
2021 updates to the World Health Organization classification of adult-type and pediatric-type diffuse gliomas: a clinical practice review
Introduction
The World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS) serves as the international standard for the diagnosis of brain and spinal cord tumors. The new fifth edition of the WHO classification was published online in 2021 and made available in print in 2022 (1). The fifth edition incorporates numerous refinements and advances since the publication of the 2016 revised fourth edition, including interim recommendations from the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy-Not Official WHO (cIMPACT-NOW) working groups (2-9).
Major updates in the fifth edition center upon tumor taxonomy, nomenclature and grading (10). Fourteen new tumor types are included, as are new tumor subtypes with particular clinical relevance. Our evolving understanding of the molecular drivers of CNS tumors, together with increasing accessibility of novel diagnostic techniques such as DNA methylation profiling, facilitated the recognition and characterization of these additional tumor types and subtypes. Integration of molecular diagnostics with histomorphologic features is now required for several tumor types, and a layered reporting structure for diagnoses is recommended to include all relevant histologic and molecular data. In practice, this translates to an initial preliminary histologic diagnosis followed by a final integrated histologic and molecular diagnosis with WHO grade.
In a new approach to the WHO classification, gliomas, glioneuronal tumors and neuronal tumors are grouped into six families. For the first time, diffuse gliomas are divided into ‘adult-type diffuse gliomas’, ‘pediatric-type diffuse low-grade gliomas’, and ‘pediatric-type high-grade gliomas’ that reflect the clinical behavior and molecular differences between tumor types that arise mostly in adults and those occurring mainly in the pediatric or infant population. The remaining three tumor families include circumscribed (non-diffuse) astrocytic gliomas, glioneuronal and neuronal tumors, and ependymal tumors.
As mentioned, diffuse gliomas in particular have undergone significant changes in nomenclature. Adult-type diffuse glioma classification has been significantly streamlined to three main tumor types, in contrast to the 15 different entities in the 2016 WHO classification. This was accomplished in part by changes in assigning tumor grades within tumor types, and eliminating the term ‘anaplastic’ to indicate a WHO grade 3 astrocytoma or oligodendroglioma. As such, IDH-mutant astrocytomas are now graded within tumor type (CNS WHO grade 2, 3 or 4) based on their histologic and molecular features. Furthermore, the increasing application of molecular diagnostics to tumor classification has decreased ‘not otherwise specified (NOS)’ and ‘not elsewhere classified (NEC)’ diagnoses that were previously included for each type of diffuse glioma in the 2016 WHO classification. Finally, the histopathologic diagnosis of oligoastrocytoma no longer exists due to the reclassification of the majority of these tumors based on their defined molecular alterations. Several excellent recent reviews highlight the changes to adult-type glioma classification and their clinical relevance (11-13).
Multiple newly recognized pediatric-type diffuse low-grade and high-grade gliomas are defined in the new WHO classification. For some of these tumor types, insufficient prospective outcome data exist to assign a definitive WHO grade. In many cases, the potential efficacy of small molecular inhibitors or other targeted therapies is unknown and may affect future prognostication. This review focuses on general updates in the 2021 edition of the CNS WHO classification specific to diffuse gliomas, with a particular focus on the histopathologic features of the newly recognized pediatric-type low-grade and high-grade tumor types. Examples of several tumor types are presented, including some representing ongoing diagnostic challenges.
Adult-type diffuse gliomas
Astrocytoma, IDH-mutant, CNS WHO grade 2, 3 or 4
The tumor type astrocytoma, IDH-mutant, encompasses all diffusely infiltrating astrocytomas with IDH1 or IDH2 mutations that lack chromosome 1p/19q codeletion. P53 and ATRX mutations are also frequently present. Astrocytoma, IDH-mutant, includes CNS WHO grade 2, 3 and 4 tumors and eliminates the prior terminology of anaplastic astrocytoma, IDH-mutant, and glioblastoma, IDH-mutant. In addition to defined histologic criteria, the updated grading scheme further incorporates CDKN2A/B status to better elucidate the expected biologic behavior of these tumors.
Most IDH-mutant tumors are supratentorial, however, IDH-mutant gliomas do rarely occur in the infratentorial compartment, most often with IDH1/IDH2 variants other than IDH1 R132H (14,15). CNS WHO grade 2 and 3 IDH-mutant astrocytomas occur most frequently in young adults in their thirties or forties (16,17). CNS WHO grade 4 tumors tend to arise slightly later in the fourth or fifth decade (17). IDH-mutant astrocytomas are very rare in the pediatric population (18).
On histopathology, IDH-mutant astrocytomas are typically composed of fibrillary glial cells showing variable degrees of nuclear atypia. In CNS WHO grade 2 tumors, the overall cellularity is low to moderate. Tumor cell nuclei are ovoid and mostly monomorphic. Occasionally tumor cell nuclei may be quite round, raising initial diagnostic consideration for an oligodendroglioma. Mitotic activity is usually absent or extremely low in CNS WHO grade 2 tumors (e.g., a single mitosis is identified in a large resection specimen). Increased mitotic activity is diagnostic of a CNS WHO grade 3 tumor, although a threshold for total mitoses has yet to be established and can complicate accurate diagnosis in some cases. CNS WHO grade 3 tumors are often more hypercellular and tumor nuclei may be more atypical. The presence of tumor necrosis and/or microvascular proliferation is required for histologically defined CNS WHO grade 4 tumors.
On immunohistochemistry, tumor cells are immunoreactive for the transcription factor OLIG2 and show variable immunoreactivity for GFAP. Tumors with an IDH1 R132H mutation stain positive with the mutation-specific antibody; other IDH1 and IDH2 variant astrocytomas are negative. The majority of tumors will show strong expression of p53 and loss of ATRX staining, consistent with underlying TP53 and ATRX mutations. Additional sequencing analysis is required to identify the approximately 10% of cases with an IDH1 or IDH2 mutation other than p.R132H to reach the appropriate diagnosis (19). DNA methylation profiling also reliably identifies IDH-mutant astrocytomas including low-grade and high-grade IDH-mutant astrocytoma subgroups (20).
