
Proton radiotherapy for glioma and glioblastoma
Introduction
Radiotherapy (RT) is an integral part of modern multimodal therapy for cancer, particularly those affecting the central nervous system (CNS). RT techniques have improved with time, and many options have become available in the interest of minimizing adverse effects and improving survival rates and quality of life. Proton therapy (PT), or proton beam radiation therapy (PBRT), offers certain advantages to other modern photon-based conformal therapy when used in the treatment of CNS malignancies. In this review, we assess existing clinical data examining the use of PT in gliomas focusing on malignant glioma including glioblastoma multiforme (GBM). We survey the foundational evidence supporting the tissue sparing effects of PT when used for targeting CNS malignancies and examine how the aggressive nature of GBM provides a uniquely challenging arena for implementing PT based therapy. We review the early clinical data of PT for GBM, high grade, low grade, and recurrent glioma. We identify the few ongoing clinical investigations directly comparing PT to photon-based therapy for GBM and low-grade glioma, and identify key areas where data is needed to influence current clinical practice. Finally, we conclude with the promise of using PT as a tool to implement FLASH RT.
PT for the treatment of glioma
Low-grade gliomas (LGG) are defined by the World Health Organization (WHO) as tumors that fall within Grades I and II, in comparison to the Grade III and IV high-grade gliomas including GBM. LGGs show less aggressive tendencies than their high-grade counterparts and generally better prognoses, but the difficulty in their treatment lies in their diversity and longevity (1). They can vary in histological staining and molecular marker mutations, as well as some chromosomal deletions. Much like in the case of high-grade gliomas, LGGs are managed mainly by resection, radiation, and chemotherapy, and studies have shown that the extent of resection is directly correlated with overall survival (2). The treatment delivered often depends on patient preference and level of risk. Radiation and chemotherapy are generally only recommended for patients deemed to be high risk either by age or resection status, and there isn’t much clinical data to confirm if RT can decrease toxicity and minimize adverse effects over other treatment options (3). Clinical studies in general regarding the management of LGG have been difficult to complete due to the longer overall survival of patients and rarity of such tumors.
GBM is considered a Grade IV glioma by the WHO. It’s the most malignant primary CNS tumor and has the worst prognosis for patients, with a 5-year survival rate less than 6% (4). As an aggressive cancer that forms from the astrocytes that support nerve cells, it is extremely difficult to treat. GBM causes blood-brain barrier (BBB) dysfunction and has intertumoral and intratumoral heterogeneity, leading to challenges in developing therapeutics and treatment approaches for the entire tumor (5). GBM is a highly invasive cancer with infiltration of the surrounding healthy brain tissue, which makes gross total resection difficult and necessitates adjuvant RT. Current standard-of-care treatment is surgical resection to debulk the tumor, followed by temozolomide and radiation, but the recurrence rate for GBM post-treatment remains high. For patients with recurrence, there is unfortunately no standard-of-care, and treatment options are limited to repeated surgery, systemic therapy, or re-irradiation (reRT) depending on the presentation. The role of RT in GBM treatment, including hypofractionation for the elderly, advances in treatment planning, and salvage reRT continues to be investigated (6). Additionally, the first small scale studies using PT and a few direct comparisons between PT and modern conformal photon-based therapies such as intensity modulated radiotherapy (IMRT) are beginning to inform the use of PT in treating GBM.
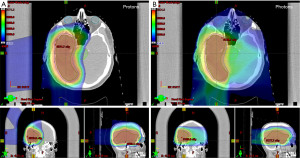
The potential benefits of PT over photon-based RT are due to the intrinsic properties of heavy particles. Conventional photon-based therapy has an inevitable exit dose of radiation as photons deposit energy along the X-ray beam path, and the energy deposition is highest near the point of entry. In contrast, protons decelerate faster than photons and deposit more energy as they slow and interact with surrounding tissue, culminating in the Bragg peak, depositing maximum dose at a depth specific to the tumor (7). Additionally, the relative biological effectiveness (RBE), or the comparison of two types of radiation to give the same biological effect, of protons is slightly higher than that of photon beams. Clinically, the accepted RBE value is 1.1, and the corresponding dose administered using PT is scaled using this factor (8). PT thus can dramatically limit the off-target dose received when used to treat CNS tumors such as GBM (Figure 1). This in turn may prevent side effects caused by normal tissue irradiation, which in the case of CNS tumors include cognitive impairment, lymphopenia, and sensory deficits. Patients can also receive higher doses in narrower beams that could increase treatment effectiveness and precision. Studies examining the use of PT in CNS tumors have suggested acceptable rates of tumor control and toxicity comparable to photon-based RT (9). PT has found a home in the treatment of CNS tumors in pediatric patients, who are the susceptible to long term toxicities such as developing secondary cancers and developmental delay (10-13).
