Current status and future direction of chemotherapy for pancreatic cancer
Introduction
Pancreatic carcinoma is a disease with a dismal prognosis; the 5-year survival rate of patients diagnosed with this cancer remains less than 5% (1). Since it is difficult to diagnose pancreatic cancer at an early stage, 70-80% patients with pancreatic cancer have unresectable disease, including locally advanced or distant metastatic disease, at diagnosis. Pancreatic cancer is currently the fifth leading cause of cancer-related mortality in Japan, with an estimated 28,017 deaths occurring from the disease in 2010 (2).
Pancreatic cancer is clinically classified to three stages, namely, resectable, unresectable locally advanced, and metastatic, regarding treatment strategy. According to the TNM classification by the UICC, resectable disease corresponds mostly to Stage I and II and in some cases, to Stage III, unresectable locally advanced disease corresponds to Stage III, and metastatic disease corresponds to Stage IV. The treatment strategy differs by the clinical stage, and it is important to determine the clinical stage in each pancreatic cancer patient to select the most appropriate treatment method.
For more than 10 years, ever since a Phase III study revealed survival benefit of gemcitabine as compared to fluorouracil therapy (3), gemcitabine has been widely used as the standard chemotherapy for unresectable pancreatic cancer. After gemcitabine chemotherapy became established as the standard therapy, many newer agents have been investigated for the treatment of unresectable pancreatic cancer, and some promising treatments have been developed. Furthermore, chemotherapy is also applied as adjuvant therapy after surgery and combined with radiotherapy for locally advanced disease.
Chemotherapy for unresectable pancreatic cancer
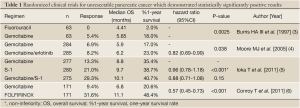
Gemcitabine has become established as the standard treatment for patients with unresectable pancreatic cancer, improving the patient survival as compared to fluorouracil (Table 1) (3). However, the anticancer activity of this drug is only modest; the reported response rate is around 10% and the median overall survival (OS) is 6 to 7 months in patients with unresectable pancreatic cancer treated with gemcitabine. Thus, the prognosis of these patients remains poor, and development of more effective treatments for pancreatic cancer is urgently needed.
Full table
S-1. which consists of tegafur, gimeracil and oteracil potassium, has been developed for pancreatic cancer in Japan. Tegafur is a prodrug of fluorouracil, and gimeracil is a competitive inhibitor of dihydropyrimidine dehydrogenase (DPD), the enzyme responsible for the degradation of fluorouracil, which allows efficacious concentrations of fluorouracil to be maintained in the plasma and tumor tissues. Oteracil potassium, a competitive inhibitor of orotate phosphoribosyltransferase (OPRT), inhibits the phosphorylation of fluorouracil in the gastrointestinal tract, and reduces the serious gastrointestinal toxicity of fluorouracil. A phase II study of S-1 demonstrated promising activity against pancreatic cancer; the response rate was 37.5%, median time to progression (TTP) was 3.7 months, median OS was 9.2 months (7). Furthermore, it was expected that S-1 administered combined with gemcitabine (GS therapy) might be more effective, and several phase II studies of GS therapy have been conducted. In a reported multi-institutional study of GS therapy, the response rate was 44%, the median progression-free survival (PFS) was 5.9 months, and the median OS was 10.1 months (8).
Thus, S-1 or GS therapy was expected to replace gemcitabine as the standard therapy for unresectable pancreatic cancer, and a phase III study was conducted comparing S-1 or GS therapy with gemcitabine alone (5). The primary endpoint was OS, and the superiority of GS therapy and the non-inferiority of S-1 were examined. It was expected that the median OS would be 7.5 months in the gemcitabine group, 8.0 months in the S-1 group, and 10.5 months in the GS group. The subjects were chemotherapy-naïve patients with locally advanced or metastatic pancreatic cancer. Patients were randomly assigned to receive only gemcitabine (1,000 mg/m2 on days 1, 8, and 15 of a 28-day cycle), only S-1 (80/100/120 mg/day according to body surface area on days 1 to 28 of a 42-day cycle), or gemcitabine plus S-1 (gemcitabine 1,000 mg/m2 on days 1 and 8 plus S-1 60/80/100 mg/day on days 1 to 14 of a 21-day cycle). In the total of 834 enrolled patients, median OS was 8.8 months in the gemcitabine group, 9.7 months in the S-1 group, and 10.1 months in the GS group. The non-inferiority of S-1 to gemcitabine was demonstrated [hazard ratio, 0.96; 97.5% confidence interval (CI), 0.78 to 1.18; P<0.001 for non-inferiority], while the superiority of gemcitabine plus S-1 was not proven (hazard ratio, 0.88; 97.5% CI, 0.71 to 1.08; P=0.15) (Table 1) (5). Both treatments were generally well-tolerated, although hematologic and gastrointestinal toxicities were more severe in the GS group than in the gemcitabine group. As a result, at present S-1 monotherapy is accepted as an alternative treatment option for unresectable pancreatic cancer in Japan.