In addition to the previously described histopathologic criteria, multiple recent studies demonstrate that clinical outcomes are highly associated with CDKN2A/B status (21,22). Homozygous deletion of CDKN2A or CDKN2B in IDH-mutant astrocytomas markedly decreases overall survival and is considered diagnostic of a CNS WHO grade 4 tumor in the updated WHO classification, even in the absence of microvascular proliferation or necrosis. Furthermore, there is evidence that CDKN2A loss in histologically defined CNS WHO grade 4 tumors is associated with worse clinical outcomes (21,23).
Oligodendroglioma, IDH-mutant and 1p/19q-codeleted, CNS WHO grade 2 or 3
Oligodendroglioma is defined as a diffusely infiltrating glioma with either IDH1 or IDH2 mutation and co-deletion of chromosomes 1p and 19q. Oligodendroglioma, IDH-mutant and 1p/19q-codeleted, is still designated CNS WHO grade 2 or 3 in the updated WHO classification depending on the presence of various histologic features described below. As previously mentioned, the terminology of anaplastic oligodendroglioma was eliminated in the 2021 CNS WHO classification. There is no oligodendroglioma tumor corresponding to CNS WHO grade 4.
Oligodendrogliomas are typically cortically-based tumors and most often arise in the frontal lobe in adult patients (24). They occur across a wide age spectrum from 20 to 75+ years, with a median age of 41 years for CNS grade 2 tumors and 47 years for CNS grade 3 tumors (25). Oligodendrogliomas are exceptionally rare in children, and most tumors formerly designated ‘pediatric oligodendroglioma’ are now classified as other pediatric-type diffuse low-grade gliomas with defined molecular features. In addition to the frontal lobe, other locations in the cerebrum include the temporal, parietal or rarely occipital lobe. Very rare case reports describe oligodendrogliomas arising in the posterior fossa, brainstem or demonstrating diffuse involvement of multiple bilateral brain areas in a gliomatosis cerebri pattern (26-28). Recurrent disease is more likely to show leptomeningeal or intraventricular spread (27,29,30).
On routine histopathology, oligodendrogliomas are predominantly composed of monomorphic glial cells with round nuclei that classically show perinuclear clearing imparting the so-called “fried-egg” appearance. Oligodendrogliomas, in particular CNS WHO grade 3 tumors, may also show gemistocytic-type cells with abundant rounded eosinophilic cytoplasm and eccentrically placed nuclei. Scattered tumor microcalcifications are common. CNS WHO grade 2 oligodendrogliomas typically show a branching network of thin-walled capillaries reminiscent of chicken wire. CNS WHO grade 3 tumors often show microvascular proliferation. Mitotic activity and Ki-67 proliferation index are low in CNS WHO grade 2 tumors and increased in CNS WHO grade 3 tumors, but definite thresholds are not established. CNS WHO grade 3 tumors typically show a combination of features that include high cellularity, marked cytologic atypia, necrosis, microvascular proliferation and brisk mitotic activity. Homozygous deletion of CDKN2A and/or CDKN2B has been reported in a small subset of CNS WHO grade 3 oligodendrogliomas with aggressive behavior and thus may represent a biomarker in cases with borderline grade 3 histologic features (22).
Immunohistochemical stains are very helpful in the diagnostic workup of oligodendrogliomas. Most tumors show immunoreactivity for IDH1 R132H. In contrast to IDH-mutant diffuse astrocytomas, P53 is typically negative and ATRX nuclear staining is retained. Additional sequencing analysis or fluorescence/chromagen-based in situ hybridization assays are required to confirm whole arm loss of chromosome 1p and 19q. Fluorescence in situ hybridization (FISH) testing may occasionally lead to false positive results in cases of partial loss of 1p or 19q. TERT promoter mutations are identified in a majority of oligodendrogliomas, but the finding of a TERT promoter mutation in an IDH-mutant glioma is not diagnostic of oligodendroglioma as it is can be present in some IDH-mutant astrocytomas (31-33). DNA methylation profiling reliably classifies oligodendroglioma, IDH-mutant and 1p/19q-codeletion (20). Unsupervised clustering analysis can demonstrate distinct methylation clusters that correlate with progression-free survival, suggesting DNA methylation profiling may have predictive value (34).
Glioblastoma, IDH-wildtype, CNS WHO grade 4
Glioblastoma, IDH-wildtype, remains the most common malignant adult-type diffuse glioma (24,35). This tumor type occurs most frequently in older adults, but can arise at any age. Tumors may arise anywhere in the CNS, but are most often supratentorial and involve the subcortical white matter and deep gray matter (25). Tumor cells widely infiltrate the CNS parenchyma and can extend to the cortical surface, cross the corpus callosum, infiltrate the brainstem and spinal cord, and appear multifocal with contiguous spread usually along white matter tracts.
Glioblastoma is histologically defined as a high-grade, diffusely infiltrating astrocytoma with microvascular proliferation and/or necrosis. Glioblastoma, IDH-wildtype, is by definition both IDH-wildtype and H3-wildtype. Tumors with these mutations are classified as astrocytoma, IDH-mutant, or as H3 K27-altered or H3 G34-mutant gliomas, respectively. Furthermore, the updated CNS WHO classification also specifies that IDH-wildtype glioblastoma may be genetically defined by the presence of TERT promoter mutation, EGFR amplification, and/or the combination of whole chromosome 7 gain with whole chromosome 10 loss (+7/−10) even in cases without microvascular proliferation or necrosis (1,4,36). An integrated diagnosis of ‘diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, CNS WHO grade 4’ is recommended to incorporate all relevant data in cases with discordant histologic and molecular features (4).
On histopathology, IDH-wildtype glioblastomas are hypercellular, diffusely infiltrative astrocytic tumors composed of cells with variable degrees of differentiation and nuclear atypia. Tumor cells widely infiltrate the parenchyma and often exhibit secondary structuring with subpial, perivascular and perineuronal aggregates of tumor cells. Glioblastomas can have markedly heterogeneous morphology even within the same tumor. Anaplastic tumor cells appear small, round and hyperchromatic. Gemistocytic tumor cells have abundant glassy eosinophilic cytoplasm and eccentrically placed irregular nuclei. Tumors cells may be spindled or epithelioid in shape and have granular, lipidized or minimal cytoplasm. Multinucleated giant tumor cells may be focal or widespread. Nodules of primitive cells showing neuronal differentiation may be present. Tumor cells are arranged in sheets, nests, or fascicles. Certain morphologic patterns are considered distinct subtypes of glioblastoma including giant cell glioblastoma, gliosarcoma, and epithelioid glioblastoma (1).