PT is additionally becoming more accessible, with the instillation of multiple single-room centers across the country, as well as increased availability in large academic centers where hybrid plans are feasible. Most glioma patients are still being treated with traditional photon-based RT, but the number being treated with PT is increasing. However, there unfortunately remains a dearth of evidence directly comparing PT to photon-based therapy due largely to difficulties in accruing patients for clinical trials.
Randomized clinical trials are crucial in determining whether new treatment modalities provide a meaningful benefit to patient care, but they have been difficult to complete for PT for various reasons including insurance coverage and patient preference of treatment (14). Due to the cost of PT patient participation in clinical studies often isn’t covered by insurance companies leading to under-recruitment of PT arms in comparative studies. The National Cancer Institute (NCI) and the Patient-Centered Outcomes Research Institute (PCORI) have attempted to fund seven randomized trials investigating PT for breast, lung, prostate, esophageal, and liver cancers, as well as GBM and low-grade glioma, but all enrolled patients much slower than expected. The breast cancer trial, RadComp, is the only one that is currently open through PCORI. Nevertheless, given the high clinical priority of GBM, there is a significant amount of early data from small studies, as well as limited data directly comparing PT to photon-based treatment that may be useful in guiding clinical decision making and future study design.
Dose escalation in GBM: balancing survival and treatment effect
PT may be best positioned to reduce toxicity in slow progressing tumors and pediatric patients. However, the extremely aggressive nature of GBM and the clear dose-response relationship for glioma led to early work focusing on dose escalation. A single institute, single arm trial of PT/photon dose escalation to 90 Gy with data from 23 GBM patients showed a median overall survival of 20 months which was improved by 5–11 months relative to contemporary studies treating with conventional doses (15). Despite suggested improved survival, virtually all patients developed new areas of gadolinium enhancement on magnetic resonance imaging (MRI). Most patients experiencing significant neurocognitive side effects and 8 required reoperation following RT—6 of whom initially showed radiation necrosis without recurrent tumor. Radiation necrosis and tumor progression are difficult to disentangle, but here the authors used detailed histological data from autopsies of 15 of the patients and demonstrated that essentially all instances of confirmed tumor recurrence were limited to the periphery of treatment fields that received less than 90 Gy. Therefore, dose escalation allowed for very effective central control, but with MRI changes within the treatment field indicating significant and often symptomatic treatment effect. The same group published a similar single arm phase I/II trial evaluating dose escalation to 68.2 and 79.7 Gy in WHO grade II and III gliomas respectively which did not suggest an overall survival, and similarly included several cases of radiation necrosis (16).
A later phase I/II trial evaluated concomitant boost PT to 96.6 Gy treated with concurrent nimustine hydrochloride and found a median overall survival of 21.6 months (17). Here, patients were selected with a max pre-treatment tumor diameter of 4 cm compared to approximately 5 cm (60 mL) in the above study by Fitzek et al. (16). Fourteen of the 20 patients in this study demonstrated MRI changes consistent with a combination of radiation necrosis and/or progression, with a median MRI-change free survival of 11.2 months. Six required reoperation within a year after RT, all of whom showed a mix of necrosis and residual tumor. A follow-up study on this series of patients found that 6 of 23 patients demonstrated long-term survival of 52.8–81 months (18). Notably, these patients were all diagnosed with radiation necrosis with 5 requiring necrotomy, and all had a clinical target volume (CTV1) receiving boost dose of <36 cc, while the median CTV1 of cases that developed recurrence was 93.5 cc.
