Molecular therapy for gastric cancer
Introduction
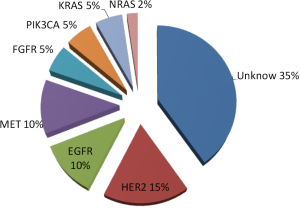
Gastric cancer can be divided into two types: differentiated, which is intestinal type in Lauren’s classification, and undifferentiated, which is diffuse type in Lauren’s classification histologically (1,2). The pathological findings can generally be divided into two metastatic patterns: peritoneal dissemination and hematogenous spread to the liver or lungs. Many types of genetic or epigenetic alterations cause these diverse phenotypes of gastric cancer (3-9). Overexpression of human epidermal growth factor receptor (EGFR, HER1), HER2, and HER3 is commonly observed by immunohistochemistry (IHC). On the other hand, gene mutations in members of the HER family are rare in gastric cancer (Figure 1). In addition, gene mutations are not commonly observed for downstream signal-transducing molecules under membrane receptors. The frequency of KRAS mutations of codon 12 or 13 was 5%, that of PIK3CA mutations in exon 9 was 5%, and that of NRAS mutations of codon 12 or 13 was in 2% in primary gastric cancer.
HER family
HER2
The HER2 gene is amplified or its product is overexpressed in 10% to 22% of gastric cancers, and is associated with enhanced cell proliferation and survival (5). Patients who highly overexpressed HER2 by IHC accounted for 10% of gastric cancers (3). HER2 is not the worse prognostic factor in gastric cancer, as opposed to breast cancer. A recent global randomized trial (ToGA) showed that trastuzumab, a humanized anti-HER2 monoclonal antibody, was effective against HER2-positive gastric cancer (5). IHC3+ and/or FISH-positive, which was defined as a HER2:EP17 ratio of 2 or more, was considered to be “HER2-positive” in the ToGA trial. In that study, the IHC3+ rate was 11.0%, and the FISH-positive rate was 23.1%. Trastuzumab exerts its anticancer effects by inducing antibody-dependent cytotoxicity that inhibits HER2-mediated signaling, and by preventing cleavage of the extracellular domain of HER2. In ToGA, patients with HER2-positive gastric cancer were randomized to receive 5-fluorouracil or capecitabine and cisplatin with trastuzumab every 3 weeks for 6 cycles, or chemotherapy alone. Tumor specimens from 3,807 patients were centrally tested to determine the HER2 status: 22.1% were HER2-positive. The median survival time (MST) was significantly improved with trastuzumab plus chemotherapy compared to chemotherapy alone (13.5 vs. 11.1 months, respectively) (P=0.0048; HR 0.74, 95% CI, 0.60, 0.91). RR was 47.3% in the trastuzumab plus chemotherapy arm and 34.5% with chemotherapy alone (P=0.0017). The safety profiles in the two groups were similar, and there were no unexpected adverse events in the trastuzumab arm. There was no difference in symptomatic congestive heart failure between the two arms. Decreases in asymptomatic left ventricular ejection fraction were reported in 4.6% of patients in the trastuzumab combined arm and in 1.1% of those in the chemotherapy arm.
Lapatinib is a tyrosine kinase inhibitor (TKI) against HER2 and EGFR. A phase II study (10,11) evaluated first-line lapatinib monotherapy in 47 patients and reported modest activity. Only 3 patients (7%) had a confirmed partial response (PR) and 2 (5%) had an unconfirmed PR. Nine patients (20%) had stable disease (SD). The median time to treatment failure (TTF) was 2 months and MST was 5 months. Another trial reported no partial response in 21 evaluable patients who had been treated with multiple prior therapies (11). In this study, gastroesophageal cancer patients were selected for EGFR-positivity using IHC and/or for HER2-positivity by FISH. Two patients had durable stable disease with 1,000 mg lapatinib, which lasted for 37 and 16 weeks. Multivariate proportional hazards modeling of IHC biomarkers revealed that higher levels of TGF-α were associated with a shorter TTP (P<0.05). Two phase III studies are currently underway for the development of second-line and first-line therapies (12,13). TYTAN is a randomized phase III study that is comparing paclitaxel with or without lapatinib as a second-line therapy in patients with HER2 FISH-amplified gastric cancer. The primary endpoint is overall survival and 260 patients will be enrolled (12). The LOGiC trial is comparing capecitabine and oxaliplatin with or without lapatinib as a first-line therapy in patients with advanced gastric cancer with HER2 amplification by FISH. The primary endpoint is overall survival (13). The results will be reported in the near future.
