Paradigm of polyendocrine therapy in endocrine responsive breast cancer: the role of fulvestrant
Endocrine therapy is the cornerstone of any treatment plan for endocrine-responsive breast cancer in both the adjuvant and metastatic settings (1). In the metastatic setting in post-menopausal patients aromatase inhibitors (AIs; anastrozole, exemestane, letrozole) are standard therapies, shown to demonstrate improved progression-free survival (PFS) and a favorable adverse effect (AE) profile compared to other endocrine agents such as tamoxifen (2,3). Tamoxifen, which is a selective estrogen receptor (ER) modulator (SERM), continues to have a large role in the management of endocrine receptor positive breast cancer in both the adjuvant and metastatic setting, in both pre- and post-menopausal women (4,5). The AIs reduce circulating estrogen levels in postmenopausal women by inhibiting peripheral conversion of androgens to estradiol. Fulvestrant (Faslodex, AstraZeneca) is an analogue of estradiol that binds the ER in such a way that disrupts the ER leading to increased receptor degradation and half-life (6,7), resulting in apoptosis and reduced proliferation of affected cells (8).
The sequencing and combination of endocrine therapy is an evolving area. For example, the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial showed that the combination of anastrozole with tamoxifen was not superior to single agent tamoxifen in the adjuvant setting (9). The Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) trial similarly did not show superiority of the combination compared with tamoxifen alone in decreasing the proliferation marker Ki-67 (10). Much of this data is from the pre-fulvestrant era and the sequencing and combination of endocrine therapy with this agent is not yet clear. Initial studies with fulvestrant 250 mg IM per month (the initial FDA approved dose) indicated equivalence, but not superiority, compared to tamoxifen (11), anastrozole (12), and exemestane (13). At a higher dose (500 mg every two weeks for one month followed by 500 mg monthly injections), fulvestrant was found to be at least equivalent to anastrozole alone in clinical benefit rate and overall response rate with improved time to failure by 13 months in the FIRST trial (14,15). Thus in September 2010, fulvestrant was approved at the higher dose level.
There is mixed pre-clinical rationale for the combination of fulvestrant with other anti-estrogen agents. As discussed by Weinberg et al. (16), multiple strategies to overcome hormonal resistance have had pre-clinical success including the combination of hormonal and growth factor blockade and dual hormonal blockade. A preclinical study in the transplanted human ER positive breast cancer cell line MCF-7, showed greater efficacy of fulvestrant compared with tamoxifen after estrogen withdrawal (17). In this model, tumor cells are injected into mice in an estrogenic environment created with an estrogen releasing subcutaneous pellet. Upon tumor formation, the estrogen pellet is removed. In this estrogen deprivation setting fulvestrant has been shown to have potent anti-tumor effects. However, if mice continue to receive estrogen, fulvestrant does not have significant anti-tumor activity (18). Later studies in the MCF-7 Ca cell model (cells that were genetically modified to express high levels of aromatase) showed equivalence of combinations of fulvestrant with anastrozole and fulvestrant with tamoxifen compared to the use of either agent alone (19). But similar studies with various doses of fulvestrant in combination with AI showed superiority of the combination (20). For example, Macedo et al. (21) studied the combination of fulvestrant and anastrozole in the xenograft mouse model and showed decreased rate of tumor growth compared to either agent alone as well as down-regulation of signaling proteins such as insulin-like growth factor type I receptor beta, mammalian target of rapamycin (mTOR) and estrogen receptor alpha in tumors exposed to both agents, hinting at a mechanism for efficacy of therapy. In summary, while the preclinical picture is mixed, there is rationale for combining an anti-estrogen with an anti-estrogen receptor drug.
Given these encouraging pre-clinical results, two randomized trials were initiated to evaluate the efficacy of combined AI and fulvestrant therapy in post-menopausal breast cancer patients. The Fulvestrant and Anastrozole Combination Therapy (FACT) trial was a Phase III, open-label, prospective randomized controlled trial that evaluated a loading-dose (LD) schedule of fulvestrant 250 mg together with anastrozole versus anastrozole alone in 514 predominantly European post-menopausal women with receptor-positive breast cancer treated at first relapse (22). FACT was a negative study with no difference in primary or secondary endpoints of time to progression (TTP, defined as time from randomization to progression or death due to any cause) or overall survival (OS) between the groups.