Overall, the above studies do indicate that dose escalation to >90 Gy may be beneficial for local control of GBM, and as such have the potential to improve overall survival. However, the risk for adverse effects, particularly radiation necrosis, is high if not certain. Nevertheless, in select patients where the treatment field can completely cover a relatively small tumor site, dose escalation may eventually prove safe enough to be a reasonable option. Notably, the above studies did not incorporate modern multimodal treatments for GBM, including chemoradiation with temozolamide as well as second line agents like bevacizumab and low-intensity tumor treating fields (Optune®), which combined have led to increases in overall survival on the order of several months. Additionally, we now know more about how the heterogenous molecular characteristics of GBM influence outcomes, with isocitrate dehydrogenase (IDH) and O6-methylguanine-DNA methyltransferase (MGMT) status associated with better prognosis (19). This begs the question as to whether PT could provide an improved neurocognitive side-effect profile and quality of life with standard (~60 Gy) doses in order to better maintain functional status and quality of life when combined with these modern approaches.
Improving side effect profiles using standard dose PT
The first ever phase II direct comparison of PT to IMRT in GBM patients (Table 1) treated to 60 Gy with concurrent and adjuvant temozolomide chemotherapy was designed to evaluate if PT delayed cognitive decline when given at standard doses (20). Dosimetric analysis demonstrated that PT was effective at reducing minimum, average, and maximum dose to essentially all analyzed structures outside the treatment field, however there was no significant difference in the primary endpoint of time to cognitive failure. Among several patient reported outcomes within 6 months of treatment, PT was only associated with a lower rate of fatigue compared to IMRT (24% vs. 58%). There was additionally no difference in progression free survival, or overall survival between groups. There was a decreased risk of grade 2 or higher toxicities in patients who received PT compared to IMRT. Notably, the progression free survival in this trial was only 6.6 months in the PT arm, reflecting an inclusive patient population with no selection based on positive prognostic factors and a majority of whom did not receive MGMT testing.
Table 1
| Study | Diagnosis | # of patients | Intervention | Outcomes | Results | |
|---|---|---|---|---|---|---|
| Primary | Secondary | |||||
| Brown et al., 2021 | GBM | 67 | PT vs. IMRT (standard dose) |
Time to cognitive failure | OS, PFS, toxicity, patient reported outcomes | Lower fatigue, reduced grade II+ toxicity in PT group |
| Mohan et al., 2021 | GBM | 67 | PT vs. IMRT (standard dose) |
Incidence of grade 3+ lymphopenia | Reduced in PT group | |
| NRG-BN001 | GBM | 624 (closed) | PT vs. IMRT (dose-escalated + boost) vs. standard dose RT |
OS (vs. conventional) | OS (vs. IMRT), PFS, MRI progression, toxicity | – |
| NRG-BN005 | Grade II or III IDH + glioma | 120 (target) | PT vs. IMRT (standard dose) |
CTB COMP score | OS, PFS, toxicity, patient reported outcomes | – |
PT, proton therapy; GBM, glioblastoma multiforme; IDH, isocitrate dehydrogenase; IMRT, intensity modulated radiotherapy; OS, overall survival; PFS, progression free survival; CTB COMP, combined standardized neurocognitive score; MRI, magnetic resonance imaging.
Subsequent analysis of the same group of patients revealed that there was a lower incidence of grade 3+ lymphopenia within one month of RT in patients treated with PT compared to IMRT (21). Here, the strongest predictor in addition to sex (women have a greater incidence) and baseline lymphocyte count was whole-brain V20 (i.e., the volume receiving at least 20 Gy), which correspondingly was reduced in the PT group. While the above data reaffirm the ability of PT to reduce off-target dose when treating GBM to standard 60 Gy, the potential long term cognitive benefits of reducing exposure of normal tissue may simply be masked by the aggressive course of GBM without selection based on prognostic factors (22). It is possible the tissue sparing benefits of PT prove useful in GBM cases selected for positive molecular and clinical features, or in LGG, which has a more favorable prognosis.
Unfortunately, there is equally sparse data available on the treatment of LGG with PT. Early retrospective data demonstrated low acute toxicity comparable to IMRT (23,24). A prospective cohort study followed 20 patients with grade 2 LGG over 5 years with a detailed battery of neuropsychiatric tests following PT and found that patients exhibited overall stability in their cognition, mood, and performance status (25,26). Additionally, patient reported quality of life showed no significant changes over time. The progression-free survival was 85% and 40% at 3 and 5 years respectively, and overall survival was 84% at 5 years. No patients in this study received concurrent chemotherapy which worsens toxicity compared to RT alone, and which has become standard of care since recent long-term phase 3 data has shown an overall and progression free survival (27). Larger head-to-head studies of PT versus photon therapy using current chemoradiation protocols with prolonged follow-up are ultimately required to identify and potential benefits in long-term side effect profiles.