T-DM1, which is a three-part immunoconjugate consisting of trastuzumab, a stable linker, and the potent maytansine derivative DM-1 combines the antitumor activity of trastuzumab with the ability to deliver a microtubule-disrupting cytotoxic agent specifically to antigen-expressing tumor cells. In the phase III study EMILIA, T-DM1 was shown to be effective for advanced HER2-positive breast cancer (14). Pertuzumab inhibits the dimerization of HER2, and suppresses multiple HER signaling pathways, which leads to a more comprehensive blockade of HER2-drivensignaling (15). In the phase III study CLEOPATRA, pertuzumab plus trastuzumab and docetaxel combination therapy conferred a survival benefit compared with trastuzumab and docetaxel for HER2-positive breast cancer (16). These two drugs may also be promising for the treatment of HER2-positive gastric cancer.
EGFR
The overexpression of EGFR occurs in 58-86% of gastric adenocarcinomas (3,17-19). Patients that highly overexpressed EGFR by IHC accounted for 24% of gastric cancers. The prognostic value of EGFR is controversial. A phase II study for gastric cancer was carried out using gefitinib, a TKI of the EGFR, but the expected therapeutic outcome was not achieved (17,18). The response rate was 0% and 18% of the patients showed stable disease (17). In another phase II trial with erlotinib, the response rate was only 9% in patients who had esophago-gastric junctional cancer (18).
Monotherapy with cetuximab, a chimeric anti-EGFR monoclonal antibody, did not induce a response in gastric cancer patients (19). In phase II studies, cetuximab plus first-line fluoropyrimidine with irinotecan or platinum compounds has shown promising activity (20,21). The results of a randomized controlled phase III study of capecitabine and cisplatin (XP) with or without cetuximab in gastric and gastroesophageal junction cancer have recently been reported (22). Nine hundred four patients were randomized to 3-week cycles of twice-daily (days 1-15) capecitabine at a dose of 1,000 mg/m2 and iv cisplatin 80 mg/m2 on day 1 every 3 weeks, and weekly cetuximab 400 mg/m2 followed by 250 mg/m2/week, or chemotherapy alone. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival, best overall response, and safety. The median PFS was 4.4 months (95% CI, 4.2-5.5 months) in the cetuximab arm and 5.6 (5.1-5.7) in the XP arm (HR 1.091, 95% CI, 0.920-1.292; P=0.3158). The MST was 9.4 months (95% CI, 8.3-10.6 months) in the cetuximab arm and 10.7 (9.4-11.3) in the XP arm (HR 1.004, 95% CI, 0.866-1.165; P=0.9547). The RR was 30% with cetuximab and 29% with chemotherapy. XP plus cetuximab showed no benefit compared to XP alone in the first-line treatment of advanced gastric cancer.
The REAL-3 trial evaluated the addition of panitumumab, a fully human anti-EGFR monoclonal antibody, to epirubicin, oxaliplatin and capecitabine (EOC) in advanced esophago-gastric cancer (23). Five hundred fifty-three patients were randomised to receive EOC, epirubicin 50 mg/m2, oxaliplatin 130 mg/m2, and capecitabine 1,250 mg/m2/day, or mEOC, epirubicin 50 mg/m2, oxaliplatin 100 mg/m2, capecitabine 1,000 mg/m2/day, and panitumumab 9 mg/kg. The primary endpoint was overall survival. The secondary endpoints were PFS, RR, and safety. The MST was 11.3 months with EOC compared to 8.8 months with mEOC plus panitumumab (HR 1.37, 95% CI, 1.07-1.76; P=0.013). The median PFS was 7.4 and 6.0 months, respectively (HR 1.22, 95% CI, 0.98-1.52; P=0.068), with the RR was 42% and 46%. Multivariate analysis demonstrated that KRAS mutation (HR 2.1: 95% CI, 1.10-4.05, P=0.025) and PIK3CA mutation (HR 3.2: 95% CI, 1.01-10.40, P=0.048) each had negative prognostic value. These results suggest that an EGFR-targeted agent alone is not effective in all patients with gastric cancer.