Mitotic activity is present in all glioblastomas, and often markedly increased. Microvascular proliferation and either pseudopalisading or geographic areas of tumor necrosis are very frequent. At least one of these two features must be present for a histologic diagnosis of glioblastoma.
IDH-wildtype glioblastomas have a distinct DNA methylation profile that is considered sufficient for diagnosis, and may be very helpful in cases that are diagnostically challenging (20). Molecular subgroups of IDH-wildtype glioblastoma can also be distinguished by their methylome profile, include RTK1, RTK2 and mesenchymal subtypes in adult patients (20,37).
Pediatric-type diffuse low-grade gliomas
Diffuse astrocytoma, MYB- or MYBL1-altered, CNS WHO grade 1
Diffuse astrocytoma, MYB- or MYBL1-altered, is a newly defined tumor type in the updated CNS WHO classification (1). These are low-grade tumors frequently diagnosed in the setting of medically refractory epilepsy since childhood and thus included among the group of long-term (low-grade) epilepsy associated tumors (LEATs) (38-40). This tumor type commonly involves cortical and subcortical regions of cerebral cortex and may arise in any lobe (38,41). On magnetic resonance imaging (MRI), the typical findings are a well-delineated, occasionally infiltrative-appearing, non-enhancing T1-hypointense, T2-fluid attenuated inversion recovery (FLAIR)-hyperintense lesion without restricted diffusion (40,41). Diffuse astrocytoma, MYB- or MYBL1-altered, is a CNS WHO grade 1 tumor. Long-term outcomes following surgical resection are good from both oncologic and seizure-control perspectives (40,41).
On histomorphology, tumors range from minimally to moderately hypercellular and show diffuse infiltration by a population of monomorphic cells with ovoid to elongated nuclei (Figure 1) (41,42). This characteristically bland morphology is reflected in previous nomenclature of isomorphic diffuse glioma (40). Mitotic activity is absent or very low and corresponds to a low Ki-67 proliferation index (40). Microvascular proliferation or necrosis are not present. Tumor cells are immunoreactive for GFAP and may show negative immunostaining for Olig2, although this does not appear to be universal (40,41).

Molecular diagnostic testing that demonstrates fusion between the MYB or MYBL1 genes and a partner gene confirms the diagnosis (6,40-43). A DNA methylation profile matching to diffuse astrocytoma, MYB- or MYBL1-altered further supports the diagnosis (20,41). Diffuse astrocytoma with MYB- or MYBL1-fusion and angiocentric glioma with MYB-QKI fusion share many morphologic, genetic, and epigenetic similarities, but are considered separate tumor types in the current WHO classification (1). In older adolescents and young adults, it is important to distinguish diffuse astrocytoma, MYB- or MYBL1-altered, from IDH-mutant and IDH-wildtype diffuse gliomas with similar histomorphologic features but more aggressive behavior.
Angiocentric glioma, CNS WHO grade 1
Angiocentric glioma is classified as pediatric-type diffuse glioma in the updated CNS WHO classification. Angiocentric gliomas are rare tumors typically affecting children and younger adults with epilepsy and are considered another LEAT (44,45). They may occur in the cerebral cortex or brainstem and on imaging characteristically show a well-delineated, non-enhancing, T2-FLAIR hyperintense lesion occasionally with a stalk-like connection to the lateral ventricle (41,46-51). On histopathology, angiocentric gliomas are moderately cellular, diffusely infiltrating tumors composed of monomorphic spindle cells with elongated nuclei at least focally arranged in single or multiple layers around blood vessels, often in a radial pattern (45). Mitotic activity is infrequent and the Ki-67 proliferation index is low, usually under 5% (1). Microvascular proliferation and necrosis are not features of this tumor type. On immunohistochemistry, the tumor cells are usually diffusely positive for GFAP but often negative for Olig2 (45,51,52). A characteristic finding is epithelial membrane antigen (EMA) immunostaining in a dot-like or ring-like pattern in the cytoplasm of tumor cells (52). Angiocentric gliomas do not show immunoreactivity for neuronal markers.
The majority of angiocentric gliomas are driven by fusion of the MYB and QKI genes (41,42,50,53,54). Rarely MYB shows other fusion partners such as PCDHGA1 (41). Occasionally angiocentric gliomas may instead have deletions or amplifications at the MYB locus on 6q23.3 (55).
Angiocentric glioma is a low-grade diffuse glioma with indolent growth and is designated CNS WHO grade 1. These gliomas share overlapping morphologic, radiologic, genetic and biologic similarities with diffuse glioma with MYB alterations. Examples of diffuse glioma with MYB-QKI fusion but without a clear angiocentric growth pattern are difficult to classify as either diffuse glioma, MYB-altered or angiocentric glioma, especially in biopsy cases with limited sampling (Figure 2).
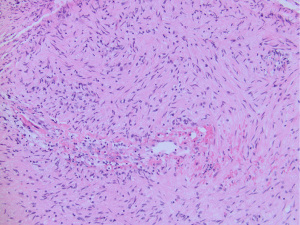
Polymorphous low-grade neuroepithelial tumor of the young (PLNTY), CNS WHO grade 1
PLNTY is a new tumor in the updated CNS WHO classification. PLNTY is a diffusely infiltrating glioma that occurs mostly in children and young adults with epilepsy but has been diagnosed in older patients in their fourth or fifth decade (56-58). PLNTY occur in the cerebral hemispheres and involve cortex and subcortical white matter. On MRI, tumors are typically solid and cystic with T2 hyperintensity and frequent calcification (59,60). Focal contrast enhancement can be present.
On histopathology, tumors show infiltrative and nodular growth, oligodendroglioma-like morphology with monomorphic cells with round nuclei and perinuclear clearing, and calcification (Figure 3) (56). PLNTY also commonly show astrocytic or pleomorphic morphology, spindle cells, and perivascular rosette-like structures. Occasional mitoses can be encountered. Microvascular proliferation and necrosis are not seen. Foci of neoplastic neuronal cells may be encountered, and it remains to be determined if PLNTY would be best classified as a glioneuronal tumors (58). The tumor cells are immunoreactive for OLIG2, and show variable immunoreactivity for GFAP. Immunostaining for CD34 may be patchy or diffuse, but is generally strongly expressed by both tumor cells and by ramified cells in the surrounding cortex (56). The Ki-67 proliferation index is low.