In the face of this negative trial, the publication of the SWOG S0226 trial results in the New England Journal of Medicine, showing an improvement not just in PFS (defined as time from randomization to progression or death due to any cause) but also OS with the combination of fulvestrant and anastrozole is intriguing (23). This Phase III trial randomized 694 predominantly American postmenopausal women with previously untreated metastatic breast cancer to either fulvestrant with anastrozole or anastrozole alone with cross over to fulvestrant alone strongly encouraged at time of progression for the anastrozole alone group. The primary endpoint was PFS which was superior in the combination arm at a median of 15.0 months [95% confidence interval (CI), 13.2 to 18.4 months] in the combination group and 13.5 months (95% CI, 12.1 to 15.1 months) in the anastrozole alone group (P=0.007). OS also favored the combination arm with median of 47.7 months (95% CI, 43.4 to 55.7 months) in the combination group compared to 41.3 months (95% CI, 37.2-45.0 months) with anastrozole alone (P=0.049). The toxicity profile of the two groups was similar.
One strength of this study is the encouragement of cross-over to fulvestrant for the anastrozole only group, which occurred in 41% of the patients in the anastrozole-only group. The overall survival benefit persisted for upfront combination therapy even in those who crossed over at progression. However this must be interpreted with caution as the cross over patients received low dose fulvestrant (without the 500 mg loading dose).
Fulvestrant dosing is an issue in both FACT and SWOG S0226. Both trials use the dosing scheme of 500 mg LD on Day 1, followed by 250 mg on days 15, 29, then monthly. However, since the design and initiation of these trials, fulvestrant has been approved for use with a higher dosing scheme, specifically 500 mg on Days 0, 14, 28 and then monthly (as opposed to 250 mg monthly), based on the results of the CONFIRM trial that showed a median 1.0 month improvement in PFS with the higher dose (24).
So how should we interpret these disparate results from similarly designed trials? First of all, it is important to note that while the sample size of the two studies are different with 514 patients in the FACT trial and 694 patients in SWOG S0226, the studies were powered differently with FACT at 80% power and SWOG S0226 at 90% power resulting in similar effect size. Thus the difference in sample size is not a key feature that can explain the disparate results. However, there were significant differences both in the patient population and the duration of follow-up between these trials that likely drove the differing results.
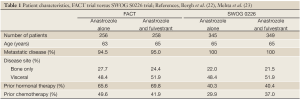
As outlined in Table 1,2, while the patients in both trials were similar in age and disease extent there were significant differences in the proportion of patients who were endocrine naïve. Roughly 30% of women in the FACT trial had not received prior endocrine therapy versus nearly 60% in SWOG S0226. The larger proportion of endocrine naïve patients is likely the main driver behind the positive results in the SWOG trial. Indeed, unplanned subgroup analysis of the SWOG study showed the PFS benefit may have been restricted to this large endocrine naïve group. Also notable is the increased proportion of patients who received prior chemotherapy in the FACT trial compared to SWOG S0226. Additionally, SWOG S0226 mandated that patients completed neoadjuvant or adjuvant chemotherapy more than 12 months before enrollment while FACT allowed patients who had recently progressed on chemotherapy with no “wash-out” period required. In general, then, the SWOG S0226 patient population had received less treatment - either endocrine or chemotherapy - and had a longer chemotherapy treatment free interval than the patients in FACT.
Full table

Full table
Another major difference between these trials is the follow-up time. Median follow up in FACT was only 8.9 months in comparison to 35 months in SWOG S0226. This becomes increasingly relevant as improvements in both PFS and OS in the SWOG trial became more pronounced over time. As reported in the New England Journal of Medicine (23), the Kaplan-Meier curves for PFS did not separate until 12 months. Similarly, the magnitude of difference in overall survival increased over time. At one year, the rate of OS was 89% with anastrozole alone and 91% with the combination. By year three, OS was 57% in the anastrozole alone group versus 62% in the combination group. Indeed, if follow-up on SWOG S0226 was only 8.9 months, it too may have been a negative study. Would longer follow-up on the FACT trial show a positive result?
The study population in SWOG S0226 was unique, including 39% of patients who had metastatic disease at presentation; in general population studies show that less than 10% (25) of patients present with metastatic disease. Perhaps there is a role for combination endocrine therapy is in this small population of previously un-treated patients with metastatic disease, however we do not have enough data to call this standard of care at this time.
What can, or should, be done to clarify the activity of combination fulvestrant and anastrozole? One possibility, particularly intriguing in light of the suggestion that combination therapy may work best in endocrine-therapy naïve patients, is investigation in the neoadjuvant setting. One small pilot study of 121 postmenopausal patients with ER-positive disease did just that, testing the combination of anastrozole plus a single 500 mg fulvestrant injection versus each agent alone. No additional Ki-67 downregulation was noted with the combination over either agent alone (26). However, in general, endocrine neoadjuvant studies have failed to produce meaningful results in part the optimal endpoint has not yet been identified.