Nimotuzumab is a humanized monoclonal IgG1 antibody against human EGFR. In a randomized phase II trial, patients received nimotuzumab plus irinotecan or irinotecan alone as a second-line therapy (24). The primary endpoint was PFS. Median PFS was 73 and 85 days, respectively (HR 0.860, 95% CI, 0.516-1.435; P=0.5668). The MST was 250.5 and 232 days in the nimotuzumab and irinotecan monotherapy groups, respectively (HR 0.994, 95% CI, 0.618-1.599; P=0.9778). The RR was 18.4% and 10.3%, respectively. A subset analysis of EGFR 2+ or 3+ patients by IHC revealed a median PFS of 118.5 and 59.0 days in the nimotuzumab and irinotecan monotherapy groups, respectively. On the other hand, a shorter median PFS was observed in EGFR 0 or 1+ patients (58.5 and 87.5 days). Nimotuzumab might show some activity in EGFR 2+, 3+ patients.
HER3
HER3 is a key dimerization partner for of the HER family that activates oncogenic signaling pathways to lead to cell survival and proliferation (25,26). Acquired resistance to anti-EGFR inhibitors may result from the activation of HER3 and/or HER2, which share overlapping signaling pathways. U3-1287 is a fully human anti-HER3 monoclonal antibody that has been shown to exhibit anticancer activity in preclinical models. In a Japanese phase I trial, it was shown to be tolerable up to 20 mg/kg. No DLTs were observed. U3-1287-related adverse events included an increase in ALT in 3 patients, and increases in thrombocytopenia, diarrhea, stomatitis, cheilitis, rash, and AST in 2 patients each (26).
c-MET/HGF
The MET proto-oncogene encodes the receptor (MET) of hepatocyte growth factor (HGF), and its amplification is observed only in advanced cancer; i.e., 19% in well-differentiated adenocarcinoma but as high as 39% in scirrhous gastric cancer (27-29). The activation of MET suppresses apoptosis and promotes tumor cell survival, gene transcription, angiogenesis, cellular proliferation, migration, mitosis, and differentiation. In gastric cancer, the activation of MET has reportedly been attributed to gene amplification (4-6). The results of a phase II study of foretinib, which inhibits several kinases including c-MET, VEGFR-2, PDGFR, RON, KIT, and TIE2, in poorly differentiated gastric cancer have been reported (30). The primary endpoint was RR. MET amplification, as determined by FISH of archival tissue, was defined as at least three copies of 7q31 including both a high level of gene amplification and a low level of aneuploidy of chromosome 7. Three of 64 (4.7%) patients showed high-level MET gene amplification. One of these three highly amplified MET showed SD, and the other two showed progressive disease. MET gene amplification is not observed solely in the poorly differentiated type. The RR was 0% with foretinib on a schedule of 5 days on/9 days off. Plasma levels of shed MET and VEGF-A tended to increase during the treatment periods compared with the drug holidays, and may reflect biological changes following foretinib (31). Tivantinib is a selective, non-ATP competitive, MET inhibitor (32). No objective response was observed, and the SD rate was 36.7% in a phase II study with tivantinib monotherapy against previously treated gastric cancer. The median PFS was only 43 (95% CI, 29-92) days. No obvious relationship was seen between outcomes and MET gene amplification, c-MET or HGF expression in tumor and serum. Four patients with MET gene amplification showed SD and PD (n=2 each). The histologic type in one of the four amplified patients was poorly to moderately differentiated adenocarcinoma, and the others were moderately differentiated adenocarcinoma.