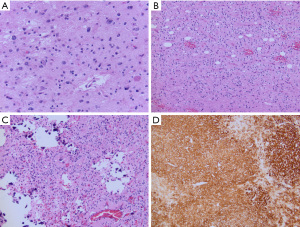
PLNTY are MAPK pathway driven tumors (58). BRAF V600E mutations are commonly identified in tumors from older patients while younger patients often have FGFR fusions, most often with FGFR2 or FGFR3 (56,58,60). Mutations in IDH, ATRX and TP53 are not in keeping with the diagnosis of PLNTY. PLNTY also has a distinct DNA methylation profile with apparent subgroups aligning to BRAF V600E mutant and FGFR fused tumors (56).
PLNTY are indolent tumors designated CNS WHO grade 1. Very rare cases of malignant transformation have been reported (61). Tumor recurrence is very rare following gross total resection, and seizure freedom is often achieved (56).
Diffuse low-grade glioma, MAPK pathway-altered
Diffuse low-grade glioma, MAPK pathway-altered, is a new tumor type in the updated CNS WHO classification (1). This group of diffusely infiltrating tumors occur most often in children (62). Diffuse low-grade glioma, MAPK pathway-altered tumors share many histomorphologic features of other diffuse low-grade gliomas and thus require confirmation of a MAPK pathway alteration for definitive diagnosis.
On histopathology, MAPK pathway-altered diffuse LGG are typically low to moderately cellular tumors composed of monomorphic cells with either oligodendroglioma-like or astrocytic-type features infiltrating the brain parenchyma (Figure 4) (42). Tumors with FGFR1 alterations typically have oligodendroglioma-like features (42). Mitotic activity is rare. Necrosis and microvascular proliferation are not seen. Tumor cells are typically immunoreactive for GFAP and OLIG2 and negative for neuronal markers. CD34 immunostaining is usually limited to vessels. IDH1 and H3K27M immunostains should be performed to help exclude adult-type oligodendroglioma and diffuse midline glioma (DMG).
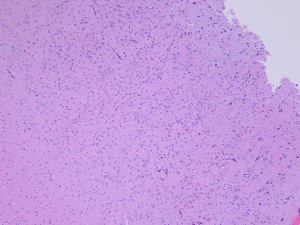
The most common MAPK pathway alterations are BRAF V600E mutation and FGFR1 alterations, typically either a duplication or mutation in the tyrosine kinase domain (6,62). Other rare MAPK pathway alterations have also been reported (42). DNA methylation profiling of these tumors does not resolve these tumors into a single group and it seems likely that this group will eventually split into several distinct tumor types (20,42).
Diffuse low-grade gliomas with MAPK pathway alterations are low-grade tumors; however a CNS WHO grade has not yet been assigned. Additional long-term follow up data are needed, especially with regard to the various molecular subgroups included in this tumor type.
Pediatric-type diffuse high-grade gliomas
DMGs, H3 K27-altered, CNS WHO grade 4
DMG terminology has been updated in the current WHO classification to reflect our growing understanding of the pathogenesis of these tumors. DMG with histone H3 K27M mutations were previously classified as DMG, H3 K27-mutant in the 2016 WHO classification. It is now evident that multiple pathogenic alterations involving the histone 3.1, 3.2 and 3.3 genes can be identified in this tumor type. The new terminology of DMG, H3 K27-altered, includes tumors with loss of H3 K27 trimethylation due to p.K27M mutation, overexpression of enhancer of zeste homolog inhibitory protein (EZHIP), or an EGFR mutation. H3 K27-altered tumors include diffuse intrinsic pontine gliomas (DIPG) in addition to diffuse gliomas arising in other midline locations. Recognizing that H3K27 alterations have also been identified in other circumscribed or low-grade tumor types, DMG must also meet criteria of being diffusely infiltrative and in a midline anatomic location.
In the pediatric population, DMG most frequently involve the brainstem, principally the pons (DIPG) (63). Tumors also often involve the bilateral thalami, and tumors with this presentation frequently harbor EGFR mutations (64). In older patients, either unilateral thalamus or spinal cord are more common sites, although DMG have been reported in most supratentorial and infratentorial midline structures. The median age at diagnosis is 7–8 years of age for H3.3 p.K27M-mutant DMG, DMG with EZHIP overexpression, and EGFR-mutant DMG. H3.1 or 3.2 p.K27M-mutant tumors are diagnosed in slightly younger children with a median age of approximately 5 years old (1).
On histopathology, DMG are diffusely infiltrative tumors with heterogeneous morphology (65,66). Many DMG are composed of bland monomorphic cells with small, ovoid nuclei. Others resemble other low-grade astrocytomas or oligodendroglioma, or show epithelioid or highly pleomorphic cytology. Rare cases have a primitive neuroepithelial cell (PNET-like) appearance with neuropil-like islands (67). Mitotic figures are often present, and microvascular proliferation and necrosis may be found. It is important to note that the histologic features of DMG may align best with a low-grade CNS WHO grade 2 or 3 glioma, however, all DMG are CNS WHO grade 4 tumors by definition. A layered reporting structure of the integrated pathologic diagnosis is recommended so both histologic and molecular information are represented.
By immunohistochemistry, DMG with H3K27M mutation or EZHIP overexpression are generally positive for OLIG2 and MAP2 and variably positive for GFAP. DMG with EGFR mutations may be GFAP positive but lack OLIG2 expression. Neuronal markers are negative in the tumor cells. Mutation-specific antibodies for H3 K27M show strong nuclear expression in the H3 K27-mutant subgroup (Figure 5A). Loss of H3 K27me3 immunoreactivity is a highly sensitive marker of tumors with either H3 K27M or H3 K27I mutations or those with EZHIP overexpression (Figure 5B). Some laboratories have the ability to stain for EZHIP overexpression using antibodies against EZHIP (Cxorf67). Additionally, many DMG cases show P53 overexpression (~50%) or loss of ATRX expression (~15%) consistent with the presence of TP53 and ATRX mutations, respectively (68,69).