PT as an option for salvage therapy of local tumor recurrence
PT has drawn interest in yet another arena; local and regional recurrence of GBM and malignant glioma presents a unique challenge due to the limits of reRT and reoperation (28). For GBM, the rate of recurrence within the prior treatment field approaches 80%, and the toxicity associated with reRT using photon-based therapy, particularly when the interval to reRT is short, can be significant (29). Nevertheless, reRT has proven feasible and potentially beneficial in the treatment of recurrent glioma and GBM, particularly when reoperation is not possible and patients fail salvage with a systemic agent such as bevacizumab (30-32). Most recently, a phase II trial demonstrated improved progression free survival at 6 months when reRT with 35 Gy combined with bevacizumab was compared to bevacizumab alone, however without showing an improvement in median overall survival (33). reRT was generally well tolerated with grade III or higher adverse events in only 5% of patients. Several lines of research are open attempting to improve image guidance and the challenge of delineating treatment effect/pseudoprogression vs. recurrent tumor which may eventually lead to better refined target volumes and less unnecessary irradiation (34-36). However, even with theoretically perfect identification of tumor, PT-based reRT may be appropriate for minimizing dose to previously irradiated tissue.
Data specifically using PT to treat recurrence in 8 mostly high-grade glioma patients with a median reRT dose of 33 Gy and initial dose of 55 Gy reported a median overall survival of 19.4 months with only two cases of uncomplicated radiation necrosis and no acute toxicity greater than grade 2 (37). Results from a separate institute, again using PT in 20 patients with a recurrence of mostly high-grade glioma, found no grade 3+ toxicities although there were 2 cases of radiation necrosis requiring hyperbaric oxygen and surgery (38). Here, the median doses were higher with an initial photon-based dose of 48–60 Gy and a reRT dose of 30–54 Gy depending on WHO grade. Median overall survival after reRT with PT was 7.8 months for GBM and 24.9 months for grade III glioma. A multicenter prospective study of 45 patients with recurrent GBM (median time of recurrence: 20.2 months) similarly found low rates of toxicity with only a single case of acute grade 3, and 4 patients with late grade 3 toxicities (39). Results from reRT of other primary CNS tumors, including several studies of pediatric patients, have generally also shown comparable survival to photon-based data along with favorable toxicity profiles (40-42).
Ongoing clinical investigations
There are ongoing clinical trials directly comparing PT to photon-based therapy which largely segregate alone the themes described above (Table 1). In terms of dose escalation, NRG-BN001 is a phase II multi-center trial of hypofractionated dose-escalated PT or photon based IMRT directly compared to conventional photon RT for new GBM. Patients will also receive concurrent and adjuvant temozolomide. At the time of this publication, the study is closed for accrual with 624 total enrolled patients. The primary outcome of this study is overall survival of either PT or IMRT based dose escalation compared to standard dose conformal photon therapy, with secondary outcomes also comparing OS of IMRT to PT, progression free survival, and toxicity including neurocognitive testing and the incidence of both tumor progression and pseudo-progression based on MRI imaging with a planned follow-up of 5 years. Overall, this study will be the first large, multicenter direct comparison between PT and photon-based therapy in GBM. However, its design will also be able to address whether dose-escalation may have a place in current multimodal therapeutic regimens. If so, PT may become a reasonable means for dose-escalation regardless of a direct benefit based on modality.
NRG-BN005 is another randomized phase II trial investigating whether PT or IMRT at standard doses with adjuvant temozolomide can preserve brain function in IDH mutant grade II or III IDH mutation positive glioma. The study is currently open to accrual with an estimated enrollment size of 120 patients. In this study the primary outcome is instead focused on long-term cognitive outcomes and quality of life. With a goal follow-up of 10 years, patients will undergo detailed cognitive testing at baseline and with each follow-up. While focusing on lower grade disease with better prognosis, as our understanding of GBM continues to improve along with better treatment options, the results of this trial may ultimately influence how the tissue sparing properties of PT are viewed for the treatment of high-grade glioma.