Rilotumumab (AMG 102) is a fully human IgG2 monoclonal antibody to HGF. A placebo-controlled randomized phase 2 study of epirubicin, cisplatin, and capecitabine (ECX) with or without rilotumumab in gastric and esophago-gastric junctional cancer showed promising results (33). Chemo-naïve patients were randomized 1:1:1 to receive ECX (50 mg/m2 iv day 1, 60 mg/m2 iv day 1, 625 mg/m2 bid orally days 1-21, respectively) plus rilotumumab 15 mg/kg (Arm A), rilotumumab 7.5 mg/kg (Arm B), or placebo (Arm C) iv on day 1 every 3 weeks. MET protein was measured in archival tumor samples by IHC. Overall survival and PFS were evaluated. The MST was 10.6 months (95% CI, 9.5-12.0 months) in Arms A+B compared to 8.9 months (95% CI, 5.7-10.6 months) in Arm C (HR 0.70, 95% CI, 0.45-1.09). The median PFS was 5.7 and 4.2 months, respectively (HR 0.60, 95% CI, 0.39-0.91). The MST in MET-positive patients by immunohistochemistry was 11.5 months (n=27; 95% CI, 9.2-12.1 months) in Arms A+B compared to 5.7 months (n=11, 95% CI, 4.5-10.4) in Arm C (HR 0.34, 95% CI, 0.15-0.78). The median PFS was 6.9 and 4.4 months, respectively (HR 0.44, 95% CI, 0.20-0.96). A planned phase III study will test the efficacy of rilotumumab plus ECX in MET-positive gastric cancer. MetMAb is a monoclonal monovalent antibody to MET (34). A 48-year-old woman with advanced gastric cancer was treated with MetMAb at a dose of 20 mg/kg as part of a phase I study and experienced CR after 3 months from the beginning of MetMAb, which lasted approximately 2 years. She then progressed with new lesions in her peritoneum. The histology was poorly differentiated adenocarcinoma with signet ring cell components. An analysis of the MET copy number of primary gastric tumors revealed high polysomy and MET protein expression by IHC.
Patients with an increase in the MET copy number of 5< accounted for 10% (21/216), and showed a significantly worse prognosis with a multivariate hazard ratio of 2.91 for overall survival (28). In another report, 10 of 489 (2%) patients harbored MET amplification. The highest frequency of MET positivity was observed in esophago-gastric junctional tumors (3%, 3/97). Two of 4 four patients with MET-amplified tumors who were treated with crizotinib showed tumor shrinkage of 30% and 16%, and PFS of 3.7 and 3.5 months, respectively (29). Few patients with gastric cancer show MET amplification, and the efficacy of TKI of MET was quite limited. The preliminary effects of antibodies to MET or HGF have been reported in MET-amplified gastric cancer.
VEGFR/VEGF
Tumor angiogenesis through vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor (VEGFR) signaling is involved in the progression of gastric cancer (35-37). VEGF-R2 is a potent regulator of vascular endothelial cells and has been directly linked to tumor angiogenesis and blood vessel-dependent metastasis. On the other hand, VEGF-R1 may contribute to pathological vascularization directly by stimulating endothelial cell function and indirectly by mediating the recruitment of bone marrow progenitor cells. Several studies have found that the expression of VEGF ligands and subtypes is correlated with the prognosis in gastric cancer (36,37) and the expression of soluble VEGF-R1 also predicts the prognosis (38). Hirashima et al. analyzed VEGF-R expression levels in primary tumors from 86 patients with advanced gastric cancer, and reported that the expression of VEGF-R1, 2 and 3 in stromal vessels in primary gastric tumors significantly predicted poor survival (35).
Multi-targeted TKIs like sunitinib were generally ineffective against gastric cancer in a phase II study in gastric cancer. The RR was 2.6% (2/78) in a phase II study of sunitinib monotherapy as second-line treatment (39). Twenty-five of 78 patients (32.1%) had SD, and 4 (5.1%) experienced SD that lasted more than 24 weeks. The median PFS was 2.3 months. In another German phase II trial with sunitinib monotherapy for chemo-refractory patients, the response rate was 3.9%, with a median PFS of 1.28 months (40). Tumor VEGF-C expression, which combines VEGFR-2 and 3, compared with no expression, was associated with a significantly shorter median PFS (1.23 vs. 2.86 months; P=0.019), however, there was no difference in the tumor control rate (P=0.142) (40). Sunitinib had an antiproliferative effect in gastric cancer cell lines with high PDGFRA expression (41).
Cediranib and sorafenib are also multi-target TKIs. Both agents were combined with cisplatin plus S-1 or capecitabine, which is a standard first-line treatment for gastric cancer (42,43). The most common adverse events were neutropenia, anorexia, nausea, fatigue, diarrhea, and hand-foot syndrome. The results of these combination studies were not so promising compared with chemotherapy alone with contiguous non-hematologic toxicities in a first-line setting.