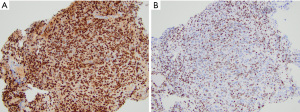
Our current understanding of DMG based on multiple lines of genomic analysis supports the division of DMG into four biologically and clinically distinct subtypes that are recognized in the updated CNS WHO classification (1,68-70). H3.3 p.K27-mutant tumors harbor a somatic mutation resulting in a substitution of lysine (K) to methionine (M), or rarely isoleucine (I), at position 27 of the histone H3 variant. This subgroup is enriched for tumors with TP53 and less frequently PPM1D cooperating or subclonal gene mutations (71). Alternatively, H3.1 and H3.2 p.K27M-mutant tumors are enriched for concomitant PI3K or MAPK pathway alterations. ACVR1 mutations are strongly associated with H3.1p.K27M tumors (72,73). H3 K27M-mutant DMG may also have gain-of-function mutations or amplifications of PDGFRA and FGFR1. H3-wildtype DMG with EZHIP overexpression are quite rare and require a combination of immunohistochemical analysis for H3 K27me3 and RNA expression analysis for diagnosis. EGFR-mutant DMG most commonly have alterations in exon 20 of the EGFR oncogene on chromosome 7p encoding the intracellular tyrosine kinase domain. The EGFR-mutant DMG subgroup is also enriched for tumors with p53 cooperating mutations.
Patients with DMG have a poor prognosis independent of anatomic location. A large study by Mackay and colleagues demonstrated a median survival of 13.5 months and a 2-year overall survival of 21.4% for non-DIPG DMG. DIPG cases had a median survival of 10.8 months and a 2-year overall survival of 21% (63). Other studies indicate that DMG with H3.1 or 3.2 p.K27M mutations or with EZHIP overexpression have a slightly increased overall survival compared to patients with H3.3 p.K27M DMG (70,74,75). Patients younger than three or older than 10 years also show somewhat improved clinical outcomes compared to older children (63,75).
Diffuse hemispheric glioma, H3 G34-mutant, CNS WHO grade 4
Diffuse hemispheric glioma, H3 G34-mutant, is a diffusely infiltrative high-grade glioma that arises in the cerebral hemispheres but may spread to include midline structures. By definition, these tumors have a missense mutation in the H3-3A (H3F3A) gene resulting in a substitution of p.G34R or p.G34V on the histone variant.
On histopathology, these high-grade gliomas are hypercellular infiltrative tumors. Tumor cells may have a glial or embryonal-type appearance. Tumor cells may be markedly pleomorphic and multinucleated, or be small, hyperchromatic cells that occasionally may form Homer Wright rosettes. Tumors cells often infiltrative the cortex and show secondary structuring around neurons and vessels. Mitotic activity is increased and the Ki-67 proliferation index is usually high. Microvascular proliferation and necrosis are typically present but not required for CNS WHO grade 4 designation. Tumor cells often show loss of ATRX expression and P53 overexpression by immunohistochemistry, which are clues that the tumor may harbor a H3.3 p.G34R/V mutation. MAP2 and FOXG1 are usually positive in the tumor cells (1,37). Mutation-specific antibodies are available in some laboratories to detect both G34R and G34V mutant proteins with high sensitivity (Figure 6). Notably, these gliomas are often OLIG2 negative and can be variably positive for GFAP unlike other HGG that typically show OLIG2 and GFAP immunoreactivity.
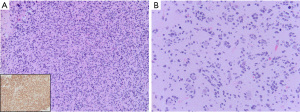
In addition to the required identification of a H3-3A gene missense mutation replacing glycine with arginine or valine at position p.G34, these gliomas have frequent mutations in ATRX (95%) and TP53 (90%) (76). PDGFRA amplification is relatively common in tumors with glial morphology while CCND2 amplification is more frequent in tumors with primitive neuroepithelial morphology (76). Mutations identified in other types of pediatric or young adult high-grade gliomas including H3 K27M, IDH1/IDH2, and BRAF are absent. Diffuse high-grade glioma, H3 G34-mutant, has a distinct DNA methylation profile that includes both G34R and G34V variants. These gliomas often show MGMT promoter hypermethylation which may be associated with improved outcomes together with the absence of PDGRFA, CCND2 or other less common oncogene amplifications reported in this tumor type (76). Patients often have multiple local tumor recurrences and the overall prognosis of H3 G4-mutant diffuse hemispheric glioma is poor (63,76).
Diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype, CNS WHO grade 4
Diffuse pediatric-type high-grade gliomas (pHGG), H3-wildtype and IDH-wildtype, are a group of malignant gliomas primarily occurring in children or young adults that by definition lack alterations in histone H3, IDH1 and IDH2 genes. Not unexpectedly, this group of tumors is heterogeneous in terms of clinical, histopathologic, genomic and epigenetic features yet remain distinguishable from adult-type high-grade gliomas.
pHGG, H3-wildtype and IDH-wildtype, may arise anywhere in the supratentorial or infratentorial compartments. On histopathology, these tumors often appear malignant with increased mitotic activity. Microvascular proliferation and necrosis may or may not be present, and likely do not have the same prognostic significance as in adult-type high-grade gliomas (77). Tumor cells many have an astrocytic or primitive embryonal-type morphology (Figure 7). In a small series of pHGG with MYCN amplification, tumors were described as having both diffusely infiltrating and circumscribed nodular tumor areas with frequent leptomeningeal involvement. Tumor cells were arranged in sheets with spindled or epithelioid morphology and prominent nucleoli, and were immunoreactive for neuronal but not glial markers (78). All pHGG, H3-wildtpe and IDH-wildtype, should not show immunoreactivity for H3K27M or IDH1 R132H or loss of immunoreactivity for H3 K27me3 on immunohistochemical stains. TP53 mutations are common so many tumors will show strong immunohistochemical staining for P53.
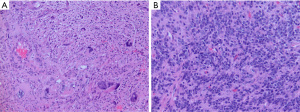
Molecular testing is needed to exclude other types of high-grade gliomas, particularly in older adolescents and infants. Differential diagnoses include DMG with H3 K27-alteration, IDH-mutant astrocytoma, adult-type glioblastoma with gain of chromosome 7 and loss of 10 and/or EGFR amplification, and epithelioid glioblastoma with BRAF mutation and CDKN2A/B homozygous deletion. In young children, CNS embryonal tumors should be a diagnostic consideration. In infant patients, infant-type hemispheric glioma and desmoplastic infantile ganglioglioma/astrocytoma should be ruled out.