Although many gemcitabine-based combination regimens have been evaluated, a statistically significant survival benefit as compared to gemcitabine alone was obtained only for erlotinib combined with gemcitabine in a phase III study (the PA. 3 study) (4). Erlotinib is an epidermal growth factor receptor (EGFR) tyrosine-kinase inhibitor and is used in the treatment of various types of solid tumors, especially lung cancer. In the PA.3 study, erlotinib plus gemcitabine therapy reduced the risk of death by 18% as compared to treatment with gemcitabine alone (hazard ratio 0.82; 95% CI, 0.69-0.99; P=0.038), with a median OS of 6.24 versus 5.91 months, respectively (Table 1) (4). As a result, combination therapy with gemcitabine plus erlotinib came to be recognized as one of the standard treatments for unresectable pancreatic cancer. In Japan, a phase II study was conducted to examine the feasibility and efficacy of gemcitabine plus erlotinib therapy in Japanese patients, and 107 patients were enrolled (9). The most common adverse events were skin rash, including acneiform dermatitis and anorexia. While interstitial lung disease-like events were of grave concern and were reported in nine patients (8.5%), all of the patients recovered or improved. The median OS and median PFS were 9.23 and 3.48 months, respectively. In Japanese patients with unresectable pancreatic cancer, erlotinib plus gemcitabine therapy showed acceptable toxicity and promising efficacy that were not inferior to the results reported from western patients.
As a chemotherapeutic regimen not including gemcitabine, FOLFIRINOX, consisting of oxaliplatin, irinotecan, fluorouracil and leucovorin, was investigated for advanced pancreatic cancer in France. A phase III study comparing FOLFIRINOX with gemcitabine demonstrated significant survival benefit of FOLFIRINOX as compared to gemcitabine in patients with metastatic pancreatic cancer (Table 1) (6). FOLFIRINOX was associated with a higher incidence of toxicity; in particular, febrile neutropenia was observed in 5.4% patients in the FOLFIRINOX group and 1.2% patients in the gemcitabine group. Based on these results, FOLFIRINOX is considered as a first-line option for metastatic pancreatic cancer as a standard care, however, appropriate selection of candidates is necessary, such as patients with a good performance status, of younger age and having no risk of cholangitis. In Japan, a small phase II study of FOLFIRINOX is currently under investigation to examine the feasibility in Japanese patients, because irinotecan is used 180 mg/m2 in this regimen, whereas only use at 150 mg/m2 or less is approved for various types of cancers in Japan.
A another promising new chemotherapy regimen is a combination of gemcitabine plus nab-paclitaxel. This combination yielded promising results in a phase I/II study; the response rate was 48% and the median OS was 12.2 months in patients with metastatic pancreatic cancer (10). This study also suggested that Stromal Secreted Protein Acidic and Rich in Cysteine (SPARC) expression may be an important marker of early activity of gemcitabine plus nab-paclitaxel in patients with advanced pancreatic cancer. A phase III study comparing gemcitabine plus nab-paclitaxel with gemcitabine alone is currently under investigation in the USA.
Treatment strategy for unresectable locally advanced pancreatic cancer
Randomized controlled trials (RCTs) conducted by Moertel et al. and the Gastrointestinal Tumor Study Group (GITSG) have shown the survival benefit of chemoradiotherapy with fluorouracil as compared to radiation alone in patients with locally advanced pancreatic cancer (11,12). Chemoradiotherapy with concurrent external-beam radiotherapy (EBRT) and systemic fluorouracil chemotherapy has become a standard treatment. Various intensive radiotherapy and/or chemotherapy schedules have been investigated in clinical trials in efforts to improve the efficacy and increase the survival rates. According to the EBM-based clinical guidelines for pancreatic cancer published by the Japan Pancreas Society, chemoradiotherapy is effective for locally advanced disease and is recommended as one of the treatment options (13).