FLASH RT: potential implementation with PT
As highlighted above, PT has intrinsic benefits that allow for more effective delivery of radiation to tumors while sparing normal tissue, even when given at standard doses and rates. Within the last ten years, FLASH RT has emerged as yet another method to decrease damage to normal tissue while maintaining similar tumor control, resulting in a widened therapeutic index. FLASH refers to the delivery of radiation with ultra-high dose rates (~40–60 Gy/s) which demonstrates remarkable normal tissue sparing in preclinical studies while maintaining similar tumor control compared to dose-matched conventional rates (~2 Gy/min) (43,44). While the radiobiology underlying this benefit is not fully understood, the ‘FLASH effect’ has been demonstrated in vivo in multiple small animal models across multiple organ systems (45). For instance, mice treated with 10 Gy whole brain electron RT at >60 Gy/s had almost complete protection from subsequent cognitive impairment (46).
A major practical hurdle has been developing delivery methods that have dose rates high enough to achieve the FLASH effect. Nearly all the initial studies describing FLASH use electron beam RT, which can deliver fast dose rates but has very poor tissue penetration. In terms of currently available clinical tools, PT delivered via pencil beam scanning (PBS) can achieve higher dose rates than photon RT, with FLASH rates possible when PBS systems are localized to individual spot positions, leading to the enticing possibility of combining the intrinsic spatial benefits of PT with the temporal advantage of FLASH to achieve unprecedented levels of normal tissue sparing (47). Interestingly, the first studies using proton-based FLASH were mixed, with several unable to reproduce the FLASH effect seen with electrons in vitro or in vivo (48-52). However, recently several in vivo studies have provided very promising results.
Researchers from our home institution developed a novel small animal model apparatus that used a 230 MeV accelerator with double-scattered protons and CT-guidance and can deliver 60–100 Gy/s as well as conventional rates ~1 Gy/s (53). Using this method, mice that received 15–18 Gy of whole abdominal irradiation showed both significantly less acute toxicity as was as long-term development of fibrosis when the treatment was given at FLASH rates. Notably, control of pancreatic tumor lines was identical between conventional and FLASH groups. Similar results using both PBS-based and scattering in mice have generally shown improvements in toxicities such as weight loss, skin damage, and muscle contractures while maintaining effectiveness against a range of tumor cell lines (54-56). Nevertheless, there is still a tremendous amount of work that needs to be done both in terms of understanding the underlying radiobiology of how FLASH works, and in standardizing and scaling the delivery of FLASH to ‘human-sized’ volumes. Regardless, PT is poised as the tool to make this translation possible. Proton systems capable of delivering FLASH rate doses are available, and the first several phase I trials of PT based FLASH are underway (57). The treatment of CNS based tumors such as GBM stands to gain immensely if the tissue sparing effects of FLASH are successfully translated to the clinic.
Conclusions
In this review, we have surveyed the available data regarding the use of PT for treating GBM and high-grade glioma, as well as both ongoing clinical and translational work that has the potential to impact proton-based approaches. The available evidence is limited, with only a single head-to-head comparison of PT vs. photon-based therapy in GBM available. At this time, there is insufficient evidence to show improved outcomes with PT in GBM, and because the disease is so aggressive, the long-term cognitive benefits of sparing tissue with PT may simply be masked by disease progression. Nevertheless, there are several distinct avenues in which PT may eventually work its way into practice.
There is no evidence currently suggesting that PT at standard doses provides any meaningful reduction in toxicity for GBM patients, and in fact, the only available direct comparison between PT and photon-based therapy in GBM indicates that there is no difference in cognitive outcomes. However, as the therapeutic options for GBM continue to improve survival, and as we become better at identifying molecular subtypes of GBM with better prognoses, using PT to spare normal tissue and improve cognitive outcomes may eventually become desirable. For low grade glioma, we have early data suggesting that PT may have a more favorable side effect profile, and NRG-BN001 will look to expand on this data with extended follow-up times.
With dose escalation, there is a fine balance between the benefit of improved local control with an increased safety risk. Early data hints at an overall survival benefit with perhaps manageable toxicity in select patients, however without the guidance of either molecular profiling or inclusion of temozolomide, both of which are now standard of care. Future studies such as NRG-BN005 will be able to better address the question of dose escalation in the context of current practice guidelines. It will be interesting to see if any secondary factors influence the outcome of high dose PT, such as IDH status or tumor size.