The molecular alterations driving tumorigenesis for these pHGG are diverse. In addition to the previously mentioned TP53 mutations and MYCN amplification, these pHGG have frequent PDGFRA alterations (mutations or amplification), ID2, NF1 alterations, and EGFR amplification (18,63,68,79). pHGG, H3-wildtype and IDH-wildtype, comprise a distinct group by DNA methylation profiling with several molecular subgroups (RTK1, RTK2 and MYCN) based on their genetic and epigenetic features (20,80). RTK1 pHGG subtype gliomas have frequent PDGFRA amplification and may be associated with prior therapeutic cranial radiation, constitutional mismatch repair deficiency or Lynch syndrome (81). RTK2 pHGG subgroup tumors are associated with EGFR amplification and TERT promoter mutations and appear to have a better prognosis than RTK1 subgroup tumors. MYCN pHGG subgroup tumors include tumors with MYCN amplification, detected in about half of tumors, and frequent ID2 amplification, detected in about 70% of tumors (78). pHGG MYCN subgroup tumors are associated with a very poor prognosis (80).
Infant-type hemispheric glioma
Infant-type hemispheric glioma is a newly defined tumor type in the CNS WHO classification with distinct molecular drivers and clinical behavior as compared to gliomas in older children. Infant-type hemispheric gliomas are comprised of multiple subgroups, most with receptor tyrosine kinase (RTK) fusions involving the NTRK, ROS1, ALK and MET genes (82,83).
Infant-type hemispheric gliomas arise in very young children typically under one year of age (82). These gliomas are large masses arising in the cerebral hemispheres, often superficially and occasionally with variably solid and cystic components. Leptomeningeal involvement is common and leptomeningeal dissemination has been reported (83,84). On histopathology, the tumors are hypercellular and often show a sharp border with adjacent brain tissue. Tumor cell morphology is variable and includes astrocytic cells with mild to moderately pleomorphic nuclei and occasionally gemistocytic cells arranged in sheets. Tumor cell nuclei may also be spindled and form fascicles (Figure 8). Increased mitotic activity, microvascular proliferation, and pseudopalisading necrosis are common. ALK-fusion tumors may show ependymal differentiation or focal ganglion cells (83,84). On immunohistochemistry, tumor cells are immunoreactive for GFAP and negative for neuronal markers. Immunostaining for ALK may be positive in some tumors with ALK fusions. NTRK immunohistochemistry is not considered useful to detect NTRK-fusion positive tumors as NTRK expression is elevated in background brain tissue.
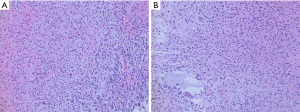
Molecular testing for gene fusions is recommended in the diagnostic workup of all infant gliomas to confirm the diagnosis and identify potential molecular targets for therapy. DNA methylation profiling reveals a distinct methylome profile for all infant-type hemispheric gliomas and is useful to help exclude other high-grade gliomas with similar or overlapping histomorphology (e.g., desmoplastic infantile ganglioglioma/astrocytoma and ependymoma).
A CNS WHO grade is not yet assigned for infant-type hemispheric gliomas as outcome data are still sparse. At least some fusion-positive subgroups appear to have a better prognosis. Guerreiro Stucklin et al. have reported that patients with ALK fusion-positive tumors carry the best prognosis, NTRK-fused tumors showed an intermediate outcome, and ROS-fused tumors were associated with the lowest five-year overall survival (82). Larger studies are needed to confirm these early observations and to assess outcomes following targeted therapy with several promising small molecular inhibitors of specific tyrosine kinase receptors.
Limitations
This review is limited by its focus on recent changes to diffuse glioma classification and includes relatively few examples of the spectrum of gliomas that arise in children and adults. As our knowledge continues to evolve, tumor classification based on these histologic and molecular features will inevitably become outdated. An overview of the current literature was the goal of this review; however, additional findings discussed in recent publications may have been inadvertently excluded.
Conclusions
The CNS WHO classification continues to evolve and is likely best considered a snapshot-in-time that reflects our current understanding based on the information and tools available. The number of pages in the print versions of the CNS WHO classification nearly doubled in past 20 years, in large part due to the development of molecular diagnostics that have refined our ability to distinguish tumor types and subtypes with clinical significance. At the same time, histopathology and immunohistochemistry remain proven methodologies for CNS tumor classification and importantly guide practical and affordable approaches to further molecular testing. The classification of adult-type and pediatric-type diffuse gliomas will undoubtedly continue to evolve as new knowledge is introduced in future CNS WHO classifications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Joshua Palmer, Iyad Alnahhas and Wenyin Shi) for the series “Recent Advances in Neuro-Oncology” published in Chinese Clinical Oncology. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-120/coif). The series “Recent Advances in Neuro-Oncology” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Central Nervous System Tumours. WHO classification of tumours series. 5th edition. Lyon: International Agency for Research on Cancer, 2021.
- Central Nervous System Tumours. WHO classification of tumours series. 5th edition. Lyon: International Agency for Research on Cancer, 2016.