AVAGAST and AVATAR were randomized placebo-controlled trials that were designed to evaluate the efficacy of adding bevacizumab to capecitabine or fluorouracil plus cisplatin in first-line treatment against advanced gastric cancer (44,45). The primary endpoint was overall survival, and 774 patients were enrolled. The MST was 12.1 months with bevacizumab plus fluoropyrimidine and cisplatin (FP) and 10.1 months with placebo plus FP (HR 0.87, 95% CI, 0.73-1.03; P=0.1002). The median PFS (6.7 vs. 5.3 months, HR 0.80, 95% CI, 0.68-0.93; P=0.0037) and RR (46.0% vs. 37.4%, P=0.0315) were both significantly improved with bevacizumab versus placebo. Although AVAGAST did not reach its primary endpoint, some anti-angiogenic activity was suggested with the addition of bevacizumab to chemotherapy, which was associated with significant increases in PFS and RR in the first-line treatment of advanced gastric cancer. Low tumor neuropilin-1 expression was associated with shorter overall survival in placebo-treated patients (46). The addition of BV seems to produce a survival benefit; patients with low tumor neuropilin-1 expression had OS treatment hazard ratio values that were better than those in patients with high neuropilin-1 expression.
Ramucirumab is a fully human IgG1 antibody to VEGFR2. A randomized phase III study of ramucirumab plus paclitaxel versus paclitaxel monotherapy as second-line treatment is ongoing (47).
FGFR
Fibroblast growth factor receptor (FGFR) 2 gene amplification in gastric cancer cell lines confers hypersensitivity to FGFR inhibitors. A copy number assay and FISH analysis revealed that 5% (7/152) of gastric cancers harbored FGFR2 amplification; histologically, five patients had diffuse type and two had intestinal type (48). The amplification of FGFR1, 3 and 4 was not detected. A FISH analysis showed that six of the seven tumors were highly amplified, while the remaining tumor had a relatively low grade of amplification. Patients with FGFR2 amplification tended to exhibit a shorter overall survival period. FGFR2 gene amplification is almost entirely mutually exclusive with HER2 and MET gene amplification. Cediranib exerted potent antitumor activity against gastric cancer xenografts overexpressing FGFR2. AZD4547 is a pan-FGFR TKI that is under clinical development (49).
IGFR
Insulin-like growth factor type 1 receptor (IGF-1R) is a cell membrane receptor that is activated by its ligands, IGF-1 and IGF-2 (50). IGF-1R participates in cell proliferation, differentiation, and the prevention of apoptosis. Since IGF-1R is also involved in malignant transformation the development of IGF-1R-directed cancer therapy has been initiated. IGF-1R is frequently overexpressed in human cancers, and the association between IGF-1R expression and outcomes has been assessed for breast cancer and other solid tumors. Patients who highly overexpressed IGF-1R by IHC accounted for 29% (25/87) of gastric cancers: 40% (16/40) of intestinal type and 19% (9/47) of diffuse type (3). About 30 agents that target the IGF-IR have been investigated, including the anti-IGF-IR antibodies IMC-A12, AMG-479, AVE1642, BIIB022, CP-751871, MK0646, and Sch717454, and the small-molecule inhibitors OSI-906 and XL228 (50).
mTOR
Everolimus is an oral inhibitor of the mammalian target of rapamycin serine-threonine kinase. A downstream component of the PI3K-AKT signaling pathway is deregulated in gastric cancer cells and everolimus has shown anti-cancer effects in both in vitro and in vivo models of gastric cancer (51). No objective responses were observed in phase II of everolimus monotherapy for previously treated patients with gastric cancer. The PD rate was 45% (24/53) and the median PFS was 83 days (95% CI, 50-91 days) (52). Everolimus did not show a significant survival benefit compared with best supportive care (BSC) in previously treated patients with advanced gastric cancer in a subsequent phase III trial. The MST was 5.39 months with everolimus and 4.34 months with BSC (HR 0.90, 95% CI, 0.75-1.08; P=0.1244) (53).