Similarly, reRT of GBM and recurrent glioma is an important method of salvage therapy that may be beneficial under the right circumstances. Given that recurrence can occur rapidly with GBM, there is an increased risk of toxicity from reRT. In general, reRT with modern photon-based therapy appears to be relatively safe and potentially effective at delaying tumor progression. While there is no direct evidence comping PT to photons in this context, early data from the treatment of GBM and recurrent glioma, as well as data from reRT of other CNS tumors indicates that PT may have a particularly favorable side effect profile without sacrificing efficacy. Prospective direct-comparison studies will be needed to better understand how PT compares to photons, and in general more phase III data of reRT in glioma is needed to guide practice.
Despite the paucity of high-level data available for PT, in our practice we find that PT may be the best tool for safely delivering radiation in select cases and may be relatively contraindicated in others. Based on the studies described above, PT stands to provide the greatest benefit when used in relatively young GBM patients with good functional status and favorable molecular profiles. Additionally, patients with increased risk of lymphopenia could likely benefit from reducing total brain irradiation using PT. Creating comparison plans with the available photon-based therapy is also useful to estimate the overall tissue sparing benefit of PT. This is particularly true when evaluating patients for reRT, or with tumors near sensitive structures. Reasons to forgo PT in GBM would include rapid progression from the time of post-operative imaging to simulation, or the potential for rapid fluctuations in anatomy that would require re-evaluation scans (presence of a ventriculoperitoneal shunt, although not prohibitive). Finally, treatment should not be significantly delayed in order to pursue PT over modern photon-based approaches.
GBM remains difficult to treat and presents patients with a disappointingly unfavorable prognosis. PT at this time has limited data however, this may change with time as we accrue more high-level data on dose escalation and cognitive sparing effects of PT and use could accelerate if PT provides a successful medium for translating FLASH to the clinical setting. There remains room for major gains in outcomes which will likely be driven by a combination of advances in the multimodal treatment of GBM including novel therapeutics and their combination with RT. PT may ultimately be an important part of those advances by expanding the therapeutic window of dose escalation, reRT, or through the development of FLASH RT. Critically, continued research is needed to effectively and productively collaborate with our partners in the insurance sector in order to expand the availability of PT. Many challenges remain with designing, implementing, and executing trials of PT. Studies like NRG-BN001 and NRG-BN005, and eventually more like them, will be crucial in guiding the integration of PT into the treatment of GBM.
Acknowledgments
We would like to acknowledge Dr. Robert A. Lustig for his meaningful review of the manuscript.
Funding: None.
Footnote
Provenance and peer review: This article was commissioned by the Guest Editors (Joshua Palmer, Iyad Alnahhas and Wenyin Shi) for the series “Recent Advances in Neuro-Oncology” published in Chinese Clinical Oncology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-92/coif). The series “Recent Advances in Neuro-Oncology” was commissioned by the editorial office without any funding or sponsorship. MAB received support for attending meetings from IBA: Travel for Proton Meeting April 2022 and Varian: Travel for User Meeting 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev 2017;40:1-14. [Crossref] [PubMed]
- Kiliç T, Ozduman K, Elmaci I, et al. Effect of surgery on tumor progression and malignant degeneration in hemispheric diffuse low-grade astrocytomas. J Clin Neurosci 2002;9:549-52. [Crossref] [PubMed]
- Brown TJ, Bota DA, van Den Bent MJ, et al. Management of low-grade glioma: a systematic review and meta-analysis. Neurooncol Pract 2019;6:249-58. [Crossref] [PubMed]
- Shergalis A, Bankhead A 3rd, Luesakul U, et al. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol Rev 2018;70:412-45. [Crossref] [PubMed]
- Noch EK, Ramakrishna R, Magge R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg 2018;116:505-17. [Crossref] [PubMed]
- Mann J, Ramakrishna R, Magge R, et al. Advances in Radiotherapy for Glioblastoma. Front Neurol 2018;8:748. [Crossref] [PubMed]
- LaRiviere MJ, Santos PMG, Hill-Kayser CE, et al. Proton Therapy. Hematol Oncol Clin North Am 2019;33:989-1009. [Crossref] [PubMed]
- Patyal B. Dosimetry aspects of proton therapy. Technol Cancer Res Treat 2007;6:17-23. [Crossref] [PubMed]
- Yerramilli D, Bussière MR, Loeffler JS, et al. Proton Beam Therapy (For CNS Tumors). In: Adult CNS Radiation Oncology Cham: Springer International Publishing; 2018:709–22.