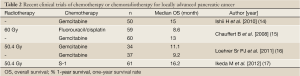
On the other hand, since gemcitabine has been applied to unresectable pancreatic cancer including locally advanced disease, the efficacy of gemcitabine in respect of survival has been reported to be comparable to that of chemoradiotherapy. In the guidelines for pancreatic cancer published by the Japan Pancreas Society, chemotherapy with gemcitabine alone is also recommended as a treatment option for patients with unresectable locally advanced pancreatic cancer (13). We first conducted a phase II study of gemcitabine alone to examine its efficacy and safety in patients with locally advanced disease of the JCOG 0506 study (14). This study was conducted to be foreseeing a phase III trial comparing gemcitabine monotherapy with chemoradiotherapy, to establish the most promising treatment for locally advanced pancreatic cancer. The primary endpoint of this study was the 1-year survival rate. Fifty patients were enrolled from January 2006 to February 2007, and the results revealed a median OS of 15.0 months and 1-year survival rate of 64.0% (Table 2) (14), which significantly exceeded expectations. The toxicities were generally mild and the drug was well-tolerated. Furthermore, a RCT of gemcitabine vs. conventional chemoradiotherapy with fluorouracil plus cisplatin failed to show any survival benefit of chemoradiotherapy (Table 2) (15). Based on these results, gemcitabine monotherapy has come to be regarded as the provisional standard therapy.
Full table
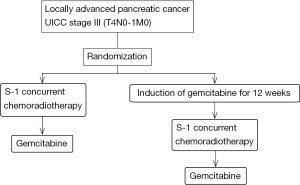
A clinical trial conducted in the USA comparing gemcitabine plus radiotherapy vs. gemcitabine alone in patients with locally advanced pancreatic cancer reported that the OS was superior in the combined treatment group as compared to the gemcitabine alone group (Table 2) (16). Furthermore, chemoradiotherapy using S-1 exhibited promising efficacy in a phase II study conducted in Japan; the median OS was 16.2 months (17). There is a possibility that new methods of chemoradiotherapy might improve the survival, especially prolonged survival of more than 2 years. Thus, in order to develop more promising new chemoradiotherapies, we conducted a randomized phase II study of two chemoradiotherapeutic methods; one consisting of S-1 chemoradiotherapy and maintenance therapy with gemcitabine, and the other consisting of induction gemcitabine chemotherapy for 3 months followed by S-1 chemoradiotherapy and maintenance therapy with gemcitabine (JCOG1106) (18).
The JCOG1106 study is a multi-institutional open-label randomized phase II study to evaluate the efficacy of induction chemotherapy of gemcitabine in combination with S-1 chemoradiotherapy and select a candidate in phase III study comparing with gemcitabine alone (Figure 1) (18). The primary endpoint is OS, and we shall select the treatment method providing the better survival between the two for use in a subsequent phase III study. The one-year survival rate of the two treatments would be expected to be more than 60% at least, because that of patients administered gemcitabine monotherapy in the JCOG 0506 study was 64%. The sample size is 100 patients and this study is currently under investigation.
Adjuvant therapy after surgery
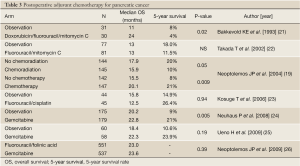
Several RCTs have been conducted to assess the efficacy of postoperative adjuvant chemotherapy. The ESPAC-01 study demonstrated the survival advantage of fluorouracil-based adjuvant chemotherapy (19). Adjuvant therapy with gemcitabine produced significant prolongation of the disease-free survival (DFS); in the CONKO-01 study, the median DFS was 13.4 months in the gemcitabine group and 6.9 months in the surgery alone group (P<0.001) (20). The survival advantage of adjuvant gemcitabine therapy was also demonstrated by the final results of this study (Table 3) (24). Furthermore, the ESPAC-03 study was conducted to examine the efficacy and safety of gemcitabine as adjuvant chemotherapy as compared to fluorouracil plus folinic acid. The study revealed no difference in the survival between the two treatments, and gemcitabine was found to be less toxic than fluorouracil plus folinic acid (26). Thus, gemcitabine was established as a postoperative adjuvant chemotherapy in patients with resectable pancreatic cancer.
Full table
In Japan, although RCTs of fluorouracil plus mitomycin C and fluorouracil plus cisplatin have been conducted, neither of these regimens showed any survival benefit (22,23). Subsequently, a RCT of the efficacy/toxicity of adjuvant chemotherapy using gemcitabine was conducted (25). Although the number of patients was smaller, the results were similar to those of the CONKO-01 and ESPAC-03 studies (Table 3). Based on these results, gemcitabine treatment also came to be recommended as postoperative adjuvant chemotherapy in Japan (13). Two large RCTs of adjuvant chemotherapy with gemcitabine are currently in progress. One is a non-inferiority study comparing S-1 with gemcitabine (the JASPAC-01 study), and the other is a superiority study comparing gemcitabine plus S-1 with gemcitabine alone (the JSAP-04). Recently, the news of an interim analysis that the JASPAC-01 study demonstrated the non-inferiority of S-1 has been released.