Conclusions
The outcomes of future clinical trials in gastric cancer should improve with advances in diagnostic technology to help us identify the right agents for specific targets.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand 1965;64:31-49. [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [PubMed]
- Matsubara J, Yamada Y, Hirashima Y, et al. Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clin Cancer Res 2008;14:3022-9. [PubMed]
- Dragovich T, Campen C. Anti-EGFR-Targeted Therapy for Esophageal and Gastric Cancers: An Evolving Concept. J Oncol 2009;2009:804108.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Hayashi M, Inokuchi M, Takagi Y, et al. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin Cancer Res 2008;14:7843-9. [PubMed]
- Zhang XL, Yang YS, Xu DP, et al. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg 2009;33:2112-8. [PubMed]
- Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol 2011;29:3030-6. [PubMed]
- Bang YJ, Kang YK, Kang WK, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs 2011;29:1449-58. [PubMed]
- Iqbal S, Goldman B, Lenz HJ, et al. A phase II SWOG study of GW572016 (lapatinib) as first line therapy in patients (pts) with advanced or metastatic gastric cancer. J Clin Oncol 2007;25:abstr 4621.
- Hecht JR, Urba SG, Koehler M, et al. Lapatinib monotherapy in recurrent upper gastrointestinal malignancy: Phase II efficacy and biomarker analyses. Gastrointestinal Cancers Symposium 2008;43:abstr 48.
- Satoh T, Bang Y, Wang J, et al. Interim safety analysis from TYTAN: A phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer. J Clin Oncol 2012;28:abstr 4057.
- LOGiC - Lapatinib Optimization Study in ErbB2 (HER2) Positive Gastric Cancer: A Phase III Global, Blinded Study Designed to Evaluate Clinical Endpoints and Safety of Chemotherapy Plus Lapatinib. ClinicalTrials.gov 2012.
- Blackwell KL, Miles D, Gianni L, et al. Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane. J Clin Oncol 2012;30:abstr LBA1.
- Yamamoto N, Yamada Y, Fujiwara Y, et al. Phase I and pharmacokinetic study of HER2-targeted rhuMAb 2C4 (Pertuzumab, RO4368451) in Japanese patients with solid tumors. Jpn J Clin Oncol 2009;39:260-6. [PubMed]
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [PubMed]
- Rojo F, Tabernero J, Albanell J, et al. Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol 2006;24:4309-16. [PubMed]
- Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol 2006;24:4922-7. [PubMed]
- Gold PJ, Goldman B, Iqbal S, et al. Cetuximab as second-line therapy in patients with metastatic esophageal cancer: A phase II Southwest Oncology Group study. Gastrointestinal Cancers Symposium 2008:abstr 96.
- Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 2010;102:500-5. [PubMed]
- Moehler M, Mueller A, Trarbach T, et al. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 2011;22:1358-66. [PubMed]
- Lordick F, Bodoky G, Chung HC, et al. Cetuximab in combination with capecitabine and cisplatin as first-line treatment in advanced gastric cancer: Randomized controlled phase III EXPAND study. 2012 ESMO Presidential Symposium I.2012.
- Waddell TS, Filho JR, Castro GD, et al. A randomised multicentre trial of epirubicin,oxaliplatin and capecitabine(EOC)+panitumumab in advanced oesophago-gastric cancer. 2012 ESMO PD667.
- Kim YH, Sasaki Y, Lee KH, et al. Randomized phase II study of nimotuzumab, an anti-EGFR antibody, plus irinotecan in patients with 5-fluorouracil-based regimen-refractory advanced or recurrent gastric cancer in Korea and Japan: Preliminary results. J Clin Oncol 2011;29:abstr 87.
- Berlin J, Keedy VL, Janne PA, et al. A first-in-human phase I study of U3-1287 (AMG 888), a HER3 inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol 2011;29:abstr 3026.
- Yamada Y, Wakui H, Yamamoto N, et al. Phase 1 study of U3-1287,a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Jpn Cancer Association 2012:abstr E-1048.
- Kuniyasu H, Yasui W, Kitadai Y, et al. Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun 1992;189:227-32. [PubMed]
- Graziano F, Galluccio N, Lorenzini P, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol 2011;29:4789-95. [PubMed]
- Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011;29:4803-10. [PubMed]
- Jhawer M, Kindler HL, Wainberg Z, et al. Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): Interim results of a multicenter phase II study. J Clin Oncol 2009;27:abstr 4509.