- Baliga S, Yock TI. Proton beam therapy in pediatric oncology. Curr Opin Pediatr 2019;31:28-34. [Crossref] [PubMed]
- Mizumoto M, Oshiro Y, Yamamoto T, et al. Proton Beam Therapy for Pediatric Brain Tumor. Neurol Med Chir (Tokyo) 2017;57:343-55. [Crossref] [PubMed]
- Chhabra A, Mahajan A. Treatment of common pediatric CNS malignancies with proton therapy. Chin Clin Oncol 2016;5:49. [Crossref] [PubMed]
- Kirsch DG, Tarbell NJ. New technologies in radiation therapy for pediatric brain tumors: the rationale for proton radiation therapy. Pediatr Blood Cancer 2004;42:461-4. [Crossref] [PubMed]
- Bekelman JE, Denicoff A, Buchsbaum J. Randomized Trials of Proton Therapy: Why They Are at Risk, Proposed Solutions, and Implications for Evaluating Advanced Technologies to Diagnose and Treat Cancer. J Clin Oncol 2018;36:2461-4. [Crossref] [PubMed]
- Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg 1999;91:251-60. [Crossref] [PubMed]
- Fitzek MM, Thornton AF, Harsh G 4th, et al. Dose-escalation with proton/photon irradiation for Daumas-Duport lower-grade glioma: results of an institutional phase I/II trial. Int J Radiat Oncol Biol Phys 2001;51:131-7. [Crossref] [PubMed]
- Mizumoto M, Tsuboi K, Igaki H, et al. Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2010;77:98-105. [Crossref] [PubMed]
- Mizumoto M, Yamamoto T, Takano S, et al. Long-term survival after treatment of glioblastoma multiforme with hyperfractionated concomitant boost proton beam therapy. Pract Radiat Oncol 2015;5:e9-16. [Crossref] [PubMed]
- Yang P, Zhang W, Wang Y, et al. IDH mutation and MGMT promoter methylation in glioblastoma: results of a prospective registry. Oncotarget 2015;6:40896-906. [Crossref] [PubMed]
- Brown PD, Chung C, Liu DD, et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol 2021;23:1337-47. [Crossref] [PubMed]
- Mohan R, Liu AY, Brown PD, et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neuro Oncol 2021;23:284-94. [Crossref] [PubMed]
- Press RH, Chhabra AM, Choi JI, et al. Proton therapy for newly diagnosed glioblastoma: More room for investigation. Neuro Oncol 2021;23:1980-1. [Crossref] [PubMed]
- Hauswald H, Rieken S, Ecker S, et al. First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol 2012;7:189. [Crossref] [PubMed]
- Wilkinson B, Morgan H, Gondi V, et al. Low Levels of Acute Toxicity Associated With Proton Therapy for Low-Grade Glioma: A Proton Collaborative Group Study. Int J Radiat Oncol Biol Phys 2016;96:E135. [Crossref] [PubMed]
- Sherman JC, Colvin MK, Mancuso SM, et al. Neurocognitive effects of proton radiation therapy in adults with low-grade glioma. J Neurooncol 2016;126:157-64. [Crossref] [PubMed]
- Shih HA, Sherman JC, Nachtigall LB, et al. Proton therapy for low-grade gliomas: Results from a prospective trial. Cancer 2015;121:1712-9. [Crossref] [PubMed]
- Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med 2016;374:1344-55. [Crossref] [PubMed]
- Verma V, Rwigema JCM, Malyapa RS, et al. Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radiother Oncol 2017;125:21-30. [Crossref] [PubMed]
- Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 2008;70:1350-60. [Crossref] [PubMed]
- Shi W, Scannell Bryan M, Gilbert MR, et al. Investigating the Effect of Reirradiation or Systemic Therapy in Patients With Glioblastoma After Tumor Progression: A Secondary Analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int J Radiat Oncol Biol Phys 2018;100:38-44. [Crossref] [PubMed]
- Straube C, Kessel KA, Zimmer C, et al. A Second Course of Radiotherapy in Patients with Recurrent Malignant Gliomas: Clinical Data on Re-irradiation, Prognostic Factors, and Usefulness of Digital Biomarkers. Curr Treat Options Oncol 2019;20:71. [Crossref] [PubMed]
- Youland RS, Lee JY, Kreofsky CR, et al. Modern reirradiation for recurrent gliomas can safely delay tumor progression. Neurooncol Pract 2018;5:46-55. [Crossref] [PubMed]