Future direction
In pancreatic cancer, major advances have been made in relation to the establishment of standard treatments in recent years. However, the survival of patients with pancreatic cancer still remains dismal. Although administration of many molecular-targeted agents in combination with gemcitabine have been investigated, none of the agents, except erlotinib, showed efficacy. In order to develop more molecular-targeted agents, it is important to find unique biomarkers or driver mutations for carcinogenesis or progression of pancreatic cancer.
Various intensive regimens such as FOLFIRINOX and gemcitabine plus nab-paclitaxel have been developed. New molecular-targeted agents are also expected to be introduced for pancreatic cancer. It would be important to identify patients that would benefit from these regimens based on clinical information about the patient and biomarkers from the point of view of establishment of an individualized treatment strategies.
In recent years, many clinical trials have investigated new chemotherapy regimens for patients with metastatic pancreatic cancer as distinct from patients with locally advanced disease, because of the differences in the characteristics and prognosis of patients with metastatic and locally advanced disease. A new chemotherapeutic regimen can be accurately evaluated only in patients with metastatic disease. On the other hand, in patients with locally advanced disease, intensive chemotherapy or chemoradiotherapy may be useful for down-staging the tumor and make the patient suitable for surgical resection.
Although currently, surgery remains the only potentially curative treatment for pancreatic cancer, most patients develop recurrence. Survival benefit of adjuvant chemotherapy was demonstrated, however, the prognosis of patients with advanced disease stages such as stage II and III is still poor. The efficacy of neoadjuvant therapy has been examined for these patients (27-30). Various neoadjuvant therapies have recently been investigated, and RCTs are needed to confirm the efficacy and safety of neoadjuvant therapy.
Since a large number of patients is required to confirm the survival benefit in RCTs, it is difficult to conduct these trials in a single country. Many clinical trials using new agents are conducted as global studies or Asian studies including Japan. Global cooperation in multinational trials is essential to achieve the goal.
Acknowwledgements
Disclosure: The authors declare no conflict of interest.
References
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17.
- Foundation for Promotion of Cancer Research. Cancer Statistics in Japan- 2011. Available online: http://ganjoho.jp/public/statistics/backnumber/2011_jp.html (access on October 20, 2012)
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13.
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6.
- Ioka T, Ikeda M, Ohkawa S, et al. Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study. J Clin Oncol 2011;29:abstr 4007.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25.
- Okusaka T, Funakoshi A, Furuse J, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 2008;61:615-21.
- Ueno H, Okusaka T, Furuse J, et al. Multicenter phase II study of gemcitabine and S-1 combination therapy (GS Therapy) in patients with metastatic pancreatic cancer. Jpn J Clin Oncol 2011;41:953-8.
- Okusaka T, Furuse J, Funakoshi A, et al. Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci 2011;102:425-31.
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54.
- Moertel CG, Childs DS Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 1969;2:865-7.
- Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981;48:1705-10.
- Yamaguchi K, Tanaka M, Committee for Revision of Clinical Guidelines for Pancreatic Cancer of Japan Pancreas Society. EBM-based Clinical Guidelines for Pancreatic Cancer 2009 from the Japan Pancreas Society: a synopsis. Jpn J Clin Oncol 2011;41:836-40.
- Ishii H, Furuse J, Boku N, et al. Phase II study of gemcitabine chemotherapy alone for locally advanced pancreatic carcinoma: JCOG0506. Jpn J Clin Oncol 2010;40:573-9.
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592-9.
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12.
- Ikeda M, Ioka T, Ito Y, et al. A Multicenter Phase II Trial of S-1 With Concurrent Radiation Therapy for Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2013;85:163-9.
- Furuse J, Ishii H, Okusaka T. The Hepatobiliary and Pancreatic Oncology (HBPO) Group of the Japan Clinical Oncology Group (JCOG): History and Future Direction. Jpn J Clin Oncol 2013;43:2-7.
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10.
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77.
- Bakkevold KE, Arnesjø B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater--results of a controlled, prospective, randomised multicentre study. Eur J Cancer 1993;29A:698-703.
- Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002;95:1685-95.
- Kosuge T, Kiuchi T, Mukai K, et al. A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol 2006;36:159-65.
- Neuhaus P, Riess H, Post S, et al. CONKO-001: Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine vs. observation in patients with resected pancreatic cancer. J Clin Oncol 2008;26: abstr LBA4504.
- Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer 2009;101:908-15.
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81.
- Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol 2007;14:2088-96.
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95.
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502.
- Heinrich S, Schäfer M, Weber A, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg 2008;248:1014-22.