- Cecchi F, Liu Y, Gagnon RC, et al. Shed MET, VEGF-A, and sVEGFR-2 are markers for foretinib treatnebt in patients with metastatic gastric cancer. EORTC-NCI-AACR 2009:abstr B210.
- Muro K, Ryu MH, Yasui H, et al. A phase II study of tivantinib monotherapy in patients with previously treated advanced or recurrent gastric cancer. J Clin Oncol 2012;30:abstr 4082.
- Davidenko I, Iveson T, Donehower RC, et al. Updated efficacy, biomarker, and exposure-response data form a phase 2 study of rilotumumab (R) plus epirubicin, cisplatin, and capecitabine(ECX) in gastric (G) or esophagogastric junction (EGJ) cancer. 2012 ESMO P687.
- Catenacci DV, Henderson L, Xiao SY, et al. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov 2011;1:573-9. [PubMed]
- Hirashima Y, Yamada Y, Matsubara J, et al. Impact of vascular endothelial growth factor receptor 1, 2, and 3 expression on the outcome of patients with gastric cancer. Cancer Sci 2009;100:310-5. [PubMed]
- Ichikura T, Tomimatsu S, Ohkura E, et al. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol 2001;78:132-7. [PubMed]
- Jüttner S, Wissmann C, Jöns T, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol 2006;24:228-40. [PubMed]
- Kosaka Y, Mimori K, Fukagawa T, et al. Identification of the high-risk group for metastasis of gastric cancer cases by vascular endothelial growth factor receptor-1 overexpression in peripheral blood. Br J Cancer 2007;96:1723-8. [PubMed]
- Bang YJ, Kang YK, Kang WK, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs 2011;29:1449-58. [PubMed]
- Moehler M, Mueller A, Hartmann JT, et al. An open-label, multicentre biomarker-oriented AIO phase II trial of sunitinib for patients with chemo-refractory advanced gastric cancer. Eur J Cancer 2011;47:1511-20. [PubMed]
- Yoon YK, Im SA, Min A, et al. Sunitinib synergizes the antitumor effect of cisplatin via modulation of ERCC1 expression in models of gastric cancer. Cancer Lett 2012;321:128-36. [PubMed]
- Satoh T, Yamada Y, Muro K, et al. Phase I study of cediranib in combination with cisplatin plus fluoropyrimidine (S-1 or capecitabine) in Japanese patients with previously untreated advanced gastric cancer. Cancer Chemother Pharmacol 2012;69:439-46. [PubMed]
- Yamada Y, Minami H, Fuse N, et al. A phase 1 study of sorafenib (BAY43-9006) in combination with S-1 plus cisplatin in patients with advanced gastric cancer. American Association for Cancer Research 2009:abstr A260.
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [PubMed]
- Shen L, Li J, Xu JM, et al. Efficacy and tolerability of bevacizumab (BEV) plus capecitabine and cisplatin (XP) in Chinese patients (pts) with locally advanced or metastatic gastric/gastroesophageal junction cancer (AGC): Results from the AVATAR study. J Clin Oncol 2012;30:abstr 73.
- Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27. [PubMed]
- Wilke H, Cunningham D, Ohtsu A, et al. A randomized, multicenter, double-blind, placebo (PBO)-controlled phase III study of paclitaxel (PTX) with or without ramucirumab (IMC-1121B; RAM) in patients (pts) with metastatic gastric adenocarcinoma, refractory to or progressive after first-line therapy with platinum (PLT) and fluoropyrimidine (FP). J Clin Oncol 2012;30:abstr TPS4139.
- Matsumoto K, Arao T, Hamaguchi T, et al. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br J Cancer 2012;106:727-32. [PubMed]
- Takeda M, Arao T, Yokote H, et al. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res 2007;13:3051-7. [PubMed]
- Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene 2009;28:3009-21. [PubMed]
- Lee KH, Hur HS, Im SA, et al. RAD001 shows activity against gastric cancer cells and overcomes 5-FU resistance by downregulating thymidylate synthase. Cancer Lett 2010;299:22-8. [PubMed]
- Doi T, Muro K, Boku N, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol 2010;28:1904-10. [PubMed]
- Van Cutsem E, Yeh KH, Bang YJ, et al. Phase III trial of everolimus (EVE) in previously treated patients with advanced gastric cancer (AGC): GRANITE-1. J Clin Oncol 2012;30:abstr LBA3.