et al. The peptide binding specificity of the MHC class II I-A molecule of the Lewis rat, RT1.BI. Int Immunol 1996;8:1825-32.
- McIntosh KR, Linsley PS, Bacha PA, et al. Immunotherapy of experimental autoimmune myasthenia gravis: selective effects of CTLA4Ig and synergistic combination with an IL2-diphtheria toxin fusion protein. J Neuroimmunol 1998;87:136-46.
- Thygesen P, Christensen HB, Hougen HP, et al. Immunity to experimental Salmonella typhimurium infections in rats. Transfer of immunity with primed CD45RC+ and CD45RC- CD4 T-cell subpopulations. APMIS 1996;104:750-4.
- Lundgren R, Holmquist B, Hesselvik M, et al. Treatment of prostatitis in the rat. Prostate 1984;5:277-84.
- Liu KJ, Chatta GS, Twardzik DR, et al. Identification of rat prostatic steroid-binding protein as a target antigen of experimental autoimmune prostatitis: implications for prostate cancer therapy. J Immunol 1997;159:472-80.
- Johnson LE, Frye TP, Arnot AR, et al. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP). Vaccine 2006;24:293-303.
- Johnson LE, Frye TP, Chinnasamy N, et al. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol Immunother 2007;56:885-95.
- Johnson LE, Frye TP, McNeel DG. Immunization with a prostate cancer xenoantigen elicits a xenoantigen epitope-specific T-cell response. Oncoimmunology 2012;1:1546-56
- Kaplan-Lefko PJ, Chen TM, Ittmann MM, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 2003;55:219-37.
- Choe BK, Pontes EJ, Bloink S, et al. Human prostatic acid phosphatases: I. Isolation. Arch Androl 1978;1:221-6.
- Zou LP, Ma DH, Levi M, et al. Antigen-specific immunosuppression: nasal tolerance to P0 protein peptides for the prevention and treatment of experimental autoimmune neuritis in Lewis rats. J Neuroimmunol 1999;94:109-21.
- Asamoto M, Hokaiwado N, Cho YM, et al. Effects of genetic background on prostate and taste bud carcinogenesis due to SV40 T antigen expression under probasin gene promoter control. Carcinogenesis 2002;23:463-7.
- Gingrich JR, Barrios RJ, Kattan MW, et al. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res 1997;57:4687-91.