On the other end of the disease spectrum, the South Korean SoFEA trial, reported first results at the European Breast Cancer Conference earlier this year. SoFEA is a phase III, partially blinded, randomized trial in which women with locally advanced or metastatic disease were allocated to fulvestrant 250 mg (with 500 mg LD) plus anastrozole versus fulvestrant plus placebo versus exemestane 15 mg daily after progressing on a non-steroidal aromatase inhibitor. PFS was 4.4 months (95% CI, 3.4 to 5.4 months) for the combination of fulvestrant and anastrozole, 4.8 months (95% CI, 3.6 to 5.5 months) for anastrozole alone and 3.4 months (95% CI, 3.0-4.6 months) for exemestane alone; all differences were non-significant (27). Given the importance of longer interval follow-up as evidenced by the SWOG trial, we eagerly await more mature data from the SoFEA experience.
In conclusion, cautious optimism regarding the combination regimen of fulvestrant with anastrozole is reasonable in light of the mixed results of FACT, SWOG S0226 and the recently reported SoFEA trial. The 6.4 month improvement in OS seen in the SWOG trial is not to be taken lightly. Nor should the negative results of the FACT trial be discounted. For the time being, further study is warranted with particular attention to sub-populations that may derive the most benefit from the combination therapy, such as endocrine naïve patients with metastatic disease, admittedly a small population, or patients receiving their initial therapy for early stage breast cancer. The robust results of SWOG S0226 certainly raise hope that such a population exists.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mauri D, Pavlidis N, Polyzos NP, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 2006;98:1285-91.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000;18:3758-67.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003;21:2101-9.
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47.
- Beslija S, Bonneterre J, Burstein HJ, et al. Third consensus on medical treatment of metastatic breast cancer. Ann Oncol 2009;20:1771-85.
- Wakeling AE. Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr Relat Cancer 2000;7:17-28.
- Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res 1991;51:3867-73.
- Bundred NJ, Anderson E, Nicholson RI, et al. Fulvestrant, an estrogen receptor downregulator, reduces cell turnover index more effectively than tamoxifen. Anticancer Res 2002;22:2317-9.
- Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002;359:2131-9.
- Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer--a study from the IMPACT trialists. J Clin Oncol 2005;23:2477-92.
- Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 2004;22:1605-13.
- Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 2002;20:3386-95.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 2008;26:1664-70.
- Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol 2009;27:4530-5.
- Robertson JFR, Lindemann JPO, Llombart-Cussac A, et al. A Comparison of Fulvestrant 500 mg with Anastrozole as First-Line Treatment for Advanced Breast Cancer: Follow-Up Analysis from the ‘FIRST’ Study. Proc SABCS 2010;70:Abstract nr S1-3.
- Weinberg OK, Marquez-Garban DC, Pietras RJ. New approaches to reverse resistance to hormonal therapy in human breast cancer. Drug Resist Updat 2005;8:219-33.
- Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst 1995;87:746-50.
- Massarweh S, Osborne K, Heidi W, et al. Estrogen deprivation is crucial for the antitumor effect of fulvestrant and adding the HER inhibitor gefitinib delays acquired resistance in a xenograft model of ER-positive breast cancer. Breast Cancer Res Treat 2007;106: abstr 2090.
- Lu Q, Liu Y, Long BJ, et al. The effect of combining aromatase inhibitors with antiestrogens on tumor growth in a nude mouse model for breast cancer. Breast Cancer Res Treat 1999;57:183-92.
- Jelovac D, Macedo L, Goloubeva OG, et al. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res 2005;65:5439-44.
- Macedo LF, Sabnis GJ, Goloubeva OG, et al. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res 2008;68:3516-22.
- Bergh J, Jönsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 2012;30:1919-25.
- Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012;367:435-44.
- Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 2010;28:4594-600.
- American Cancer Society. Breast Cancer Facts & Figures 2011-2012. Atlanta: American Cancer Society, Inc.2011.
- Robertson J, Dixon J, Sibbering D, et al. Tumor biomarker changes following pre-surgical treatment with 500 mg fulvestrant plus anastrozole versus 500 mg fulvestrant and 1 mg anastozole alone. Cancer Res 2009;69: abstr 24.
- Johnston S, KL, Ellis P, et al. Fulvestrant Alone or with Concomitant Anastrozole Vs Exemestane Following Progression on Non-Steroidal Aromatase Inhibitor- First Results of the SeFEa Trial. Eur J Cancer. Presented at 8th European Breast Cancer Conference, Vienna, Austria 2012;48: Page ii, S2).