- Brat DJ, Aldape K, Colman H, et al. CimpacT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol 2020;139:603-8. [Crossref] [PubMed]
- Brat DJ, Aldape K, Colman H, et al. CimpacT-NOW update 3: recommended diagnostic criteria for“”Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade I Acta Neuropathol 2018;136:805-10. [Crossref] [PubMed]
- Ellison DW, Aldape KD, Capper D, et al. CimpacT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol 2020;30:863-6. [Crossref] [PubMed]
- Ellison DW, Hawkins C, Jones DTW, et al. CimpacT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol 2019;137:683-7. [Crossref] [PubMed]
- Louis DN, Giannini C, Capper D, et al. CimpacT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol 2018;135:639-42. [Crossref] [PubMed]
- Louis DN, Wesseling P, Aldape K, et al. CimpacT-NOW update 6: new entity and diagnostic principle recommendations of the CimpacT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol 2020;30:844-56. [Crossref] [PubMed]
- Louis DN, Wesseling P, Paulus W, et al. CimpacT-NOW update 1: Not Otherwise Specified (NOS) and Not Elsewhere Classified (NEC). Acta Neuropathol 2018;135:481-4. [Crossref] [PubMed]
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23:1231-51. [Crossref] [PubMed]
- Whitfield BT, Huse JT. Classification of adult-type diffuse gliomas: Impact of the World Health Organization 2021 update. Brain Pathol 2022;32:e13062. [Crossref] [PubMed]
- Berger TR, Wen PY, Lang-Orsini M, et al. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol 2022;8:1493-501. [Crossref] [PubMed]
- Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022;128:47-58. [Crossref] [PubMed]
- Banan R, Stichel D, Bleck A, et al. Infratentorial IDH-mutant astrocytoma is a distinct subtype. Acta Neuropathol 2020;140:569-81. [Crossref] [PubMed]
- Poetsch L, Bronnimann C, Loiseau H, et al. Characteristics of IDH-mutant gliomas with non-canonical IDH mutation. J Neurooncol 2021;151:279-86. [Crossref] [PubMed]
- Brat DJ, Verhaak RG, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med 2015;372:2481-98. [Crossref] [PubMed]
- Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol 2015;129:867-73. [Crossref] [PubMed]
- Korshunov A, Ryzhova M, Hovestadt V, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 2015;129:669-78. [Crossref] [PubMed]
- Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 2009;118:469-74. [Crossref] [PubMed]
- Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature 2018;555:469-74. [Crossref] [PubMed]
- Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol 2018;136:153-66. [Crossref] [PubMed]
- Appay R, Dehais C, Maurage CA, et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol 2019;21:1519-28. [Crossref] [PubMed]
- Korshunov A, Casalini B, Chavez L, et al. Integrated molecular characterization of IDH-mutant glioblastomas. Neuropathol Appl Neurobiol 2019;45:108-18. [Crossref] [PubMed]
- Ostrom QT, Patil N, Cioffi G, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro Oncol 2020;22:iv1-96. [Crossref] [PubMed]
- Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol 2019;21:v1-100. [Crossref] [PubMed]
- Agopyan-Miu AHCW, Banu MA, Miller ML, et al. Synchronous supratentorial and infratentorial oligodendrogliomas with incongruous IDH1 mutations, a case report. Acta Neuropathol Commun 2021;9:160. [Crossref] [PubMed]
- Hodges SD, Malafronte P, Gilhooly J, et al. Rare brainstem oligodendroglioma in an adult patient: Presentation, molecular characteristics and treatment response. J Neurol Sci 2015;355:209-10. [Crossref] [PubMed]
- Herrlinger U, Jones DTW, Glas M, et al. Gliomatosis cerebri: no evidence for a separate brain tumor entity. Acta Neuropathol 2016;131:309-19. [Crossref] [PubMed]
- Erdogan O, Sakar M, Bozkurt S, et al. Low grade oligodendroglioma seeding around the 4th ventricle. Br J Neurosurg 2019; Epub ahead of print. [Crossref] [PubMed]
- Andersen BM, Miranda C, Hatzoglou V, et al. Leptomeningeal metastases in glioma: The Memorial Sloan Kettering Cancer Center experience. Neurology 2019;92:e2483-91. [Crossref] [PubMed]
- Pekmezci M, Rice T, Molinaro AM, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol 2017;133:1001-16. [Crossref] [PubMed]
- Arita H, Narita Y, Fukushima S, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol 2013;126:267-76. [Crossref] [PubMed]
- Nonoguchi N, Ohta T, Oh JE, et al. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol 2013;126:931-7. [Crossref] [PubMed]
- Mair MJ, Leibetseder A, Heller G, et al. Early Postoperative Treatment versus Initial Observation in CNS WHO Grade 2 and 3 Oligodendroglioma: Clinical Outcomes and DNA Methylation Patterns. Clin Cancer Res 2022;28:4565-73. [Crossref] [PubMed]
- Ostrom QT, Cioffi G, Waite K, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol 2021;23:iii1-105. [Crossref] [PubMed]
- Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the CimpacT-NOW criteria. Neuro Oncol 2020;22:515-23. [Crossref] [PubMed]
- Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012;22:425-37. [Crossref] [PubMed]
- Slegers RJ, Blumcke I. Low-grade developmental and epilepsy associated brain tumors: a critical update 2020. Acta Neuropathol Commun 2020;8:27. [Crossref] [PubMed]
- Thom M, Blümcke I, Aronica E. Long-term epilepsy-associated tumors. Brain Pathol 2012;22:350-79. [Crossref] [PubMed]
- Wefers AK, Stichel D, Schrimpf D, et al. Isomorphic diffuse glioma is a morphologically and molecularly distinct tumour entity with recurrent gene fusions of MYBL1 or MYB and a benign disease course. Acta Neuropathol 2020;139:193-209. [Crossref] [PubMed]
- Chiang J, Harreld JH, Tinkle CL, et al. A single-center study of the clinicopathologic correlates of gliomas with a MYB or MYBL1 alteration. Acta Neuropathol 2019;138:1091-2. [Crossref] [PubMed]
- Qaddoumi I, Orisme W, Wen J, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 2016;131:833-45. [Crossref] [PubMed]
- Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 2013;45:602-12. [Crossref] [PubMed]
- Ampie L, Choy W, DiDomenico JD, et al. Clinical attributes and surgical outcomes of angiocentric gliomas. J Clin Neurosci 2016;28:117-22. [Crossref] [PubMed]
- Wang M, Tihan T, Rojiani AM, et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol 2005;64:875-81. [Crossref] [PubMed]
- Kurokawa R, Baba A, Emile P, et al. Neuroimaging features of angiocentric glioma: A case series and systematic review. J Neuroimaging 2022;32:389-99. [Crossref] [PubMed]
- Covington DB, Rosenblum MK, Brathwaite CD, et al. Angiocentric glioma-like tumor of the midbrain. Pediatr Neurosurg 2009;45:429-33. [Crossref] [PubMed]
- Weaver KJ, Crawford LM, Bennett JA, et al. Brainstem angiocentric glioma: report of 2 cases. J Neurosurg Pediatr 2017;20:347-51. [Crossref] [PubMed]
- Chan E, Bollen AW, Sirohi D, et al. Angiocentric glioma with MYB-QKI fusion located in the brainstem, rather than cerebral cortex. Acta Neuropathol 2017;134:671-3. [Crossref] [PubMed]
- ’’Aronco L, Rouleau C, Gayden T, et al. Brainstem angiocentric gliomas with MYB-QKI rearrangements. Acta Neuropathol 2017;134:667-9.