- Tsien C, Pugh S, Dicker AP, et al. Randomized Phase II Trial of Re-Irradiation and Concurrent Bevacizumab versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma (NRG Oncology/RTOG 1205): Initial Outcomes and RT Plan Quality Report. Int J Radiat Oncol Biol Phys 2019;105:S78. [Crossref]
- Breen WG, Youland RS, Giri S, et al. Initial results of a phase II trial of (18)F-DOPA PET-guided re-irradiation for recurrent high-grade glioma. J Neurooncol 2022;158:323-30. [Crossref] [PubMed]
- Hygino da Cruz LC Jr, Rodriguez I, Domingues RC, et al. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 2011;32:1978-85. [Crossref] [PubMed]
- de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 2004;63:535-7. [Crossref] [PubMed]
- Mizumoto M, Okumura T, Ishikawa E, et al. Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Technical considerations based on experience at a single institution. Strahlenther Onkol 2013;189:656-63. [Crossref] [PubMed]
- Galle JO, McDonald MW, Simoneaux V, et al. Reirradiation with Proton Therapy for Recurrent Gliomas. Int J Part Ther 2015;2:11-8. [Crossref]
- Saeed AM, Khairnar R, Sharma AM, et al. Clinical Outcomes in Patients with Recurrent Glioblastoma Treated with Proton Beam Therapy Reirradiation: Analysis of the Multi-Institutional Proton Collaborative Group Registry. Adv Radiat Oncol 2020;5:978-83. [Crossref] [PubMed]
- Eaton BR, Chowdhry V, Weaver K, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol 2015;116:301-8. [Crossref] [PubMed]
- Farnia B, Philip N, Georges RH, et al. Reirradiation of Recurrent Pediatric Brain Tumors after Initial Proton Therapy. Int J Part Ther 2016;3:1-12. [Crossref] [PubMed]
- McDonald MW, Linton OR, Shah MV. Proton therapy for reirradiation of progressive or recurrent chordoma. Int J Radiat Oncol Biol Phys 2013;87:1107-14. [Crossref] [PubMed]
- Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014;6:245ra93. [Crossref] [PubMed]
- Vozenin MC, De Fornel P, Petersson K, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res 2019;25:35-42. [Crossref] [PubMed]
- Hughes JR, Parsons JL. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int J Mol Sci 2020;21:6492. [Crossref] [PubMed]
- Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 2017;124:365-9. [Crossref] [PubMed]
- Diffenderfer ES, Sørensen BS, Mazal A, et al. The current status of preclinical proton FLASH radiation and future directions. Med Phys 2022;49:2039-54. [Crossref] [PubMed]
- Beyreuther E, Brand M, Hans S, et al. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother Oncol 2019;139:46-50. [Crossref] [PubMed]
- Buonanno M, Grilj V, Brenner DJ. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol 2019;139:51-5. [Crossref] [PubMed]
- Grilj V, Buonanno M, Welch D, et al. Proton Irradiation Platforms for Preclinical Studies of High-Dose-Rate (FLASH) Effects at RARAF. Radiat Res 2020;194:646-55. [Crossref] [PubMed]
- Schmid TE, Dollinger G, Hauptner A, et al. No evidence for a different RBE between pulsed and continuous 20 MeV protons. Radiat Res 2009;172:567-74. [Crossref] [PubMed]
- Schmid TE, Dollinger G, Hable V, et al. Relative biological effectiveness of pulsed and continuous 20 MeV protons for micronucleus induction in 3D human reconstructed skin tissue. Radiother Oncol 2010;95:66-72. [Crossref] [PubMed]
- Diffenderfer ES, Verginadis II, Kim MM, et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int J Radiat Oncol Biol Phys 2020;106:440-8. [Crossref] [PubMed]
- Cunningham S, McCauley S, Vairamani K, et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers (Basel) 2021;13:1012. [Crossref] [PubMed]
- Zhang Q, Cascio E, Li C, et al. FLASH Investigations Using Protons: Design of Delivery System, Preclinical Setup and Confirmation of FLASH Effect with Protons in Animal Systems. Radiat Res 2020;194:656-64. [Crossref] [PubMed]
- Zlobinskaya O, Siebenwirth C, Greubel C, et al. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat Res 2014;181:177-83. [Crossref] [PubMed]
- Taylor PA, Moran JM, Jaffray DA, et al. A roadmap to clinical trials for FLASH. Med Phys 2022;49:4099-108. [Crossref] [PubMed]