- Lellouch-Tubiana A, Boddaert N, Bourgeois M, et al. Angiocentric neuroepithelial tumor (ANET): a new epilepsy-related clinicopathological entity with distinctive MRI. Brain Pathol 2005;15:281-6. [Crossref] [PubMed]
- Ni HC, Chen SY, Chen L, et al. Angiocentric glioma: a report of nine new cases, including four with atypical histological features. Neuropathol Appl Neurobiol 2015;41:333-46. [Crossref] [PubMed]
- Ramkissoon LA, Horowitz PM, Craig JM, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A 2013;110:8188-93. [Crossref] [PubMed]
- Bandopadhayay P, Ramkissoon LA, Jain P, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet 2016;48:273-82. [Crossref] [PubMed]
- Tatevossian RG, Tang B, Dalton J, et al. MYB upregulation and genetic aberrations in a subset of pediatric low-grade gliomas. Acta Neuropathol 2010;120:731-43. [Crossref] [PubMed]
- Huse JT, Snuderl M, Jones DT, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol 2017;133:417-29. [Crossref] [PubMed]
- Riva G, Cima L, Villanova M, et al. Low-grade neuroepithelial tumor: Unusual presentation in an adult without history of seizures. Neuropathology 2018;38:557-60. [Crossref] [PubMed]
- Ida CM, Johnson DR, Nair AA, et al. Polymorphous Low-Grade Neuroepithelial Tumor of the Young (PLNTY): Molecular Profiling Confirms Frequent MAPK Pathway Activation. J Neuropathol Exp Neurol 2021;80:821-9. [Crossref] [PubMed]
- Johnson DR, Giannini C, Jenkins RB, et al. Plenty of calcification: imaging characterization of polymorphous low-grade neuroepithelial tumor of the young. Neuroradiology 2019;61:1327-32. [Crossref] [PubMed]
- Chen Y, Tian T, Guo X, et al. Polymorphous low-grade neuroepithelial tumor of the young: case report and review focus on the radiological features and genetic alterations. BMC Neurol 2020;20:123. [Crossref] [PubMed]
- Bale TA, Sait SF, Benhamida J, et al. Malignant transformation of a polymorphous low grade neuroepithelial tumor of the young (PLNTY). Acta Neuropathol 2021;141:123-5. [Crossref] [PubMed]
- Ryall S, Zapotocky M, Fukuoka K, et al. Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell 2020;37:569-83.e5. [Crossref] [PubMed]
- Mackay A, Burford A, Carvalho D, et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017;32:520-37.e5. [Crossref] [PubMed]
- Mondal G, Lee JC, Ravindranathan A, et al. Pediatric bithalamic gliomas have a distinct epigenetic signature and frequent EGFR exon 20 insertions resulting in potential sensitivity to targeted kinase inhibition. Acta Neuropathol 2020;139:1071-88. [Crossref] [PubMed]
- Buczkowicz P, Bartels U, Bouffet E, et al. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 2014;128:573-81. [Crossref] [PubMed]
- Solomon DA, Wood MD, Tihan T, et al. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol 2016;26:569-80. [Crossref] [PubMed]
- Gao Y, Feng YY, Yu JH, et al. Diffuse midline gliomas with histone H3-K27M mutation: A rare case with PNET-like appearance and neuropil-like islands. Neuropathology 2018;38:165-70. [Crossref] [PubMed]
- Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 2014;46:451-6. [Crossref] [PubMed]
- Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 2015;130:815-27. [Crossref] [PubMed]
- Castel D, Philippe C, Kergrohen T, et al. Transcriptomic and epigenetic profiling of‘’diffuse midline gliomas, H3 K27M-mutan’’ discriminate two subgroups based on the type of histone H3 mutated and not supratentorial or infratentorial location. Acta Neuropathol Commun 2018;6:117. [Crossref] [PubMed]
- Nikbakht H, Panditharatna E, Mikael LG, et al. Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nat Commun 2016;7:11185. [Crossref] [PubMed]
- Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 2014;46:444-50. [Crossref] [PubMed]
- Hoffman LM, DeWire M, Ryall S, et al. Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun 2016;4:1. [Crossref] [PubMed]
- Castel D, Kergrohen T, Tauziède-Espariat A, et al. Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3-K27M mutation. Acta Neuropathol 2020;139:1109-13. [Crossref] [PubMed]
- Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 2018;36:1963-72. [Crossref] [PubMed]
- Korshunov A, Capper D, Reuss D, et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol 2016;131:137-46. [Crossref] [PubMed]
- Varlet P, Le Teuff G, Le Deley MC, et al. WHO grade has no prognostic value in the pediatric high-grade glioma included in the HERBY trial. Neuro Oncol 2020;22:116-27. [Crossref] [PubMed]
- Tauziède-Espariat A, Debily MA, Castel D, et al. The pediatric supratentorial MYCN-amplified high-grade gliomas methylation class presents the same radiological, histopathological and molecular features as their pontine counterparts. Acta Neuropathol Commun 2020;8:104. [Crossref] [PubMed]
- Sturm D, Orr BA, Toprak UH, et al. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell 2016;164:1060-72. [Crossref] [PubMed]
- Korshunov A, Schrimpf D, Ryzhova M, et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol 2017;134:507-16. [Crossref] [PubMed]
- López GY, Van Ziffle J, Onodera C, et al. The genetic landscape of gliomas arising after therapeutic radiation. Acta Neuropathol 2019;137:139-50. [Crossref] [PubMed]
- Guerreiro Stucklin AS, Ryall S, Fukuoka K, et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun 2019;10:4343. [Crossref] [PubMed]
- Clarke M, Mackay A, Ismer B, et al. Infant High-Grade Gliomas Comprise Multiple Subgroups Characterized by Novel Targetable Gene Fusions and Favorable Outcomes. Cancer Discov 2020;10:942-63. [Crossref] [PubMed]
- Olsen TK, Panagopoulos I, Meling TR, et al. Fusion genes with ALK as recurrent partner in ependymoma-like gliomas: a new brain tumor entity? Neuro Oncol 2015;17:1365-73. [Crossref] [PubMed]


